The variety of intraocular lens (IOL) types has grown dramatically during the past decade, which has spurred much debate about appropriate IOL choices in various clinical situations. Aging populations in the developed world are growing, as is their demand for cataract surgery. Older patients are increasingly presenting with existing or potential multiple ocular comorbidities that may impact IOL performance. This raises the question of whether we should consider which IOL types should or shouldn't be offered to patients with early age-related macular degeneration (AMD) or who are at high risk for developing it.
Effects of cataract surgery
Combined data from three large population-based studies (the Beaver Dam, Rotterdam and Blue Mountains eye studies) suggest that cataract surgery increases the risk for AMD. However, this issue remains contentious, and increased AMD risk in these patients may also be linked to other factors.1 Nevertheless, it appears that this increased risk may relate to the removal of the natural ultraviolet (UV) and short-wavelength visible light-blocking effect of the natural crystalline lens. Both epidemiological and experimental evidence suggest that macular degeneration may, in part, be a light-induced maculopathy.2 One hypothesis is that shorter, energetic wavelengths of light induce retinal toxicity with an inflammatory reaction mediated by oxidative stress, leading to AMD amidst a background of aberrant complement activation.3,4 In other words, in the face of genetic "repair protein" defects, high energy illumination may exceed the repair capacity of the retina for older individuals. This hypothesis also explains why some dietary supplements appear to offer a protective role in what may be cofactor-dependent enzymatic-type genetic errors.
Genetics and AMD risk prediction
Although the full story has yet to be established, the risk of developing AMD can now be predicted fairly accurately on the basis of smoking history and specific inherited genetic variations that can be measured from birth.5 Genetic variations in complement factor H, complement component 3, complement factor I, as well as specific sequence variations in a locus containing the age-related maculopathy sensitivity 2 (ARMS2) gene account for over 60 percent of the risk of developing this disease. Interpretive algorithms have been developed that can now accurately assign individuals to a low AMD risk category, with less than a 5 percent lifetime risk of developing AMD-related visual impairment (50 percent of individuals), average risk (30 percent of individuals) and high risk (20 percent of individuals), with the most extreme group having more than a 65 percent likelihood of developing visual impairment from AMD.6
Although ophthalmologists now suspiciously search for the early signs of macular degeneration, we are looking at phenotypes that may often mislead us in assessing genotype, which now appears to be the ultimate determinant of AMD development. The following case illustrates this.
Click on image to enlarge.
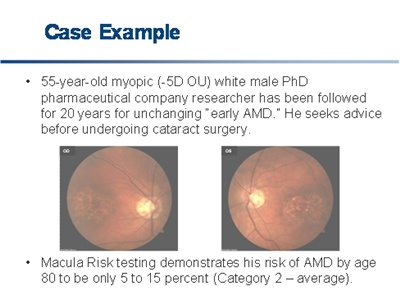
Investigators from both Tufts University and Oregon Health and Science University have advanced predictive algorithms for the progression from dry to wet AMD. Both predictive algorithms are based on a core marker set that includes the complement factor H and the mitochondrial protein, ARMS2.7, 8 The most recent publication on these algorithms has advanced a receiver operating characteristic curve having an AUC ("C statistic") of 0.83, which meets the criteria for clinical utility and is consistent with other tests commonly used in medicine, such as the prostate-specific antigen (PSA) test for prostate cancer (AUC = 0.585) and the Troponin T biomarker for myocardial infarction (AUC = 0.85).7, 9-10 Physicians now have a powerful tool to predict the future onset of AMD. The challenge is how to use it.
IOL selection
Since patients undergoing cataract extraction are on average a decade younger than the age when AMD becomes manifest, choosing an appropriate IOL can be partially guided by AMD risk assessment. Independent of IOL selection, patients with the greatest risk of developing AMD should be enrolled in follow-up programs designed to detect foveal choroidal neovascularization at inception. Treatment with intraocular injections of VEGF inhibitors, such as bevacizumab (Avastin) or ranibizumab (Lucentis), is more effective when given earlier, a treatment window often missed.11
Multifocal IOLs are often used to achieve acceptable distance and near visual acuity in selected patients. However, they are limited in application by reduced contrast sensitivity under mesopic and scotopic conditions.12 After multifocal lens implantation, patients with central scotomata rarely return to the same level of visual acuity experienced with monofocal refractive lens exchange. Older eyes, in particular those with macular degenerative changes, have reduced scotopic vision and contrast sensitivity. These patients should not be further impaired by the choice of an IOL that may exacerbate their problems.13 Many authors consider AMD to be a contraindication to the use of multifocal IOLs, suggesting the importance of selecting patients who are less likely to develop macular deterioration later in life.
Blue-filtering lenses have been developed to protect eyes from subsequent age-related macular degeneration. While potentially beneficial, their universal choice for refractive lens exchange would be problematic. Possible side effects include color-vision disturbance and decreased scotopic sensitivity. Sleep-wake timing disruption due to melatonin suppression by the absence of retinal stimulation by light in the 460 nm to 500 nm range may be particularly problematic for individuals living in low-light conditions, such as nursing homes.14 Although these potential downsides of blue-filtering IOLs have not been definitively confirmed, some practitioners and researchers believe that the benefits of tinted IOLs can be optimized through careful patient selection.
Using genetics to optimize IOL choice
The clinical circumstances of patients have thus far guided IOL choice. Recent genetic advances in AMD risk prediction provide one more discriminator for cataract surgeons to optimize IOL selection. It may be preferable to favor blue-filtering lenses over multifocal IOLs for patients at high risk of developing AMD. Low-risk individuals who desire both near and distance vision without further correction may benefit from multifocal IOLs, knowing that they are unlikely to develop AMD. They may also choose to forego tinted IOLs, thus avoiding possible color vision alteration and sleep pattern disruption. This decision-making algorithm is illustrated in the figure below.
Click on image to enlarge.
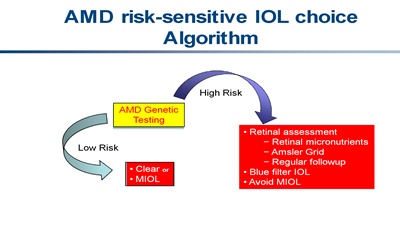
While it is unquestionably important to stratify surveillance protocols for patients at risk for advanced AMD, the advances in genetic testing for AMD and its commercial availability may prove to be of value in guiding IOL selection.
References
- Bockelbrink A, Roll S, Ruether K, Rasch A, Greiner W, Willich SN. Cataract surgery and the development or progression of age-related macular degeneration: a systematic review. Surv Ophthalmol. 2008;53(4):359-367.
- Fletcher AE, Bentham GC, Agnew M, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol. 2008;126(10):1396-1403.
- Szaflik JP, Janik-Papis K, Synowiec E, et al. DNA damage and repair in age-related macular degeneration. Mutat Res. 2009; 669(1-2):169-176.
- Thurman JM, Renner B, Kunchithapautham K, et al. Oxidative Stress Renders Retinal Pigment Epithelial Cells Susceptible to Complement-mediated Injury. J Biol Chem. 2009;284(25):16939-16947.
- Swaroop A, Branham KE, Chen W, Abecasis G. Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Human Molecular Genetics. 2007;16 Spec No 2:R174-182.
- Zanke B, Hawken S, Carter R, Chow D. A genetic approach to stratification of risk for age-related macular degeneration. Can J Ophthalmol. 2009;45(1):22-27.
- Seddon JM, Reynolds R, Maller J, Fagerness JA, Daly MJ, Rosner B. Prediction Model for Prevalence and Incidence of Advanced Age-Related Macular Degeneration Based on Genetic, Demographic, and Environmental Variables. Invest Ophthalmol Vis Sci. 2009; 50(5):2044-2053. .
- Seddon JM, Francis PJ, George S, Schultz DW, Rosner B, Klein ML. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297(16):1793-1800.
- Benecchi L, Pieri AM, Destro Pastizzaro C, Potenzoni M. Optimal measure of PSA kinetics to identify prostate cancer. Urology. 2008;71(3):390-394.
- Keller T, Zeller T, Peetz D, , et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868-877.
- Arias L, Armada F, Donate J, et al. Delay in treating age-related macular degeneration in Spain is associated with progressive vision loss. Eye. 2009;23(2):326-333.
- Martinez Palmer A, Gomez Faina P, Espana Albelda A, et al. Visual function with bilateral implantation of monofocal and multifocal intraocular lenses: a prospective, randomized, controlled clinical trial. J Refract Surg. 2008;24(3):257-264.
- Augustin AJ. The physiology of scotopic vision, contrast vision, color vision, and circadian rhythmicity: can these parameters be influenced by blue-light-filter lenses? Retina. 2008;28(9):1179-1187.
- Cuthbertson FM, Peirson SN, Wulff K, Foster RG, Downes SM. Blue light-filtering intraocular lenses: review of potential benefits and side effects. J Cataract Refract Surg. 2009;35(7):1281-1297.