Cataract/Anterior Segment, Comprehensive Ophthalmology, Cornea/External Disease
Scientists have shown that human cells can be reprogrammed to become stem cells from which sheets of corneal tissue can be grown, and that stem cells in the human eye can be coaxed to regrow clear lenses after cataract surgery.
The 2 studies, published last week in Nature, were likened to science fiction.
“I can guarantee the first reaction is going to be disbelief,” said J. Fielding Hejtmancik, an ophthalmic geneticist at the National Eye Institute. “The work in both studies is exciting but exploratory, and must be followed up with much more detailed clinical studies.”
In the first study, scientists from Osaka and Cardiff Universities cultivated human induced pluripotent stem cells (iPSCs) to produce eyelike structures in lab dishes. The cells grew, forming concentric rings of tissue, with each ring having characteristics of different parts of the eye: cornea, retina and lens. Essentially, the researchers mimicked eye development in a lab dish.
The team then extracted and purified the cornea cells to grow sheets of corneal epithelium that they used to repair damaged corneas in rabbits. The animals recovered with a healthy corneal barrier function and continued to express cornea-specific proteins. Although previous studies have generated retinal or corneal tissue using iPSCs, none has produced a variety of eye cell types in a single experiment. The researchers hope to start clinical trials in 2 to 3 years.
In the second study, scientists developed a surgical procedure that activates the body’s own stem cells to regenerate a clear, functioning lens in infants after cataract surgery. This technique doesn’t require culturing cells outside the body or transplanting material into the body. Instead, scientists left behind enough lens epithelial stem cells (LECs) after cataract surgery to allow the lens to regrow.
Researchers from the University of California at San Diego and Guangzhou University postulated that LEC regrowth after cataract surgery may mean that the body is capable of regenerating an entire lens. Beginning with in vitro studies, they showed that LECs can differentiate into lens fiber cells. Encouraged by the results, the team developed a surgical technique and tested it in rabbits, macaque monkeys and then 12 human infants.
Instead of using a 6 mm-diameter opening at the center of the anterior capsule, resulting in a large wound area and destruction of LECs, they create a 1.5 mm incision on periphery of the lens, preserving the integrity of the lens capsule and the endogenous LECs. By 3 months, the cells grew from the periphery of the capsular bag towards the center in a curvilinear symmetrical pattern, creating a new lens.
The first infant treated 2 years ago has retained good vision. Visual axis transparency was achieved in 95.8% of all eyes. Visual function testing showed the regenerated lenses were functional, with visual acuity that was comparable to those who underwent conventional cataract surgery. Study authors also expect that the regenerated lens should grow as the child grows.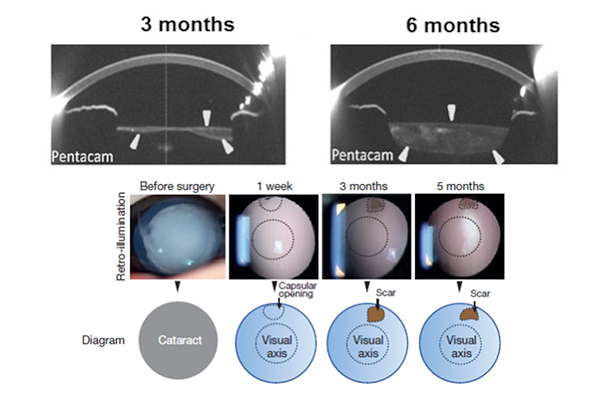
Dr. Rahul Bhola, a pediatric ophthalmologist at the University of Louisville who is not involved in the research, said preserving a few layers of LEC's circumferentially will be technically challenging. He also notes that comparing visual acuity with control eyes using Teller’s Visual Acuity Cards or other grading methods would be interesting. But his biggest concern is with amblyopia prevention as the lens regrows.
“The biggest criticism of this paper for me will be prevention of amblyopia during this critical period as the optical blur will be gradually changing during the entire course of healing,” Dr. Bhola said.
Others point out that the regenerated lenses could become cloudy again.
The study authors remain optimistic. "The success of this work represents a new approach in how new human tissue or organ can be regenerated and human disease can be treated, and may have a broad impact on regenerative therapies by harnessing the regenerative power of our own body," said study author Kang Zhang of the UCSD School of Medicine.