Download PDF
Stanley Holloway* presented to the ophthalmology department at Mayo Clinic for management of bilateral central retinal vein occlusions. The 75-year-old white man had come to Rochester, Minn., to be evaluated by the hematology-oncology department at the Mayo Clinic, and ophthalmic consultation was requested.
His History
Mr. Holloway’s ocular history was extensive. It included anti–vascular endothelial growth factor (anti-VEGF) injections in both eyes for bilateral central retinal vein occlusions; panretinal photocoagulation (PRP) in the left eye for this condition; uncomplicated cataract extraction in both eyes; and timolol maleate (Istalol) in each eye once daily for open-angle glaucoma.
His medical history was significant for a squamous cell carcinoma of the left cheek (with local metastases to the left parotid gland), renal transplant for polycystic kidney disease, transplant-related diabetes mellitus, hyperlipidemia, and hypertension due in part to chronic steroid use.
The patient was scheduled to undergo radiation treatment of the left cheek in the weeks following this visit. Systemic medications at the time of presentation included antirejection medications (mycophenolate mofetil, tacrolimus, sirolimus, and prednisone), as well as metoprolol, losartan, clonidine, metolazone, furosemide, calcium, and vitamin D. On review of systems, the patient reported recent excessive bruising and weakness.
Examination
The ocular examination revealed visual acuity of 20/400 in both eyes without improvement by pinhole or refraction in either eye. Intraocular pressure measured 13 mm Hg in each eye. Confrontation visual fields were full in the right eye and constricted in the left eye. Ocular motility was full bilaterally. Pupils were normal, without afferent pupillary defects in either eye.
The slit-lamp examination revealed dermatochalasis, normal anterior segments without iris neovascularization, and posterior chamber intraocular lenses bilaterally.
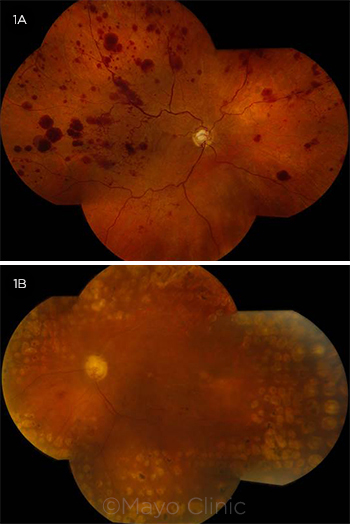
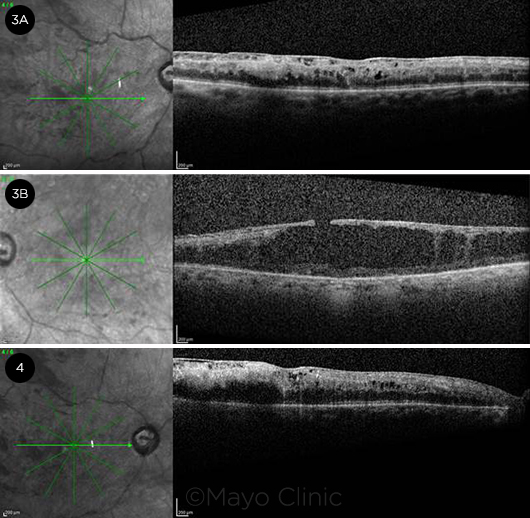
Dilated funduscopic exam of the right eye showed an optic disc with a cup-to-disc ratio of 0.6 without disc hemorrhages or neovascularization of the disc. Intraretinal hemorrhages were identified in the macula, along with an epiretinal membrane (ERM) associated with cystoid macular edema (CME) and diffuse retinal thickening (Fig. 3A). The retinal vessels demonstrated mild vascular tortuosity and diffuse large blot hemorrhages throughout the posterior pole as well as the midperiphery without evidence of retinal neovascularization (Fig. 1A).
Dilated funduscopic examination of the left eye showed optic disc pallor, along with a cup-to-disc ratio of 0.7 without disc hemorrhages or neovascularization of the disc. The macula showed degenerative-appearing cystoid changes along with an ERM and an associated pseudohole (Fig. 3B). There were only rare dot hemorrhages, one sclerotic vessel superotemporally, and no retinal neovascularization. PRP scars were noted for 360 degrees around the macula (Fig. 1B).
 |
|
FUNDUS PHOTOGRAPHS. (1A) In the right eye, there were numerous large retinal hemorrhages throughout the posterior pole and midperiphery, along with mild vascular tortuosity. (1B) In the left eye, there was optic disc pallor with rare retinal hemorrhages in the posterior pole, a superotemporal sclerotic vessel, and 360-degree panretinal photocoagulation scars.
|
Office Evaluation
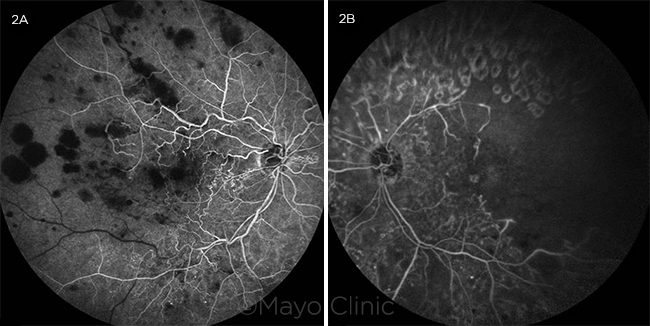
Fluorescein angiography (FA) of the right eye revealed hypofluorescent blocking defects from the retinal blot hemorrhages without retinal vascular leakage (Fig. 2A). FA of the left eye identified hypofluorescent hypoperfusion of the superotemporal arcade and temporal macula as well as hyperfluorescent window defects corresponding to PRP scars in the periphery (Fig. 2B).
 |
|
FLUORESCEIN ANGIOGRAMS. (2A) Fluorescein angiogram in the transit phase of the right eye indicated hypofluorescent blocking defects corresponding to the large retinal hemorrhages and absence of vascular hyperfluorescent leakage. (2B) Fluorescein angiogram in the late phase of the left eye showed moderate capillary dropout in the temporal macula, a hypofluorescent filling defect in the superotemporal distribution, and hyperfluorescent window defects corresponding to panretinal photocoagulation scars.
|
Workup
The unusual and asymmetric nature of the retinal hemorrhages prompted additional evaluation. Carotid duplex studies did not reveal any evidence of flow-limiting stenosis on either side. Laboratory evaluation included complete blood count, glycated hemoglobin (HbA1c), serum protein electrophoresis, protein C and S, and evaluation for factor V Leiden deficiency. Complete blood count was notable for a hemoglobin level of 11.5 g/dL (mild anemia), a white blood cell count of 11,400 cells/mm3 (mild leukocytosis), and a normal platelet count of 286,000 cells/mm3. Factor V Leiden mutation was negative, and protein C and S levels were normal. Serum protein electrophoresis revealed an elevated M-spike in the gamma fraction.
The patient was referred back to hematology for additional workup, which included bone marrow aspiration and flow cytometry studies. The evaluation confirmed the diagnosis of monoclonal gammopathy of undetermined significance.
 |
|
OCT AT PRESENTATION AND AFTER 1 MONTH. At presentation (3A), OCT of the right eye showed an epiretinal membrane with diffuse cystoid macular edema and retinal thickening; (3B) OCT of the left eye revealed diffuse degenerative cystoid changes in the macula, along with an epiretinal membrane and associated pseudohole. One month later (4), OCT of the right eye demonstrated increased cystoid macular edema compared with the prior visit.
|
Discussion
Monoclonal gammopathy of undetermined significance (MGUS) is present in 3% to 4% of the general population older than 50 years of age.1 MGUS is mostly asymptomatic and is often detected as a laboratory finding in the absence of the symptoms that are seen in more aggressive blood dyscrasias.
MGUS can transform into multiple myeloma, the most common symptoms of which are fatigue and bone pain. The diagnosis is confirmed by laboratory testing, which should include serum and urine protein electrophoresis with immunofixation, serum free light chain assay, and metabolic panels to investigate for hypercalcemia and elevated creatinine. Bone marrow biopsy is typically performed in addition to magnetic resonance imaging (MRI), which is a useful diagnostic adjunct to assess for lytic lesions affecting the spinal cord. Treatment involves systemic chemotherapy under the guidance of a hematologist-oncologist.1
Ocular findings. Ophthalmic features that may be present in MGUS, multiple myeloma, or other blood dyscrasia retinopathies are protean. The findings occur on a spectrum from asymptomatic retinal hemorrhages, cotton-wool spots, and retinal venous dilation to more aggressive retinal or optic disc neovascularization and vitreous hemorrhage. Factors contributing to the development of retinal hemorrhages in blood dyscrasias include anemia, thrombocytopenia, leukocytosis, and blood or serum hyperviscosity, which can affect the fragile retinal capillary beds and produce the findings that were seen in Mr. Holloway’s case.2
The retinal hemorrhages associated with blood dyscrasias can occur in all layers of the retina. Dot-blot hemorrhages in the inner nuclear layer, along with flame-shaped hemorrhages in the nerve fiber layer, are common. Microaneurysms in blood dyscrasia retinopathies are often seen in the midperipheral to peripheral retina, as opposed to the posterior location usually seen in diabetic retinopathy.3 Rarely, retinal vein occlusions, macular edema, and retinal neovascularization have been reported to occur in MGUS.4 Disc pallor may be identified in severe anemia, and white-centered hemorrhages can typically be seen in leukemia but only occasionally in MGUS.5
Workup. Blood pressure should be recorded. Office evaluation should include fundus photography to monitor changes over time, as well as optical coherence tomography (OCT) to detect macular edema. FA is particularly useful to assess for regional or widespread hypoperfusion (hypofluorescence) or retinal neovascularization (hyperfluorescent leakage).
The basic laboratory workup should include a metabolic panel, including serum glucose, HbA1c, lipid panel, and a complete blood count to identify anemia, leukemia, or thrombocytopenia. In the absence of 1) known history of diabetes, or 2) typical appearance of retinal vein occlusion, or if there is concern for widespread or asymmetric retinal ischemia, serum protein electrophoresis can detect elevations of a single protein fraction (i.e., in MGUS) or myeloma. Additional diagnostic studies might include 2-dimensional echocardiography and carotid duplex ultrasound studies to assess for downstream ischemia, particularly in patients with risk factors for cardiovascular disease.
Treatment. Management begins with identification of the systemic blood dyscrasia or serum gammopathy. Such findings should be conveyed to the primary care provider, who may then consider referral to a hematologist-oncologist. Additional evaluation might include bone marrow biopsy and MRI to identify skeletal lytic lesions affecting the spine as detailed above.
Ocular therapy for such retinopathies is not as well delineated as it is for proliferative diabetic retinopathy or retinal vein occlusions, but treatment with anti-VEGF agents for macular edema and PRP for retinal and/or iris neovascularization might be prudent. Ultimately, the ocular care of these patients should be coordinated with systemic medical care by the primary care provider and/or hematologist-oncologist. Prognosis may improve with chemotherapy or plasmapheresis as the retinal vascular circulation is improved, so delay of PRP might be considered if active systemic treatment is pursued and if no ominous ocular findings such as neovascularization of the iris are present.
Back to Mr. Holloway
Mr. Holloway continued to receive treatment for his squamous cell carcinoma of the left cheek, but no chemotherapy or plasmapheresis was pursued for his MGUS, as he had no bone pain or significant fatigue as would typically be the case in multiple myeloma. The absence of large blot retinal hemorrhages in the left eye was an interesting finding and was likely related to prior PRP, which had decreased the amount of perfused retina. In contrast, the patient’s right eye had not received prior PRP; thus, there was enough remaining retinal vasculature to manifest the large blot hemorrhages, most likely resulting from damage to the fragile retinal capillaries by elevated serum proteins. His bilateral central retinal vein occlusions likely occurred, then, in the context of largely asymptomatic MGUS.
Upon follow-up 1 month after his initial presentation, the patient had stable visual acuity of 20/400 in both eyes with persistent large retinal hemorrhages in the right eye. OCT revealed a slight increase in macular edema in the right eye (Fig. 4), so intravitreal bevacizumab was injected into the right eye, and 2 subsequent monthly treatments were recommended. There was no retinal or iris neovascularization necessitating PRP treatment in the right eye. Examination and imaging of the left eye were stable. The patient returned to his home state after treatment for his squamous cell carcinoma of the cheek. Recommendations were given to follow up with his local ophthalmologist to receive further anti-VEGF injections and to monitor for development of iris or retinal neovascularization, which could prompt PRP in the right eye.
___________________________
* Patient name is fictitious.
___________________________
1 Rajkumar SV, Kumar S. Mayo Clin Proc. 2016:91(1):101-119.
2 Rosa RH Jr, Cunningham RD. Retinopathy of blood dyscrasias. In: Lee GR et al, eds. Wintrobe’s Clinical Hematology. 10th ed. Baltimore: Lippincott Williams and Wilkins; 1999.
3 http://www.westcoastretina.com/sept-2011.html. Accessed July 1, 2016.
4 www.aaopt.org/proliferative-retinopathy-patient-monoclonal-gammopathy-undetermined-significance-mgus. Accessed July 1, 2016.
5 Kataria VC et al. Indian J Ophthalmol. 1983;31(7):899-902.
___________________________
Dr. Yamanuha is an assistant professor of ophthalmology at the Mayo Clinic in Rochester, Minn., and he also practices medical retina at the Mayo Clinic Health System location in La Crosse, Wisc. Dr. Raja is a cornea and external disease fellow at the University of California, San Diego. Relevant financial disclosures: None.
___________________________
WEB EXTRA. A stepwise approach to the patient with atypical or asymmetric retinal hemorrhages (PDF).