Download PDF
Complications covered the gamut from the common to the rare, and cases ranged from the spectacular save to the demoralizing outcome.
This past November, the 14th annual Spotlight on Cataract Surgery Symposium at the Academy’s annual meeting was entitled “M&M Rounds: Learning From My Mistakes.” Cochaired by Mitchell Weikert, MD, and myself, this 4-hour case-based video symposium was focused on cataract and intraocular lens (IOL) surgical complications.
Each and every one of us suffers surgical complications, but whether and how we learn from our mishaps is what helps us to improve as cataract surgeons. For this symposium, 18 experts presented a video case in which something went wrong and a complication occurred that taught them valuable lessons. At critical decision points during the case, the video was paused and the attendees were then asked to make clinical decisions using electronic audience response pads. Next, 2 discussants (who had never before viewed the case) were asked to make their own management recommendations and to comment on the audience responses before the video of the outcome was shown.
The 18 video case presentations covered the full spectrum of surgical complications, including anterior capsular tears (both with and without posterior extension), incision burn, femtosecond (FS) laser and chopper snafus, damaged or subluxated IOLs, suprachoroidal hemorrhage, descending nuclei and IOLs, IOL exchange complications, and capsules or zonules torn at virtually every stage of surgery. Even the panelists who thought that they’d “seen it all” were shaking their heads at some of these cases. The audience also voted on some special awards.
Warren E. Hill, MD, concluded the event by delivering the 11th annual AAO Charles D. Kelman Lecture. Dr. Hill’s presentation, “IOL Power Selection: Think Different,” highlighted innovative new approaches to determining IOL power and hitting emmetropia. The entire symposium with videos is available at AAO Meetings on Demand.
This EyeNet article reports the results of the audience response questions, along with written commentary from presenters and panelists. Because of the anonymous nature of this polling method, the audience opinions are always honest and forthright and were discussed in real time during the symposium. Finally, I want to especially thank our 18 audacious video presenters. It is always easier to present your best cases instead of your complications in front of several thousand attendees. We all appreciate their humility and generosity in sharing these cases with us so that we might all learn important surgical lessons from them.
—David F. Chang, MD
Cataract Spotlight Program Cochairman
Case 1: Fibrosis, Fibrosis, Fibrosis
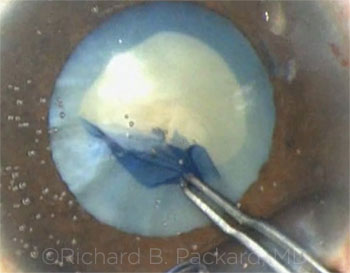
Richard Packard’s case involved a mature cataract and a small pupil. During manual capsulorrhexis, the anterior capsule tore peripherally, despite the use of capsular dye and the Little tear-out rescue maneuver.
Q1 At this point, you have an anterior capsular tear and a white brunescent lens. What would be your strategy?
| No change: Continue intracapsular phaco with single radial anterior chamber tear |
19.6%
|
| Add 1 or 2 relaxing continuous curvilinear capsulotomy (CCC) cuts, then continue intracapsular phaco |
28.7%
|
| Convert to a can-opener capsulotomy, then continue intracapsular phaco |
24.5%
|
| Prolapse the nucleus for supracapsular phaco |
15.2%
|
| Convert to manual extracapsular cataract extraction (ECCE) |
11.9%
|
Q2 After the nucleus and cortex were removed, the anterior capsular tear extended out to the equator. At this point, what IOL would you implant?
| 1-piece IOL in the bag |
9.8%
|
| 3-piece IOL in the bag |
10.0%
|
| 3-piece IOL in the sulcus (unsutured) |
68.0%
|
| 3-piece IOL in the sulcus (sutured) |
6.9%
|
| ACIOL or iris claw IOL |
5.4%
|
Richard Packard The anterior capsular tears were caused by strips of unseen and therefore unsuspected fibrosis in the capsule. Once you hit them during the capsulotomy, they cannot be passed without being cut. The Little rescue maneuver does not work here, as the tears just extend. Although the tears were present, there seemed to be little stopping the nucleus from rotating—and once it was mobilized, I felt confident that I could remove the nucleus safely despite its density by using a vertical chop technique. The anterior capsular tears were away from the area where I would be chopping, and the majority of these tears did not extend through the equator. The tear extension occurred later, when the extensive fibrous ring at the edge of the epinucleus proved difficult to remove. It required visco-elevation to free it from the capsular bag to enable removal using bimanual irrigation and aspiration (I&A).
With regard to the second question, the critical issue is the stability of the IOL. On checking through 360 degrees under the iris, I established that the anterior capsule was intact—apart from the area of the anterior capsular and equatorial tear—and would be able to support a 3-piece IOL placed in the sulcus with the haptics at 90 degrees from the anterior capsular tear. I used viscoelastic to separate the anterior capsule from the back of the iris and thus open up the sulcus. The implantation was done using a Monarch C cartridge. The leading haptic was stretched out by placing an instrument into the mouth of the cartridge and pulling it forward. The trailing haptic hung down and was not caught in the injector. As the haptic was advanced into the eye, I took care to make sure it was placed above the anterior capsule; I then rotated the injector counterclockwise to allow the optic to unfold without disturbing the leading haptic’s position. I placed the trailing haptic under the iris and in the sulcus with angled McPherson forceps by supinating the hand to cause the haptic to flex downward. I also placed the optic in the bag to add further stability.
Rosa Braga-Mele This was a very interesting case, and I believe the first lesson to be learned is to make certain that there is enough viscoelastic in the anterior segment to properly flatten the anterior capsule when you are presented with a white mature cataract. It is also important to have an armamentarium of instruments available to help deal with capsular scars or adhesions before you even begin.
However, sometimes even the best plans can lead to surprises, and one must then feel confident in the decision to move forward at that point. It was interesting to see that the largest percentage of the audience voted to proceed with a few relaxing CCC cuts and then continue with intracapsular phaco. This was how Dr. Packard proceeded, with a successful outcome. I, however, would have taken a bit of a different approach that combined answers 2 and 4. First, I would have attempted to complete the best CCC possible; then, I would have prolapsed the nucleus into the anterior chamber. Next, I would have put in some dispersive viscoelastic behind the nucleus (to protect the posterior capsule) and underneath the cornea. I would have used a second instrument behind the nucleus to provide a sort of scaffold while phacoing in the anterior chamber. This is all sparked by my fear of an incomplete capsulorrhexis extending posteriorly during phaco and my desire to minimize any nuclear or vitreous loss.
Once the nucleus is removed and we do in fact have a tear that extends completely out to the equator, we are faced with the decision of where to place the IOL. In this instance, I agree with the audience’s preference for implanting a 3-piece IOL into the sulcus unsutured, as there seemed to be enough capsular support. If there was any question of stability, I would have opted to suture or glue a 3-piece IOL to be scleral fixated. However, it is important to never use a single-piece IOL in the sulcus, as this could lead to iris chafing and pigment dispersion. With regard to sulcus IOLs, I prefer to use one with a longer diameter and, preferably, a rounded anterior edge. It is also important to change the calculated diopter power by 0.5 D because of the slightly more anterior placement of the lens.
 |
|
CASE 1: The anterior capsular tear ran peripherally.
|
Case 2: Balancing Act
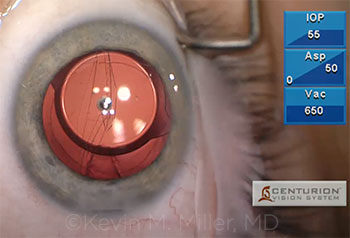
Kevin Miller successfully implanted a single-piece acrylic IOL into the capsular bag despite the presence of an anterior capsular radial tear. However, as he aspirated an ophthalmic viscoelastic device (OVD) behind the IOL with an I&A tip, a sudden wraparound tear that extended across the posterior capsule occurred.
Q3 You now have a wraparound posterior capsular tear extending behind an intracapsular single-piece acrylic IOL. What would you do?
| Leave it alone |
45.4%
|
| Explant the IOL and leave the eye aphakic |
0.7%
|
| Exchange for an unsutured 3-piece IOL in the sulcus |
33.7%
|
| Exchange for a sutured 3-piece IOL in the sulcus |
9.0%
|
| Exchange for a glued (scleral tunnel) 3-piece IOL in the sulcus |
6.2%
|
| Exchange for an ACIOL |
5.0%
|
Kevin Miller My patient developed a progressive nuclear cataract following a pars plana vitrectomy (PPV) and epiretinal membrane peel. Accelerated nuclear sclerosis is a well-known complication of PPV, and the patient had been appropriately informed of that possibility before that surgery. Cataract removal proceeded uneventfully under topical anesthesia until I noted a subincisional radialization in the anterior capsule during cortex removal. I am not certain when it happened, but would venture a guess that the phaco probe struck the subincisional anterior capsule sometime during downslope sculpting. I was able to remove the cortex without difficulty and implant a single-piece acrylic IOL within the confines of the capsular bag. I oriented the haptics 90 degrees away from the radial tear. I thought I was pretty much done at this point. However, while I was removing the dispersive-cohesive OVD from beneath the IOL, the radialization suddenly wrapped around to the equator on the other side, leaving the IOL balanced between the 2 halves of the capsule. Fortunately, the tear did not extend to involve the anterior capsule on the other side, so there was a little sliver of capsule holding the 2 halves together.
I spent a few moments staring at the eye, figuring out what to do next. The options I pondered were the following: 1) Do nothing and hope for the best; or 2) take out the lens, perform a limited anterior vitrectomy, place a 3-piece lens in the sulcus, and hope the radialization did not extend anteriorly to make this location precarious. I could see that the IOL appeared to be fairly stable in its current location, supported by the capsular halves and the remaining anterior cortical vitreous gel—and as I didn’t want to subject the patient to the trauma of an IOL exchange under topical anesthesia, I decided to close up and hope for the best.
I informed the patient of the complication and told her to “walk like a cat” for a few days and to be careful about sudden head and eye movements. Fortunately, the day after surgery, several weeks later, and 1 year later, the lens was perfectly positioned and her vision was excellent. The implant is now fibrosed into the bag and should be stable for life. In retrospect, my decision was the correct one. However, had I been wrong, I was prepared to take her back to the OR and perform a lens exchange.
Roger Steinert Nearly half of the respondents, and Dr. Miller, chose the route of the least amount of subsequent manipulations. This proved to be a good choice. The only other popular alterative was the third option, which involved more manipulation but also more opportunity to ensure a stable long-term outcome. While the majority, and Dr. Miller, were proven to have been correct, it was a choice that had significant risk of reoperation. In the end, the surgeon has to be a bit of a Dirty Harry: Are you feeling lucky?
 |
|
CASE 2: A wraparound tear after OVD removal left a single- piece acrylic IOL precariously balanced between the 2 halves of the capsule in a previously vitrectomized eye.
|
Case 3: FLACS Can Be a Moving Experience
Sonia Yoo’s patient moved during the FS laser nucleotomy. The laser inscribed a grid pattern onto the cornea. Phaco was performed, and the rest of the case was completed without further complication.
Q4 What would you tell the patient immediately after surgery?
| I would not disclose the complication until later |
9.8%
|
| I would disclose the complication and say, “I’m not sure what happened” |
23.6%
|
| I would say that the complication was caused by FS laser malfunction |
20.7%
|
| I would say that the complication was caused by movement of the patient’s eye |
40.5%
|
| I would say that the complication was caused by surgeon error |
5.3%
|
Q5 Would you apologize to the patient on postop day 1?
| Yes |
45.6%
|
| No; it wasn’t my fault |
6.2%
|
| No; the patient could still have a good outcome |
45.0%
|
| No; that would increase the likelihood of a lawsuit |
1.1%
|
| I would first consult my malpractice carrier |
2.1%
|
Sonia Yoo I had difficulty docking my patient due to her narrow interpalpebral fissure, deep-set orbit, and high nasal bridge. But I was determined to perform the femtosecond laser–assisted cataract surgery (FLACS) as I had planned. On the third try, I was successful at obtaining vacuum. The imaging and capsulotomy were uneventful, but suction was lost two-thirds of the way through the lens segmentation. Due to the high speed of laser treatment and slightly slower response time of the laser sensing vacuum loss, inadvertent laser grid treatment was applied to the patient’s cornea.
I was fortunate that there was no capsular damage, and the lens nucleus and cortex could be removed without incident. At the end of the case, the corneal grid pattern was visible in the inferotemporal third of the cornea. Amazingly, the patient’s uncorrected vision was 20/20 on postop day 1 and stayed stable over the next year. More than a year later, the corneal grid pattern still is visible on slit-lamp examination, and the patient has no untoward visual side effects.
Samuel Masket I had a remarkably similar occurrence during an early experience with FLACS. In my case, suction broke during the capsulotomy and the laser “treated” the posterior cornea. Over several months, the laser marks fully disappeared, and no harm came to the patient.
In the case at hand, Dr. Yoo aborted multifocal IOL placement in favor of a standard implant, as she was not certain of the long-term sequelae of the suction break. Given that, a discussion with the patient is mandated. The patient should be informed that the suction failed to hold the eye steady and that a standard IOL was implanted for safety reasons. An apology is neither necessary nor appropriate. However, the patient is entitled to a frank discussion of the course of the event, the judgment that was made at the moment, and the current management options. While blame should not be assigned to the patient, he or she can be made aware that eye movement or squeezing may contribute to suction break.
 |
|
CASE 3: The FS laser grid pattern was evident on postop day 1.
|
HIT THE JACKPOT AWARD
Which surgeon had the best luck?
| Richard Packard |
8.6%
|
| Kevin Miller |
79.1%
|
| Sonia Yoo |
12.3%
|
Case 4: Burn Baby Burn
Terry Kim noted a wound burn during nuclear emulsification. The first decision was how to proceed, as much of a very dense nucleus still remained. After he completed the removal of the nucleus, his initial attempts to close the incision with interrupted 10-0 nylon sutures did not produce a completely watertight incision.
Q6 You have a dense nucleus plus an incision burn. What next?
| Abort surgery and refer the patient |
0.5%
|
| Continue phaco through the same incision |
56.1%
|
| Continue phaco through a new incision |
21.6%
|
| Manually remove the nucleus by enlarging the same incision |
14.8%
|
| Manually remove the nucleus via a new incision |
7.0%
|
Q7 The incision is not secure. What next?
| Leave the eye alone with a patch or bandage contact lens |
16.3%
|
| Add interrupted sutures |
26.6%
|
| Remove sutures and add horizontal mattress suture |
16.7%
|
| Use hydrogel sealant (e.g., ReSure) or cyanoacrylate glue |
34.1%
|
| Do a scleral relaxing incision and resuture |
6.3%
|
Terry Kim A 72-year-old white female presented with dense brunescent cataracts (4+++) in both eyes. Her visual acuity (VA) was counting fingers in her right eye and hand movements in her left. She also had a long-standing history of severe age-related macular degeneration (AMD) in both eyes, which limited her visual potential. Along with the brunescent nuclear sclerotic cataracts, the slit-lamp exam revealed clear corneas, shallow anterior chambers, and poor dilation in both eyes. Her intraocular pressures (IOPs) were normal. Keratometry revealed minimal (less than 1 D) astigmatism, and her biometry showed moderately short axial lengths. The surgical plan was to proceed with phacoemulsification using torsional ultrasound and horizontal chop technique with instillation of copious OVD in the left eye.
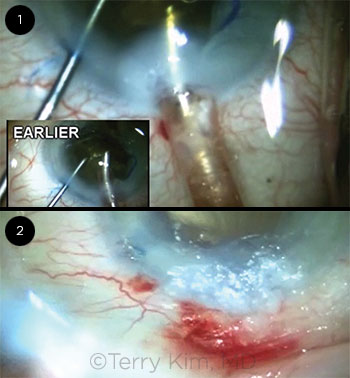
Because of the density of the lens and corresponding poor red reflex, I instilled trypan blue to stain the anterior capsule. Next, I placed a Malyugin ring to address the small pupil. After I completed the capsulorrhexis, I proceeded with a horizontal chop technique to disassemble the lens and immediately noticed the extreme density of the lens and the leathery posterior plate. This combination resulted in a prolonged phaco time with high cumulative dissipated ultrasound energy throughout the procedure. I stopped several times to reinsert more dispersive viscoelastic to re-form the shallow chamber and to protect the corneal endothelium. However, after about two-thirds of the lens material was removed, I immediately noticed a whitening of the cornea around my temporal 2.2-mm clear corneal incision (Fig. 1), which coincided with the presence of copious fluid on my lap. The fluid was cool and clear and resulted from the irrigation tubing becoming disconnected from the phaco handpiece.
In addressing the first question on what to do next, I decided to continue phaco through the same incision, and the majority of the audience agreed with me. Since I was able to identify the cause of the phaco wound burn, I made sure that the connection of the irrigation tubing was securely attached to the phaco handpiece and proceeded with phaco through the same incision, especially since the majority of the lens had already been successfully removed. I did not feel the need to convert to manual lens extraction, which would have required enlarging the original (and distorted) incision or creating a new large incision.
After complete lens removal, cortical cleanup, and IOL implantation, I realized that the phaco wound burn was severe enough to result in fish-mouthing of the wound and difficult wound closure. After a combination of radial interrupted and cross-stitch sutures of the wound, I noticed continuous leakage from the incision.
With regard to the second question, and the audience’s mixed responses, I didn’t think that the first option (leaving the wound alone with only a patch or bandage CL) was going to be sufficient to seal the leaking incision. I decided not to add additional interrupted sutures, because this approach didn’t work (which is why I added a cross-stitch). I think that removing all the sutures and adding a horizontal mattress suture as well as resuturing with a scleral relaxing incision are both reasonable options.1,2 The fact that this patient had limited visual potential from severe AMD factored into my decision not to change my suturing approach to the incision. Ultimately, I opted to leave the sutures I had in place and add cyanoacrylate glue, since the suture closure was successful in reapproximating the wound edges, and the residual leakage from the incision was minimal. Hydrogel sealant was not commercially available at the time of this case, but the cyanoacrylate glue was effective in sealing the wound (Fig. 2).
Our patient did well. Immediately postoperatively, she noted some “brightening” of her vision but no improvement in her Snellen acuity. Although her cornea was clear centrally, she did have temporal corneal edema with a copious amount of cyanoacrylate glue over her sutured incision, which eventually cleared with a month of topical corticosteroid therapy. Manifest refraction and keratometry revealed about 7 D of corneal astigmatism. Her glue eventually fell off after about 2 months; at this time, her sutures were removed. On her 6-month postop visit, her astigmatism had decreased to about 2.5 D and her vision had improved to 20/400.
She is now eager to pursue cataract surgery in her right eye. In preparation, we will make sure that all the connections to the phaco handpiece are secure and will also be aware of the risk factors for phaco wound burn (i.e., dense cataracts, prolonged phacoemulsification time, tighter wounds, clear corneal incisions, and use of certain OVDs, such as Viscoat and Healon5).3
Stephen Lane The majority of the respondents favored continuing surgery through the burned incision. This is a reasonable response, given the mild nature of the burn. In this case, continuing phaco through the same incision was not likely to further compromise the wound—and the wound gape was not so significant that the anterior chamber would be lost or significantly shallowed during phaco or I&A. In cases in which there is a more severe burn with significant wound gaping, making a second (new) incision is often the best choice, as maintenance of the chamber would be compromised due to excessive outflow through the gaping wound.
With regard to the second question, ultrasonic energy is associated with heat generation that can result in ocular tissue damage. As a result of tissue damage to the primary incision site wound, closure can be challenging due to the inability to reappose the incision edges. In this case, the audience was about equally divided between adding more interrupted sutures and using tissue glue. Both are reasonable solutions. However, depending upon the amount of wound gaping, interrupted sutures may not be a viable solution due to excessive tissue damage. Oftentimes, the use of 1 or more horizontal mattress sutures along with radial interrupted sutures is effective in accomplishing wound closure. Finally, using a tissue glue (either cyanoacrylate or hydrogel sealant) along with sutures is an effective closure technique and provides a “belt and suspenders” approach to this difficult problem. Regardless of the choice of closure technique, against-the-rule astigmatism occurs long term, and it can be quite profound, depending on the degree of tissue damage. Patient counseling is critical in these situations.
___________________________
1 Osher RH. J Cataract Refract Surg. 2005;31(3):640-642.
2 Osher RH. Video Journal of Cataract and Refractive Surgery. 1993;IX(3).
3 Floyd M et al. J Cataract Refract Surg. 2006;32(7):1222-1226.
 |
|
CASE 4: (1) Whitening of the cornea was evident around the clear corneal incision. (2) The wound was effectively sealed by adding cyanoacrylate glue.
|
Case 5: The Road Not Taken
Abhay Vasavada presented a case of posterior polar cataract. After successful phaco and IOL implantation, he used bimanual I&A instrumentation to aspirate OVD behind the IOL. However, this suddenly caused a posterior capsular rent with vitreous prolapse.
Q8 The patient has vitreous prolapse. What now?
| Triamcinolone plus limbal vitrectomy |
45.7%
|
| No triamcinolone plus limbal vitrectomy |
19.2%
|
| Triamcinolone plus PPV |
22.1%
|
| No triamcinolone plus PPV |
3.9%
|
| Scissors to amputate the vitreous |
9.0%
|
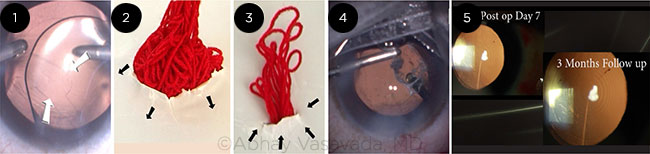
Abhay Vasavada This situation is a tricky one; the surgeon is faced with the challenge of dealing with a posterior capsular rupture and vitreous prolapse in an eye that already has a multifocal IOL in the capsular bag (Fig. 1). When posterior capsular rupture occurs, it is crucial to identify the vitreous prolapse and its extent into the anterior chamber by staining it with preservative-free triamcinolone. It also helps confirm adequacy of anterior vitrectomy.
The main goal of the vitrectomy should be to minimize further enlargement of the rupture and achieve a symmetrical support for the IOL. Although the most popular option involved performing a triamcinolone-assisted limbal anterior vitrectomy, I would prefer taking the pars plana approach. When we introduce the vitrector through the pars plana, the prolapsed vitreous drains into the vitrector port without producing significant traction or drag on the vitreous body. The removal process becomes more effective and predictable. It allows the surgeon to remove the vitreous from behind the entire optic very adequately and thus creates a symmetric support for the IOL.
In contrast, when we perform a limbal anterior vitrectomy, we are in fact applying upward traction on the vitreous body that lies below the posterior capsular rupture. This may lead to more vitreous being dragged through the rupture and further enlargement of the rupture (Fig. 2). In addition, the consequences of the vitreous traction may result in posterior segment complications later on. But importantly, this “top-down” approach makes it difficult to remove the anterior vitreous uniformly and adequately from behind the IOL. This could create asymmetric support for the IOL, making it unstable.
To perform the pars plana entry, one needs a lancet or any other sharp knife. However, there are very good trocar systems available with most newer phaco machines; these can be used to perform a 23-gauge or even a 25-gauge sutureless vitrectomy. Furthermore, these vitrectors offer very high cut rates, which will make it a less traumatic procedure. The entry should be 3.0 or 3.5 mm peripheral to the limbus, and the knife or trocar should be directed to the center of the globe and not to the center of the pupil. The surgeon can keep the irrigation cannula inside the anterior chamber by employing a paracentesis. I was able to successfully complete this case (Figs. 4 and 5).
Most cataract surgeons find the pars plana approach rather intimidating. I feel it is more of a mind-set. Interacting with a retina specialist or an experienced cataract surgeon will make the learning curve less steep.
Edward Holland The combination of a torn posterior capsule and vitreous prolapse is fortunately an uncommon one, but all cataract surgeons should be adept at handling it. Two issues were posed to the audience: use of triamcinolone and surgical technique of the vitrectomy.
It is surprising that 23% of respondents would not use triamcinolone. Triamcinolone highlights the vitreous and is a valuable tool for assisting in the complete removal of vitreous from the anterior chamber.
With regard to surgical technique, 65% of respondents chose limbal vitrectomy, while 26% indicated that they would choose a PPV approach. The number of anterior segment surgeons who perform PPV has continued to increase over the years. It is understood that the PPV technique is a safer and more efficient way to remove vitreous. It results in the vitreous being pulled posteriorly and away from the corneal incision and anterior chamber. Moreover, it reduces the amount of vitreous that needs to be removed and is less likely to extend the posterior capsule tear.
However, most anterior segment surgeons are not trained in PPV and thus are not comfortable with it. A limbal vitrectomy is still an acceptable method and can be very effective if key surgical principles are followed. First, the use of triamcinolone is especially important. Then, a bimanual method should be employed—and, to best control the anterior chamber, the main incision should be closed and 2 limbal incisions should be used (this separates irrigation and cutting/aspiration). Finally, a high cut rate and low aspiration setting is preferred (this lessens the risk of pulling more vitreous anteriorly).
It is somewhat of a surprise that 9% of respondents would choose scissors to amputate the vitreous. This approach is the least preferred and, in most cases, does not completely remove vitreous from the anterior chamber. Inadequate removal of vitreous from the anterior chamber increases the risk for vitreous traction, cystoid macular edema, and retinal detachment.
 |
|
CASE 5: (1) Posterior capsular rupture noticed during OVD removal with the multifocal IOL in the bag. (2) This simulation shows how limbal vitrectomy can induce a drag on the vitreous body and enlarge the original posterior capsular rupture. (3) This simulation shows how PPV pulls the vitreous down through the posterior capsular rupture and thereby avoids both traction and potential enlargement of the rupture. (4) PPV being performed after the vitreous was stained with triamcinolone. (5) Postoperative images show a stable in-the-bag IOL.
|
Case 6: Power Chop and—Oops!
In Geoff Tabin’s case, the assisting resident accidentally hit the surgeon’s hand, which caused the chopper to abruptly penetrate through the posterior capsule. Some of the nucleus descended posteriorly and was left. After an anterior vitrectomy was performed, a 3-piece IOL was placed in the ciliary sulcus.
Q9 Have you ever accidentally torn the posterior capsule because of one of the following?
| Sudden patient head movement |
33.4%
|
| Something hitting your arm/hand/instrument |
4.3%
|
| Someone jarring your chair or OR table |
0.6%
|
| At least 2 of the above |
25.3%
|
| None of the above |
36.5%
|
Q10 What would you tell the patient immediately postop?
| I would not plan to disclose the complication |
2.6%
|
| I wouldn’t disclose unless the patient developed a complication later |
4.5%
|
| I would disclose the torn posterior capsule but not give a cause |
80.4%
|
| I would say that the complication was caused by my assistant hitting my hand |
7.8%
|
| I would say that the complication was caused by an instrument mistake |
4.7%
|
Geoff Tabin The case I presented is a classic example of a routine cataract surgery with an unexpected complication. The assistant, a resident surgeon, was going to place a drop of balanced salt solution (BSS) on the eye and inadvertently hit the second instrument, which drove it through the posterior capsule and deep into the back of the eye.
The first take-home message is stay calm. The damage has been done. The goal is to salvage the situation and provide the best possible care for the patient. When I watched the replay of this case on video, I saw that one mistake was made immediately after the accident: We withdrew the instrument from the eye without instilling an OVD. Viscoelastic could have kept the vitreous from prolapsing into the anterior chamber. Once the instrument was out, everything was stable. It was time to take deep breaths, relax, and assess the situation.
The anterior capsulorrhexis was intact, with a large rupture of the posterior capsule. Epinuclear fragments were in the vitreous, and vitreous was in the anterior chamber. The case then proceeded nicely with a bimanual anterior vitrectomy, cortical cleanup with the vitrector on I&A cut mode, and placement of a 3-piece (Alcon MA 60BD) lens in the sulcus with optic capture in the capsulorrhexis.
The next question was what to tell the patient. I believe in being as straightforward and honest as possible. I waited to speak with the patient in the recovery room, not wanting any extra stress making a case under topical anesthesia more difficult. I did not say anything about the assistant, and I explained the situation exactly as I would for any posterior capsular rupture. Referral was made to our retinal service. Fortunately, there was no retinal trauma or damage. After a PPV with removal of lens fragments, this patient had uncorrected 20/25 acuity 6 months after the surgery.
Deepinder Dhaliwal This case presented a dramatic example of a common occurrence: An unexpected event—one that is completely out of the control of the surgeon—happens, resulting in a suboptimal result. Almost two-thirds of respondents noted that they have had a torn posterior capsule due to a sudden movement of some kind. As surgeons, we need to expect the unexpected and have a knee-jerk reaction to stay calm and not pull the instruments out of the eye. We should reach for viscoelastic with our nondominant hand and fill up the anterior chamber prior to removing the phacoemulsification handpiece. This is not an easy maneuver, and it should be practiced before one really needs it.
Initially, the irrigation fluid will force the viscoelastic back out through the paracentesis if it is injected close to the side-port incision. Therefore, the viscoelastic cannula should be inserted across the anterior chamber, then viscoelastic should be inserted. The irrigation needs to be stopped after there is enough viscoelastic to keep the anterior chamber formed, then more viscoelastic should be injected into the anterior chamber to fill it. Then—and only then—should the surgeon remove the phaco handpiece from the eye. Because vitreous will follow the pressure gradient, as long as the anterior chamber pressure is higher than the posterior segment pressure, vitreous will not present anteriorly. After the instruments are safely removed from the eye, a careful assessment can be made and a treatment plan can be formulated.
In terms of how to disclose these intraoperative challenges to the patient, I agree with the majority of the respondents (80%) and would disclose the torn posterior capsule without assigning blame. Open communication is essential.
SNAKE EYES AWARD
Which surgeon had the worst luck?
| Terry Kim |
5.9%
|
| Abhay Vasavada |
5.4%
|
| Geoff Tabin |
88.8%
|
Case 7: Denial at High Noon
Rich Hoffman presented a case in which the posterior capsule tore during intracapsular implantation of a single-piece acrylic IOL. As the IOL and haptics unfolded, insufficient posterior capsular support was available for fixating the lens.
Q11 You are presented with a single-piece IOL plus a torn posterior capsule. What now?
| Leave it there and address later if it subluxates |
12.2%
|
| Levitate the IOL into the ciliary sulcus |
9.6%
|
| Levitate the IOL into the ciliary sulcus, but suture the haptics |
1.8%
|
| Use the CCC to capture the optic |
43.5%
|
| Exchange with a 3-piece IOL in the sulcus |
32.8%
|
Rich Hoffman This was a routine 2+ nuclear sclerotic cataract extraction that was going well until shortly after I implanted a single-piece acrylic IOL into the capsular bag. A crease in the posterior capsule was identified after centration of the IOL. In retrospect, this was a tear that was created during aggressive implantation of the IOL. At the time of the procedure, I elected to ignore the discontinuity in the capsule, as the IOL was centered. As I removed the OVD, vitreous presented in the anterior chamber and was visible in the bimanual aspirating cannula. After I removed the instruments, the IOL was centered but had a slight tilt due to the lens attempting to migrate through the large opening in the posterior capsule.
Although 12% of the audience believed it might be all right to leave the IOL in place and see what happened, I believed that it would eventually decenter and need to be addressed. Interestingly, close to 10% of respondents felt that levitating the entire single-piece IOL into the ciliary sulcus was an appropriate maneuver, despite repeated reports in the literature of complications from single-piece acrylic IOLs being placed in the ciliary sulcus without support. Many of these lenses went on to cause pigment dispersion and uveitis-glaucoma-hyphema (UGH) syndrome.
I believe an IOL exchange with a 3-piece IOL in the sulcus (with optic capture) would be an adequate response; however, a simpler maneuver would be reverse optic capture. This was, in fact, what I did in this case: I injected a large bolus of a dispersive OVD behind the IOL in order to attempt to reposition the vitreous behind the capsule and then prolapsed the IOL optic in front of the anterior capsule through the intact anterior capsulorrhexis. This was performed quickly and easily and was then followed by removal of the anterior chamber OVD with a 23-gauge bimanual vitrector/infusion. After I removed most of the OVD, I injected triamcinolone into the anterior chamber to stain for any residual vitreous. None was present.
Placement of a single-piece acrylic IOL in the ciliary sulcus is contraindicated—but many surgeons may not realize that it may be appropriate to prolapse the optic of a single-piece IOL anteriorly through the rhexis if the IOL is already in the bag and is decentering due to a compromised posterior capsule. In one study of reverse optic capture with a single-piece acrylic IOL, the researchers found no sight-threatening complications with 19 months of follow-up.1
Nick Mamalis The issue of a torn posterior capsule during insertion of an IOL into the lens capsular bag is a difficult one. The audience responses illustrate several different options for dealing with this issue. For instance, 12.2% of the respondents stated that it is all right to leave the lens where it is and to address it later if it subluxates. This will depend on the amount of remnant capsular support in the posterior capsule and periphery of the lens capsular bag, which is needed to keep the IOL from decentering or dislocating into the vitreous.
It is somewhat concerning to see that 9.6% of the respondents stated that it is all right to levitate a single-piece hydrophobic acrylic IOL into the ciliary sulcus. These implants have a very thick, square edge to the IOL haptics, and the haptics are relatively sharp, which makes placement into the ciliary sulcus problematic. Levitating the IOL in the ciliary sulcus may lead to problems with pigment dispersion/glaucoma and chronic UGH syndrome.
The largest number of respondents (43.5%) voted to use the intact CCC to capture the optic. This is a reasonable solution, in that the haptics would remain sequestered from the posterior iris surface within the capsular bag, and capturing the optic with the intact anterior capsulorrhexis would help prevent dislocation or decentration of the IOL. The second most common solution chosen by the respondents was exchanging the IOL with a 3-piece PCIOL in the sulcus (32.8%). This is also a reasonable solution if the original IOL requires explantation.
It is important that any IOL placed into the ciliary sulcus is one that either is designed for the sulcus or will not cause any additional complications. A 3-piece silicone IOL with a relatively broad overall diameter and rounded anterior optic edge is an ideal choice. In addition, there are 3-piece hydrophobic acrylic IOLs that have a relatively rounded anterior optic edge; these can also be considered for placement within the ciliary sulcus in a case of a torn posterior capsule. Finally, these sulcus-fixated, 3-piece acrylic IOLs can then undergo optic capture with placement of the optic in the capsular bag posterior to the intact anterior capsulotomy.
___________________________
1 Jones JJ et al. Ophthalmic Surg Lasers Imaging. 2012;43(6):480-488.
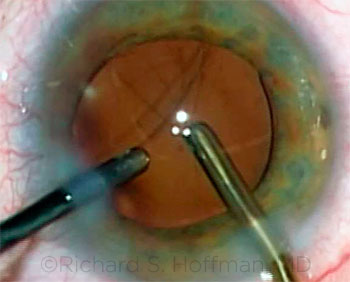 |
|
CASE 7: Vitreous presented in the anterior chamber and was visible in the bimanual aspirating cannula as the OVD was removed.
|
Case 8: You, Scott, Are No David Chang!
Scott Barnes presented a case with high astigmatism in which the posterior capsule tore. He still implanted a toric IOL into the capsular bag, but it immediately subluxated laterally by the first postop day.
Q12 You have a posterior capsular tear and a subluxated toric IOL. What now?
| Use pilocarpine to avoid further surgery |
0.9%
|
| Attempt to reposition the toric IOL |
19.4%
|
| Exchange the toric IOL for a 3-piece foldable IOL in the sulcus |
47.9%
|
| Exchange the toric IOL for a 3-piece IOL in the sulcus (with a limbal relaxing incision) |
26.1%
|
| Exchange for a nonfoldable IOL via a large on-axis incision |
5.7%
|
Scott Barnes In this case, the audience was probably correct in what to do, but if I had done a better job initially, I would not have been in this situation. You see, I have watched so many of David Chang’s cases that I was feeling that this time I could be the superhero.
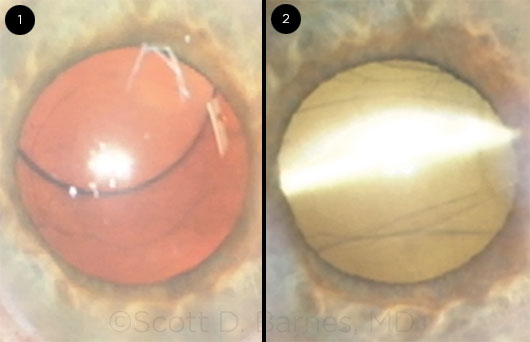
So in this case of a 64-year-old computer engineer who had 13 D of myopia, almost 3 D of corneal astigmatism, and an axial length of 29.87 mm, and who wanted to be able to see up close and at a distance after cataract surgery, I found myself facing a very small (they all are small, aren’t they?) posterior capsular tear nasally. I decided that David Chang would certainly figure out a way to place the 1-piece toric lens in the posterior capsule, so this is what I did. And the IOL appeared to stabilize—sort of—with the haptics perpendicular—almost—to the nasal-based tear.
That is until the next morning, when, predictably, the IOL was nicely subluxated toward the rent (Fig. 1). The patient did not want to give up the toric lens and did not want laser surgery to address his astigmatism. As I was unsure whether I was up for another unsuccessful attempt at channeling David Chang, I had planned on an exchange with a nontoric 3-piece IOL in the sulcus. However, the ASCRS meeting took place 1 week after this surgery, and I discussed the case with Samuel Masket, who suggested a reverse optic capture. Fortunately, I was able to leave the haptics in the capsular bag while bringing the optic through the anterior capsulorrhexis. I was even able to align the toric IOL in the proper 95-degree meridian (Fig. 2). The patient was very happy and achieved a 20/20 uncorrected outcome, although he still isn’t excited that he has to use glasses for near work.
Roberto Bellucci There are 2 ways of dealing with decentration such as this. The first is to change the IOL for a nontoric IOL to be implanted in the ciliary sulcus and then correct any residual astigmatism either by a relaxing incision or by subsequent LASIK. This is probably the safer option, although it involves more complex surgery for the patient. And although LASIK is an attractive option—because it can also correct for some residual spherical error—surface problems related to dry eye may limit patient satisfaction in the postoperative period. Moreover, the patient must be willing to accept a secondary procedure.
The second option is to discuss the decentration with the patient and to ask for his cooperation in the attempt to recenter the IOL. If the patient understands that relatively simple maneuvers can solve the problem but that further surgery may be needed, the IOL can be recentered and properly oriented via reverse optic capture. I have successfully used reverse optic capture with a multifocal IOL in a second eye surgery
 |
|
CASE 8: (1) The nasally decentered toric IOL the morning after the initial surgery. (2) After reverse optic capture, the well-centered toric IOL’s haptics are in the bag, and the optic is captured through the anterior capsulorrhexis.
|
Case 9: Feeling Pressure
Tom Oetting presented 2 cases, both of which involved iris prolapse. The questions here were prompted by the second case, in which sudden chamber shallowing and iris prolapse occurred after the phaco tip was withdrawn following nuclear emulsification. The patient complained of feeling pressure.
Q13 You observe anterior chamber shallowing and iris prolapse. What now?
| Use an OVD to reposit the iris, and resume surgery |
12.3%
|
| Start mannitol and resume surgery if the anterior chamber deepens |
35.0%
|
| Do a vitreous tap and resume surgery after the anterior chamber deepens |
21.9%
|
| Make new incision and resume surgery |
2.6%
|
| Abort surgery and send the patient home |
11.1%
|
| Abort surgery and go back to the OR in 1 to 2 hours |
17.2%
|
Q14 The patient is left aphakic with some residual cortex. On postop day 1, the eye is soft, with no visible choroidals. What now?
| Refer to another cataract surgeon |
2.0%
|
| Refer to a retina specialist for advice on rescheduling surgery |
32.9%
|
| Return to the OR within 1 week (no retina consult) |
43.4%
|
| Return to the OR after waiting at least 2 weeks |
15.0%
|
| Return to the OR after waiting at least 4 weeks |
6.8%
|
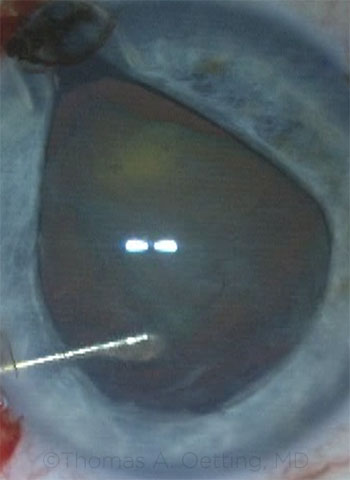
Tom Oetting In the first case, the iris prolapse occurred early, right after hydrodissection. The eye became firm, and the anterior chamber narrowed as the iris prolapsed. When iris prolapse occurs early in a case around the time of hydrodissection, the cause is almost always trapped fluid under the lens. The trapped fluid pushes the lens forward, narrowing the angle and pushing the iris out of the eye. The treatment is usually very simple and involves going first through the paracentesis and rocking the lens to release the trapped fluid. Once the fluid is released, you can gently reposition the iris into the deeper anterior chamber.
The second case was different. The iris prolapse occurred later, just after nucleofractis. The patient had pain and began to move due to this discomfort. The eye became firm with a narrow angle.
In this scenario, the diagnostic differential is broader and includes choroidal effusion, choroidal hemorrhage, and misdirection of fluid, which can become temporarily trapped behind the posterior capsule. In this particular case, we used an indirect ophthalmoscope to directly see the effusion, which on ultrasound was hemorrhagic. We stopped the case and waited for a few weeks before removing the residual cortical material and placing the IOL.
Randall Olson A sudden shallowing of the anterior chamber at any time with the eye open has to be presumed to be a suprachoroidal hemorrhage until proven otherwise. That means, get the eye closed as soon as possible and assess! A bad situation can turn into a disaster in a matter of seconds. Even if an indirect exam does not show any choroidals, they can be missed if they are far peripherally, and it is best to reassess them the next day if there is any doubt. I prefer not to go back until after the choroidals have completely resolved plus a safety window of at least 2 weeks has passed—and then, I go back with the aim to keep the eye pressurized to the extent possible and to get my business done as soon as possible.
Many audience members suggested that they wanted to lower IOP in this scenario. Generally, this is not a good thing, as lowering pressure may cause the hemorrhage to expand. A much safer approach is to close the eye firmly with sutures and assess.
Another even more common cause of late shallowing, as in this case, is zonular block syndrome. This is most common in exfoliation syndrome or other conditions in which the zonules are torn or damaged. It usually occurs with irrigation pointed out to the zonules, and it often occurs during the I&A part of the procedure. I still close the eye—but the difference from a choroidal hemorrhage is that the chamber re-forms with normal IOP in a couple of hours and there is no evidence of a choroidal.
If I am confident this is not a choroidal hemorrhage (the shallowing in this case is so sudden and only with far peripheral I&A, that I can feel more certain of this diagnosis), I will go back later the same day—or the next day, if I am at all unsure—and I will do dry I&A until all the cortex is central, place the IOL under this (OVD raises the cortex out of the bag), and then do careful central removal of cortex and OVD, as OVD removal can cause the problem to reoccur if one is not careful.
 |
|
CASE 9: In the first case, iris prolapse occurred immediately following hydrodissection; fluid was trapped behind the crystalline lens.
|
ACE UP YOUR SLEEVE AWARD
Which surgeon had the best save?
| Rich Hoffman |
19.6%
|
| Scott Barnes |
48.0%
|
| Tom Oetting |
32.3%
|
Case 10: I’d Love More Support
Soosan Jacob presented a challenging case of a white cataract with phacodonesis. After she used a capsule retractor and completed a CCC following capsular dye, implantation of a capsular tension ring (CTR) resulted in posterior dislocation of the entire nasal pole of the lens. She further suspended the capsular bag with more capsular hooks in order to resume phaco, but the posterior capsule tore as the nucleus was removed.
Q15 The CTR has dislocated the lens. What now?
| Use capsule retractors and continue phaco |
13.7%
|
| Perform the posterior assisted levitation (PAL) technique and phaco with scaffold |
11.6%
|
| Perform PAL and convert to manual ECCE |
43.5%
|
| Let the nucleus drop, implant IOL, and refer |
19.9%
|
| Let the nucleus drop, leave aphakic, and refer |
11.4%
|
Q16 The patient has posterior capsular rupture and zonular dialysis. Which IOL would you choose?
| ACIOL or iris-fixated IOL |
40.0%
|
| 3-piece IOL in the sulcus, with iris suture fixation |
6.5%
|
| 3-piece IOL in the sulcus, with scleral suture fixation |
16.7%
|
| 3-piece IOL in the sulcus, with glued/scleral tunnel fixation |
28.7%
|
| Leave aphakic and refer |
8.0%
|
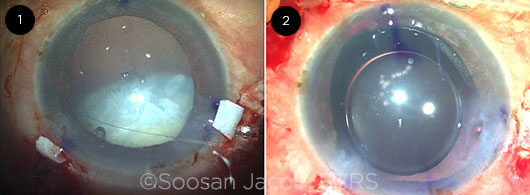
Soosan Jacob In this case of extensive subluxation, when I implanted the CTR, the remaining zonules gave way, resulting in a fully luxated cataract that was prevented from dropping into the vitreous cavity by just the 2 superior transsclerally placed capsular hooks and the terminal end of the CTR, which was still outside the eye (Fig. 1).
At this point, my options were to do PAL and remove the cataract either 1) after enlarging the incision or 2) by emulsifying the nucleus over a glued IOL scaffold. My third option was to give the capsular bag (with intact rhexis) a third point of support inferiorly. The first option would lead to disadvantages associated with a large wound—and the second involved a chance of nuclear fragments dropping around the IOL optic, thus necessitating a PPV. I therefore chose the third option, as it preserves the posterior capsule and would give me enough support for performing phacoemulsification, thus allowing in-the-bag IOL placement. As seen in the video, this was indeed the case, and the third transscleral capsular hook did give good support for completing the insertion of the CTR as well as for phacoemulsification and cortex aspiration.
My plan was to then fix the IOL/capsular bag complex by performing the glued capsular hook technique.1 In this scenario, the haptic of a modified transsclerally passed capsular hook is tucked into an intrascleral Scharioth tunnel made at the edge of the scleral flap. This allows sutureless transscleral fixation of the IOL/bag complex. Once the posterior capsular rupture happened, my natural choice was to go in for a glued IOL2 because of the extensive experience we have had with this technique in cases with insufficient capsular support. I therefore made an additional flap so that I had 2 scleral flaps diametrically opposite of each other. My tendency in such cases is to implant a 3-piece IOL in the bag so that I always have the option of easy conversion to a glued IOL if necessary. I therefore was prepared, and it was easy for me to perform a closed chamber translocation of this IOL into a glued IOL using the handshake technique.3 The patient had a well-centered IOL (Fig. 2) and did well postoperatively.
Mike Snyder Although it is uncommon, zonulopathy is a problem encountered at some point by nearly every eye surgeon. The timing of when to put in a CTR varies from surgeon to surgeon. I prefer Ken Rosenthal’s now-classic suggestion: “…as late as you can, but as soon as you must.” Supporting the capsule with multiple hooks or Ahmed segments can permit phacoemulsification to proceed.
Faced with a dangling lens and the CTR partway in, 43% of the audience preferred converting to a manual extracapsular procedure. This surprises me, since retrieving the dangling cataract with a large incision while viewing it through an infolded cornea would be exceedingly difficult. With no clear understanding of whether vitreous gel may or may not be just under or even overlying the lens, traction on the vitreous base with a resulting giant retinal tear is a notable possibility. The next most common response was to place an IOL and refer to a vitreoretinal colleague for PPV and lensectomy. Although no surgeons want to tell a patient that they need another procedure, that pathway maximizes the likelihood of a safe, controlled removal of lens material and excellent visual outcome.
With regard to the second question, the audience clearly preferred an ACIOL. This likely represents a higher degree of comfort among surgeons with ACIOL surgical techniques. While ACIOL placement is familiar, ACIOLs do directly contact the ciliary body, and possible low-grade cyclitis with resultant cystoid macular edema may ensue. It is reassuring that most audience members preferred scleral fixation over angle fixation.
___________________________
1 Jacob S et al. J Cataract Refract Surg. 2014;40(12):1958-1965.
2 Kumar DA et al. J Cataract Refract Surg. 2013;39(8):1211-1218.
3 Agarwal A et al. J Cataract Refract Surg. 2013;39(3):317-322.
 |
|
CASE 10: (1) Completely luxated cataract with a partially implanted CTR and superiorly placed transscleral capsular hooks holding it in place. (2) The well-centered glued IOL is evident.
|
Case 11: Go Ahead, Make My Day
David Chang presented a cataract with a traumatic zonular dialysis in which the capsular bag was preserved and a CTR was implanted. The first question was where to place the IOL and whether additional long-term bag/IOL support with suture fixation was advisable. A 3-piece IOL was implanted into the ciliary sulcus because one side of the bag tended to tip posteriorly. Following I&A removal of the OVD, the CTR no longer seemed to be symmetrically placed, which prompted the second question.
Q17 You have zonular dialysis plus a CTR. Which IOL would you choose?
| PCIOL in the bag |
49.9%
|
| Sutured CTR plus in-the-bag IOL |
12.5%
|
| Sutured Ahmed capsular tension segment (CTS) plus in-the-bag IOL |
13.9%
|
| 3-piece IOL in the sulcus |
21.0%
|
| Scleral- or iris-fixated PCIOL (sulcus) |
2.0%
|
| ACIOL or iris claw IOL |
1.0%
|
Q18 The CTR moved. What now?
| Leave/add OVD; end the case |
11.6%
|
| Insert Miochol-E; end the case |
16.6%
|
| Spatula sweep, with or without vitrectomy |
5.7%
|
| Inject triamcinolone, perform anterior vitrectomy from limbal approach |
25.5%
|
| Inject triamcinolone, perform anterior vitrectomy from pars plana approach |
40.6%
|
David Chang Capsule retractors support the weakened capsular bag in the anterior-posterior direction, provide rotational stability when zonular counter-traction is lacking, and restrain the equatorial capsular bag from being aspirated by the phaco or I&A tip. If possible, delaying CTR placement avoids trapping the cortex within the bag equator. If the dialysis is large enough, or the zonulopathy is diffuse enough, there may not be sufficient support for bag-IOL fixation. An underutilized alternative to suturing an intracapsular device is to place a 3-piece IOL in the sulcus. This provides additional 2-point fixation thanks to haptic-ciliary body contact. I generally capture the optic with the capsulorrhexis to assure IOL centration, prevent capsulophimosis, maintain the same effective lens position, and prevent late rotation of the haptics into and through the zonular dialysis.
In this case, the CTR changed position following IOL placement, and I confirmed and visualized the suspected vitreous prolapse by injecting triamcinolone into the anterior chamber. A pars plana approach for the anterior vitrectomy is ideal if vitreous is prolapsing through a zonular dialysis in a pseudophakic eye. The bag-IOL complex acts like a 1-way valve through which vitreous can be pulled forward (by a vitrectomy tip located in the anterior chamber) but will not recede posteriorly. Introducing the vitrectomy cutter through a pars plana sclerotomy 3.5 mm posterior to the limbus keeps the tip behind the IOL. In this way, vitreous is drawn from the anterior chamber back into the vitreous cavity, and no further anterior prolapse will occur. The anterior chamber is maintained with a separate self-retaining limbal infusion cannula.
Robert Cionni Placement of an IOL within the capsular bag is typically preferred when the capsular bag is centered and stable. When a posterior capsular tear is present, sulcus placement of a 3-piece IOL with optic capture by the anterior CCC is then preferred. However, when the posterior capsule is intact with significant zonular compromise, as in this case, zonular support by a CTR—and, if needed, a sutured Ahmed CTS—with placement of an IOL in the bag would be the preferred option. Half of the audience chose to place the IOL in the bag, but the type of IOL chosen is also important. The single-piece IOL is the easiest to place in these situations, and provided that the bag is centered and stabilized with an appropriate device, this is the best option. The severe bag tilting would likely not have occurred if a single-piece IOL had been chosen. In cases of trauma like this, which likely occurred at a young age, the remaining zonules are quite often strong enough (due to the flattened lens equator) to support the IOL long term when a standard CTR is placed.
With regard to the second question, there are 2 possible reasons for the CTR to have moved. The first, and most frightening, is a peripheral capsular bag tear. Fortunately, this was not the etiology in this case. A second would be vitreous prolapse around the lens equator at the site of the zonular defect. A sutured Ahmed segment may have helped to prevent late vitreous prolapse. My former partner at the Cincinnati Eye Institute, Scott Burk, MD, introduced the use of triamcinolone to identify, or “stain,” prolapsed vitreous.1 This technique works quite well to identify the previously invisible vitreous present, as it did in this case. I am thrilled to see that the majority of the audience chose this answer.
Once identified, the vitreous needs to be removed. An anterior approach can be used as long as the irrigation and vitrectomy handpieces are separated and neither is placed through the large main incision but rather through side-port incisions to prevent fluid or vitreous egress. The vitrectomy tip needs to be placed posterior to the equator to encourage vitreous to move posteriorly and prevent pulling more vitreous anteriorly. This would be challenging using an anterior approach in this case due to the inferior location of the zonular dialysis. A better choice, and the audience’s preferred choice, would be vitrectomy after triamcinolone staining via the pars plana. The only caveat here would be that hitting the posterior capsule with the vitrectomy cutter would be disastrous, as the CTR would no longer support the bag and IOL. Therefore, I prefer to aim the vitrectomy port posteriorly to avoid hitting the posterior capsule.
___________________________
1 Burk SE et al. J Cataract Refract Surg. 2003;29(4):645-651.
 |
|
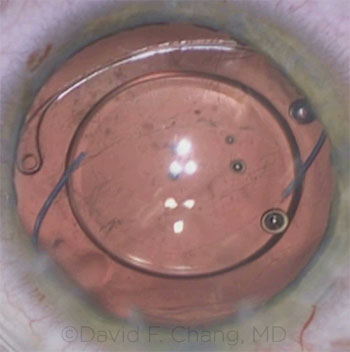
CASE 11: A gap formed between the CTR and the pupil margin.
|
Case 12: A Disinsertion Dilemma
Ike Ahmed noted a zonular dialysis during phaco while lens material still remained.
Q19 What’s your strategy for iatrogenic zonular dialysis?
| Cautious intracapsular phaco without devices |
4.2%
|
| Implant a CTR |
23.5%
|
| Implant a sutured Cionni CTR or an Ahmed CTS |
35.6%
|
| Insert capsule retractors |
19.0%
|
| Prolapse nucleus, do supracapsular phaco |
7.5%
|
| Convert to ECCE |
10.2%
|
Ike Ahmed In this case, an iatrogenic large nasal zonular dialysis greater than 6 clock hours occurred (Figs. 1 and 2). After it was identified, viscoelastic was injected to maintain the anterior chamber while the I&A tip was removed from the eye. Residual cortex was removed using a dry technique with a 27-gauge cannula, and additional viscoelastic was injected to expand the capsular bag and prevent vitreous prolapse. At that point, as the remaining zonules appeared strong, and with a 6-clock-hour nasal dialysis present, I injected a CTR followed by an in-the-bag single-piece PCIOL. The capsular bag complex appeared stable, and the PCIOL was well centered.
Retention of the capsular bag and prevention of vitreous prolapse was paramount in this case. A sutured CTS or Cionni modified CTR could be considered, but with the remaining zonules being of good strength, and with a 6-clock- hour nasal dialysis, it seemed to be unnecessary.
Furthermore, if decentration occurred postoperatively, one could return to the OR and suture fixate the existing capsular bag by suturing around the CTR through the capsule to the sclera.
Elizabeth Yeu In the case of 180 degrees of zonular loss during cataract surgery, the majority of the audience (78.1%) would have used some form of reinforced capsular support through a capsular tension device or capsular hooks. This reinforces how cataract surgery is continually evolving to best serve our patients, particularly in the realm of zonulopathy management, as this question would have likely evoked a very different majority opinion even 5 years ago. I also agree strongly that it is in the best interest of such a patient to save the capsular bag and place an IOL in the bag, if possible.
What I do to stabilize the bag intraoperatively depends largely on what step I’m at in the surgery. If the majority of the lens is still within the bag and the lens is obviously unstable, I get capsular hooks in as early as possible, as it is more difficult to deal with a tension ring when the lens is still intact. If the zonular instability becomes more pronounced during the lens disassembly itself, and I’m able to mobilize most of the endonucleus from the bag safely to the supracapsular space, I use at least a CTR to stabilize the bag. If a significant amount of the cortex and/or the epinucleus still adheres to the capsular equator, a standard CTR can make cortical removal quite challenging. This is where a corrugated CTR (Henderson CTR) can provide the optimal balance of distributed stabilization of the capsular bag and allow for greater ease with the cortical removal. One could also consider using a Cionni sutured CTR as well, in order to provide greater support.
I generally opt to start with a CTR and gauge the bag status from there. If the capsulorrhexis appears well centered, or similar to its original position, the IOL may center quite well, and no further intervention would be required. As a rule of thumb, I generally will add a sutured Ahmed CTS after a CTR when there is at least 6 clock hours of zonular loss. I’ll also suture a CTS into place after a CTR if I’m concerned about the bag stability after seating the CTR within the capsule.
Regarding sutured CTRs, it is easier to suture a single-eyelet CTR than a 2-eyelet CTR. I rarely use a 2-eyelet CTR and have only required it in cases with gross generalized instability of the capsular bag.
 |
|
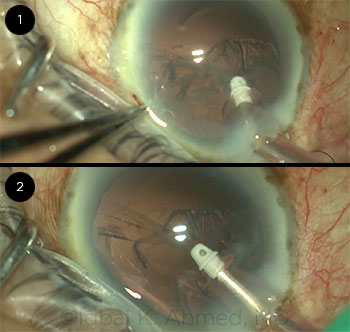
CASE 12: An iatrogenic 6-clock-hour zonular dialysis was unwittingly created because of aspiration of the capsular bag by the I&A tip.
|
WHEN TO HOLD OR FOLD AWARD
Which surgeon had the best judgment?
| Soosan Jacob |
17.3%
|
| David Chang |
51.4%
|
| Ike Ahmed |
31.3%
|
Case 13: Entering the Lion’s Den
Bonnie Henderson presented a case of a cataract that developed rapidly after a PPV (pars plana vitrectomy). As she suspected a capsular defect, she intentionally omitted hydrodissection, but she was struggling with the epinucleus after removing the nucleus.
Q20 What’s your strategy for adherent epinucleus?
| Hydrodissect it |
18.9%
|
| Viscodissect it |
63.7%
|
| Switch to bimanual I&A (no hydrodissection) |
15.9%
|
| Create a new incision for I&A (no hydrodissection) |
1.0%
|
| Abandon and let absorb |
0.6%
|
Bonnie Henderson The standard advice for removing a cataract that develops rapidly after a PPV is to avoid hydrodissection and only hydrodelineate the nucleus. Usually that advice is sufficient, and after the nucleus has been safely removed, the epinuclear shell and cortex are removed easily with the automated aspiration.
However, in this patient, that was not the case. After multiple attempts of trying to remove the residual material, I gambled that a gentle hydrodissection might be tolerated, since the capsule seemed to have stayed intact while I removed the central nucleus. I was wrong. Although I believed that the additional space and laxity created by removal of the nucleus would prevent a rupture of the posterior capsule under gentle hydrodissection, I had misjudged the fragility of the capsule. I learned that it is never safe to hydrodissect the lens when tackling a cataract that develops rapidly after a PPV.
Sophie Bakri I agree with Dr. Henderson. It is best to proceed cautiously with a postvitrectomy cataract. If a posterior capsular rent occurs, or the crystalline lens drops into the vitreous during cataract surgery, the cataract surgeon must safely close the eye and consider placement of an IOL. Vitreous and cortex should be removed from the anterior chamber first; intracameral triamcinolone may be needed to help with visualization of the vitreous. If there is enough capsular support, placement of a lens in the bag is the best option. If not, a sulcus-fixated IOL, such as a 3-piece foldable PCIOL, is a good choice. An anterior chamber lens can also be placed.
Referral to a vitreoretinal surgeon usually determines the next approach. Small cortical fragments can be observed while inflammation is controlled and IOP increases. Larger cortical fragments or the nucleus will need to be removed. The key is to provide a good view for vitrectomy. Sometimes 1 week or longer of careful observation is needed to control inflammation, corneal edema, and IOP before vitrectomy can be performed. With a good view, the risk of retinal detachment is decreased, as the vitreoretinal surgeon will be able to visualize the periphery and prophylactically treat any retinal tears or lesions predisposing to retinal detachment.
 |
|
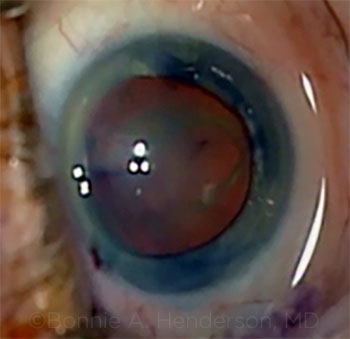
CASE 13: Cataract after recent PPV.
|
Case 14: A Sinking Feeling About Multifocal IOLs
Thomas Kohnen’s case involved posterior capsular rupture and a dropped nucleus following FS laser capsulotomy. The problem prompting the first question was that the patient already had a multifocal IOL in her first eye, and the planned IOL was a multifocal plate haptic design. There was no 3-piece multifocal backup available. The eye was left aphakic, and a second procedure was done to remove the retained nucleus and to implant a newly obtained 3-piece multifocal IOL in the sulcus. Optic capture was performed, but it didn’t hold, and the patient was very disappointed with a 2.5 D myopic error postoperatively.
Q21 The patient has a posterior capsular rupture, a dropped nucleus, and a multifocal IOL in the fellow eye. What now?
| Place a 3-piece multifocal IOL (I always have a backup) in the sulcus |
37.6%
|
| Place a 1-piece multifocal IOL in the bag |
4.0%
|
| Leave the patient aphakic and order a 3-piece multifocal IOL |
19.6%
|
| Place a 3-piece monofocal IOL in the sulcus |
38.1%
|
| Use an ACIOL or an iris claw IOL |
0.7%
|
Q22 You have myopic surprise with a multifocal IOL. What now?
| Perform multifocal IOL exchange |
6.0%
|
| Do LASIK/PRK |
71.6%
|
| Place a piggyback IOL |
5.2%
|
| Reattempt CCC/optic capture |
4.2%
|
| No more surgery!! Use glasses or a soft contact lens |
13.0%
|
Thomas Kohnen As we don’t usually have a spare 3-piece IOL available, we had to postpone the second intervention until the next day. I chose a 3-piece bifocal IOL, as a 3-piece trifocal was not available to match the trifocal IOL in her first eye. Because the anterior capsulotomy was perfectly round and intact following the FS laser procedure, anterior optic capture was achieved during the combined pars plana vitrectomy and IOL implantation.
The reason for the myopic refractive error of 2.5 D seemed to be the extrusion of the IOL optic into the sulcus.
Although 2.5 D is quite high, it may be explained by the fact that the eye was vitrectomized. Instead of performing excimer surgery or an IOL exchange, I decided to do an IOL displacement, repositioning the IOL optic through the anterior capsulotomy. This solved the problem.
The patient ended up slightly myopic with a very satisfactory outcome; her VA was excellent for far, intermediate, and near distances. She was very satisfied with the minimally invasive approach to solve the final problem after the complicated presbyopia-correcting IOL procedure.
Alan Crandall A case like this reminds us of a couple of points: First, the only way to completely avoid any complications is to never do surgery. Second, we must think about the management of such complications ahead of time and be prepared.
When I look at the first question, the first answer would be an obvious solution. I am surprised that so many audience members would have a full range of backup 3-piece IOLs. The choice of a 3-piece monofocal IOL—in a patient who wants a multifocal lens—would be a safe one, but this involves cleaning up the anterior segment and then performing a second surgery later on, usually in a few days. (This is the preferred timing in our center, based on discussions with our retina colleagues.) My choice would be to leave the patient aphakic and order a 3-piece multifocal lens.
The audience response to the next question is quite surprising. If one converts to a sulcus IOL, a power conversion depends on the axial length of the eye, but a 2.5 D surprise is quite a surprise!
Usually with capture of the optic by CCC, no adjustment is necessary, so a reattempt would not fix the power issue, and no more surgery would be unacceptable to most patients. And placing a piggyback IOL also is not an optimal solution: With 2 lenses in the sulcus, one would worry about iris chafing and secondary glaucoma. Moreover, most lenses used as piggyback IOLs (at least in the United States) come in whole diopters.
LASIK/PRK seems the most acceptable to the audience, and that would be fine. An early IOL exchange would also be a viable option, and I would try to bring the patient into the equation.
Case 15: He Really Doesn’t Want Glasses
Amar Agarwal’s patient wanted a multifocal IOL, but the posterior capsule tore despite earlier CTR placement. Part of the nucleus descended posteriorly.
Q23 You are dealing with posterior capsular rupture, a CTR, and a dropped nucleus. What now?
| Levitate the nucleus (e.g., PAL) |
10.9%
|
| Leave the nucleus and CTR and implant an IOL |
27.6%
|
| Leave the nucleus and CTR and leave aphakic |
4.9%
|
| Remove the CTR and implant an IOL |
10.9%
|
| Call a vitreoretinal colleague into the OR |
45.9%
|
Q24 There is no capsular support. Which IOL would you choose?
| Leave the patient aphakic |
0.5%
|
| Use an ACIOL or an iris claw IOL |
27.2%
|
| Iris fixate a 3-piece IOL |
7.4%
|
| Scleral fixate a 3-piece IOL |
41.4%
|
| Scleral fixate a 3-piece multifocal IOL |
23.6%
|
Amar Agarwal A patient with a subluxated cataract came to our center asking for a multifocal IOL. When I started the surgery, I used an endocapsular ring to fixate the bag. I should have used a Cionni CTR, an Ahmed CTS, an Assia anchor, a Jacob capsular hook, or Mackool capsule retractors to do a better job of fixating the capsular bag. As I did not use one of these options, I had a rent, and a nucleus piece fell into the vitreous cavity. I fixed a trocar infusion cannula in the pars plana and made my scleral flaps for a glued IOL. I then injected triamcinolone—a trick taught to me by Abhay Vasavada—to clear the vitreous properly, and I removed the endocapsular ring. I also did a posterior vitrectomy and removed the dropped nuclear piece using the sleeveless phacotip assisted levitation (SPAL) technique. Now the issue was how to fix a multifocal IOL in this patient who did not have any capsular remnants.
I decided to do a multifocal glued IOL using the handshake technique. The advantage of the multifocal glued IOL is that we can tuck and untuck the haptics on either side as much as we want and center the rings of the IOL exactly where we want them. The one lens we cannot glue in is a single-piece foldable IOL, as we need something firm in the haptics to tuck into the Scharioth pockets. I used a 3-piece foldable multifocal IOL from AMO. Another advantage of the multifocal glued IOL is that there is no pseudophakodonesis, and so the patient’s quality of vision is very good. At the end the patient was happy with a well-centered multifocal glued IOL.
Eric Donnenfeld Any time a complication occurs during cataract surgery, it can be unsettling to the surgeon and to the patient. However, the concern is even greater when a patient has high expectations for a refractive outcome. For this reason, it is imperative that every patient considering cataract surgery with a premium IOL should be told that there is a possibility that this surgeon will be unable to deliver the desired lens depending upon his or her expert intraoperative decision.
In this case, Dr. Agarwal was presented with a patient who wanted a multifocal IOL but did not have capsular support, and a piece of nucleus fell into the vitreous. His decision to perform a PPV and glued IOL with a multifocal lens was an aggressive one. The majority of the audience would not have placed a multifocal IOL, and almost half of them would have called in a vitreoretinal colleague to remove the nucleus. This is a completely reasonable decision for those who have not had vitreoretinal training, and I would have expected more surgeons to ask for a specialist’s assistance. However, in the hands of Amar Agarwal—who is as skilled a surgeon as any I know—his surgery and results are to be admired.
 |
|
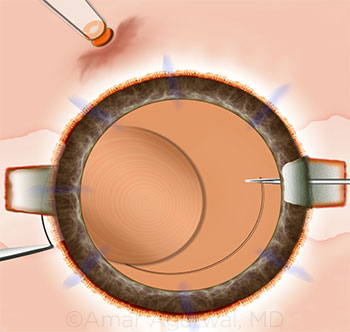
CASE 15: Multifocal glued IOL, placed using the handshake technique.
|
ROLL THE DICE AWARD
Who was the most daring surgeon?
| Bonnie Henderson |
3.7%
|
| Thomas Kohnen |
11.2%
|
| Amar Agarwal |
85.1%
|
Case 16: Always Have a Back-up Plan
In George Beiko’s case, a sulcus-fixated 3-piece IOL was to be exchanged because of an IOL power surprise. During the IOL exchange, the replacement IOL was first placed underneath the original IOL to serve as a protective scaffold. After the original lens was removed, George realized that the other IOL had disappeared, and it had descended back onto the retina.
Q25 The posterior IOL is dislocated. What now?
| Leave it and leave the patient aphakic |
5.2%
|
| Leave it and implant another PCIOL |
5.9%
|
| Leave it and implant an ACIOL |
13.4%
|
| Attempt to levitate the dropped IOL |
23.3%
|
| Call a vitroretinal surgeon into the OR |
52.2%
|
George Beiko This 85-year-old pseudophakic patient had diplopia due to anisometropia. He had a 3-piece IOL in the sulcus of one eye with a refraction of –5.00 D; his other eye had a plano refraction with an in-the-bag IOL. The plan was to exchange the sulcus IOL for one that would target plano. During the IOL exchange, the replacement IOL dropped into the posterior segment.
Fishing for an IOL in the vitreous increases the risk of retinal breaks and is not something that would be considered unless a thorough vitrectomy could be ensured. Normally, I would have referred the case to a vitreoretinal surgeon for appropriate management. However, this individual had a somewhat complicated surgical history: 15 years before this point, his retinal detachment was repaired with a buckle. Then, 13 years ago, during cataract surgery, he had a dropped nucleus, which necessitated a vitrectomy with endolaser and the placement of the sulcus lens.
Given that he had a vitrectomized posterior segment supported by a buckle and laser scars, I opted to have him lie face down on his stomach, with a lid speculum in place. I positioned myself under the stretcher—and waited. The lens floated down into the anterior chamber. Wearing surgical loupes for visualization, and using a cannula, I was able to externalize one of the haptics and fill the chamber with OVD. We then rolled the patient back into position under the microscope; I manipulated the lens into the sulcus, and stable placement of the IOL was secured.
When I was a resident, facedown positioning was in vogue for management of giant retinal tears; that memory remained with me and certainly was a factor in my decision in this case.
Tarek Hassan Despite the remarkable outcome of George’s case (one that I have never seen before!), I am happy to see that the majority of respondents would contact a vitreoretinal surgeon to approach the dislocated IOL. Although any of the other listed options would be theoretically acceptable, the standard of care today is to safely achieve a permanent repair with optimal visual results. I believe that having a vitreoretinal surgeon retrieve the dislocated IOL via a posterior pars plana approach is the best way to achieve this with minimal trauma.
Depending on the type of lens that is involved, and the anatomy of the eye, the next decision is whether to place it in the sulcus, suture fixate it, sclerally fixate it, or even exchange it with another PCIOL or ACIOL if absolutely necessary. If a vitreoretinal surgeon is not immediately available, it is preferable that the eye be left aphakic, so that the rescue can be easily performed at a later date.
In this day and age, the least-acceptable solution is to not remove the dislocated IOL and then place an additional PCIOL or ACIOL that would interfere with the vitreoretinal surgeon’s ability to remove the dropped IOL through the anterior chamber.
 |
|
CASE 16: A creative solution to a dislocated IOL: facedown positioning of the patient.
|
Case 17: It’s Not Over ’til It’s Over
Susan MacDonald’s case involved a monocular patient with a mature cataract in her only good eye. A large zonular dialysis developed while most of the ultrabrunescent nucleus was still present.
Q26 There is zonular dialysis and lots of nucleus. What now?
| Continue careful intracapsular phaco |
8.5%
|
| Perform intracapsular phaco after placing CTR |
22.6%
|
| Perform intracapsular phaco after placing capsule retractors |
23.4%
|
| Prolapse nucleus to anterior chamber, do supracapsular phaco |
37.0%
|
| Convert to ECCE |
8.4%
|
Susan MacDonald This patient had avoided cataract surgery for many years because her ophthalmologist recommended against it. It was her only seeing eye, and the cataract was very dense with pseudoexfoliation (PXF), which put her at greater risk of experiencing an intraoperative complication. But as her 98th birthday drew near, her good eye was no longer seeing well enough for her to read or perform simple daily tasks, and she decided it was time to proceed. So she was referred for cataract surgery.
On exam she had a dense 4+ nuclear sclerotic cataract with PXF, a small pupil, and a shallow chamber. Topical anesthesia was used, and the pupil was adequately enlarged with viscoelastic. The capsule was easy to tear, suggesting adequate zonular support.
However, during phacoemulsification, there was a clear peripheral red reflex. I couldn’t tell whether it was a posterior capsule rupture or zonular dehiscence. I chose to consider the worst-case scenario and assumed there was a posterior capsular rupture. I placed a dispersive viscoelastic under and over the nucleus and gently lifted it into the anterior chamber. I also placed a Sheets glide under the nucleus and over the iris to keep the nucleus in the anterior chamber while it was being emulsified. However, as I was emulsifying the nucleus, the glide was pushed into the angle, which caused iris trauma and hemorrhage. When I noticed this, I pulled the glide out of the angle.
It was only after the nucleus was removed that I was able to confirm that the capsular bag was intact but that there was a zonular dehiscence for about 2 clock hours. I used triamcinolone to identify a small strand of vitreous, which was removed via bimanual vitrectomy. I decided to place a 3-piece lens in the sulcus and used a CTR to expand the capsular bag and reverse capture the lens.
The key point in this case is that I did not know the condition of the capsular bag. If I had taken the time to inspect the bag, I might have diagnosed the zonular dehiscence and placed capsule hooks and a CTR. And if I had known it was a dehiscence, I might have used the 3-piece IOL as a glide under the nucleus. I was so concerned about not dropping the nucleus that I did not keep my eye on the leading edge of the Sheets glide. I could have prevented the angle trauma by pulling the glide out of the angle when it was moved forward.
Two additional points from this case: 1) It is critical to stay focused and calm when managing complications. This can be done by slowing down and defining the issue and proposing a step-by-step solution. 2) Watch your complication videos, as this is the best way to improve surgical judgment and skills.
Richard Lindstrom A 97-year-old with an ultrabrunescent cataract and PXF is at very high risk for zonular disinsertion, a capsular tear, and vitreous loss. I usually do these cases with a large-incision, planned ECCE. This is somewhat of a lost skill among U.S. cataract surgeons, but for me, it would be a safer approach for this patient.
Most U.S. surgeons would plan for phacoemulsification of this dense cataract. In this instance, trypan blue staining of the anterior capsule can be helpful. I prefer a larger anterior capsulotomy, and good insight into the zonular strength is usually apparent when creating the anterior capsulotomy. If the zonules are extremely loose, capsular support hooks can be placed early in the case. I will perform gentle hydrodissection with BSS using a pulsatile stream, and I like to subluxate the nucleus out of the capsular bag on one side and tilt it up. I will then inject a dispersive viscoelastic such as Viscoat to open the bag, reduce stress on the zonules, and protect the posterior capsule. A careful supracapsular phacoemulsification with replacement of the viscoelastic several times to protect the capsule and corneal endothelium gives me my best results. If the zonules disinsert during nucleus removal, the nucleus can be viscolevitated into the anterior chamber. As there is little to no cortex in these patients, a CTR can be placed under the remaining nucleus if the capsular bag is intact.
If there is a capsular tear with or without vitreous loss, as occurred in Dr. MacDonald’s patient, I agree with the largest percentage of the audience that the best plan is to prolapse the remaining nuclear fragments into the anterior chamber. Now the most critical need is to remove the nuclear remnants without losing any into the posterior segment. I like to place some form of scaffold under the nuclear remnants if I am going to remove them with phacoemulsification. The Sheets glide selected by Dr. MacDonald is an excellent choice, and I have frequently utilized this in the past.
If I am confident that I will be able to place a 3-piece IOL into the sulcus after the lens material is removed, I will implant the IOL into the eye with the haptics either in the sulcus or, if I am uncertain as to the anterior capsular rim status, into the anterior chamber. I find the IOL optic to be an excellent scaffold for protecting from loss of nuclear material into the posterior segment, and it is readily available in all ORs. Adding dispersive viscoelastic several times is often helpful. Of course, the incision can also be enlarged and the nuclear remnants removed with a lens loop. Once the retained lens material is removed, the surgeon can determine capsular and vitreous status and take appropriate steps to complete the case. When possible, I prefer a 3-piece PCIOL in the sulcus with optic capture. In patients with doubtful capsular support, I do not hesitate to suture the haptics to the iris utilizing a polyester suture and closed-chamber technique.
Case 18: Shattered Dreams
After he implanted a single-piece acrylic IOL into the capsular bag, Bob Osher noted a large crack/tear across the junction of one of the haptics.
Q27 There is postoperative refractive surprise and a damaged haptic. What now?
| Perform an IOL exchange |
70.0%
|
| Place a piggyback IOL |
5.2%
|
| Attach the haptic with a stitch |
1.2%
|
| Laser the other haptic for symmetry |
1.8%
|
| Perform keratorefractive surgery |
21.8%
|
Robert Osher This patient underwent uneventful phacoemulsification followed by cortical removal. When I injected the single-piece IOL, I noted a severe fracture along the haptic-optic junction. Even though the haptic seemed to be severed about 90% across its width, the IOL centered well, and I did not perform an IOL exchange. On the first postoperative day, the patient’s uncorrected vision was 20/70, improving to 20/20 with a +1.50 sphere. A target of plano had been planned.
I resisted all temptation to take the patient back to the OR and instead exercised conservative observation. The patient returned 2 weeks later, and the lens was well centered. The haptic appeared fused to the optic, and the patient’s uncorrected vision had improved to 20/20. Sometimes it is better to be lucky than good!
John Hovanesian This is the kind of challenge that every surgeon will come across from time to time: A damaged lens is implanted into the eye, and the surgeon must decide whether to leave it in place or remove it. Removing an implanted lens has its own risks, such as tearing the capsule, snagging the iris, and damaging the endothelium during manipulation and explantation. If we leave it in place, we risk that the lens will not occupy its intended position and will become tilted, decentered, or vaulted in a manner that produces unwanted optical side effects.
As a general rule, it’s best to remove and replace a lens at the time of implantation any time we have significant doubts about its stability. In this particular case, however, it was a reasonable choice to leave this 1-piece lens in place because of the unique configuration of the damage to the haptic. The haptic-optic junction was torn about halfway through, leaving the haptic connected by a bridge of intact lens material at the periphery of the optic.
Dr. Osher presumably judged that compressive forces on the lens during capsular healing would push the torn edges on the more central aspect of the haptic together, not apart. Therefore, the lens position would not deviate from what it would be if the haptic were normal. If the damage had been, by chance, on the outside (periphery) of the haptic, one would more likely expect compressive forces to bend the damaged haptic more easily than the other, normal haptic, and this asymmetry of haptic strength would potentially cause the optic to decenter. As it turned out, Dr. Osher’s choice was at least correct, if not overly brave.
In the audience response question, we are asked how to address this patient’s hyperopic surprise 1 day after cataract surgery. At this early time point, refractive surprises can be caused by a host of issues unrelated to the haptic’s integrity, so any intervention would be best delayed. Appropriately, Dr. Osher waited for a more stable result and was rewarded for his patience with a satisfactory outcome.
WHAT I PRESENTED IN VEGAS, STAYS IN VEGAS AWARD
Who was the most courageous presenter?
| George Beiko |
62.0%
|
| Susan MacDonald |
24.6%
|
| Bob Osher |
13.4%
|
Financial Disclosures
Amar Agarwal, MD: Abbott Medical Optics: C; Bausch + Lomb: C; Dr. Agarwal’s Pharma: O; Slack: P; Staar Surgical: C; Thieme Medical Publishers: P. Iqbal K. Ahmed, MD: Abbott Medical Optics: C,L,S; Accelerated Vision: C; Ace Vision Group: C; AdeTherapeutics: C; Aerie Pharmaceuticals: C; Alcon: C,L,S; Allergan: C,L,S; AqueSys: C,S; Bausch + Lomb: C; Carl Zeiss Meditec: C,L,S; Clarity: C,S; Evisia: C; Eyelight: C; ForSight Labs: C; Glaukos: C,S; Iantech: C; InnFocus: C; Iridex: C; Ivantis: C,L,S; LayerBio: C; New World Medical: S; Oculus: C; Omega Ophthalmics: C; Ono Pharmaceutical: C; PolyActiva: C; Sanoculis: C; ScienceBased Health: C; Stroma: C; Transcend Medical: C; TrueVision: C. Sophie J. Bakri, MD: Allergan: C. Scott D. Barnes, MD: Abbott Medical Optics: L; Staar Surgical: L. George Beiko, MD: Abbott Medical Optics: C,S; Bausch + Lomb: C,S; Infinite Vision: C. Roberto Bellucci, MD: Bausch + Lomb: C; PhysIOL: C; Sifi Medtech: C. Rosa Braga-Mele, MD, FRCSC: Alcon: C,L; Allergan: L. David F. Chang, MD: Abbott Medical Optics: C; Allergan: L; Calhoun Vision: C,O; Clarity: C,O; Icon Bioscience: C,O; LensAR: C,O; Minosys: C,O; PowerVision: O; RevitalVision: O; Slack: P; Transcend Medical: C,O; Versant Ventures: O. Robert J. Cionni, MD: Alcon: C,L,O; ClarVista: C; Mile High Ophthalmics: C; Morcher: P; Ocumetics: C; Omeros: C; ReVision Optics: C; Sony Medical: C. Alan S. Crandall, MD: Alcon: C,L; AqueSys: C; Asico: C; Glaukos: C; iScience: C; Ivantis: C; Mastel: C; Omeros: C; Transcend Medical: C. Deepinder K. Dhaliwal, MD, LAc: Abbott Medical Optics: S; Avedro: S; Eleven Biotherapeutics: S; Imprimis: S; NovaBay Pharmaceuticals: C. Eric D. Donnenfeld, MD: Abbott Medical Optics: C,L,S; AcuFocus: C; Alcon: C,L,S; Allergan: C,L,S; AqueSys: C,O; Bausch + Lomb: C,L,S; Cataract and Refractive Surgery Today: C; Elenza: C,O; Glaukos: C,O; Icon Bioscience: C; Kala Pharmaceuticals: C; Katena: C; LacriPen: C,O; LenSx: C; Mati Pharmaceuticals: C,O; Mimetogen Pharmaceuticals: C,O; NovaBay Pharmaceuticals: C,O; Ocuhub: O; Odyssey: C; PRN: C,O; RPS: C,O; Strathspey Crown: O; TearLab: C,O; TLC Laser Eye Centers: L,O; TrueVision: C,O; Versant Ventures: O; WaveTec: C. Tarek S. Hassan, MD: Alcon: C; Allergan: C; ArcticDx: O; Bayer Healthcare Pharmaceuticals: C; Genentech: C; Insight Instruments: C,P; Janssen: C; Novartis: C; Regeneron: C; Roche: C. Bonnie A. Henderson, MD: Abbott Medical Optics: C; Alcon: C; Bausch + Lomb: C; ClarVista: C; Massachusetts Eye and Ear Infirmary: P; Regeneron: C; Stealth BioTherapeutics: C. Richard S. Hoffman, MD: Carl Zeiss Meditec: C; MicroSurgical Technology: C. Edward J. Holland, MD: Alcon: C,L,S; Bausch + Lomb: C,L; Kala Pharmaceuticals: C; Mati Therapeutics: C; PRN: C,S; RPS: C; Senju Pharmaceutical: C,L; Shire: C; TearLab: C; TearScience: C,L. John A. Hovanesian, MD: 1-800-Doctors: C,O; Abbott Medical Optics: C,L,O,P; Allergan: C; Bausch + Lomb: C,L,S; Glaukos: S; Halozyme Therapeutics: C; IOP Ophthalmics: C,L,S; Ocular Therapeutix: C,L,O,S; Reata Pharmaceuticals: C; ReVision Optics: C; SARcode: C,L,S; Sarentis Ophthalmics: C; Sight Sciences: C,O; Slack: C,L; TearScience: C,L,S; TLC Laser Eye Centers: C,L,O; TrueVision: C,L,S; Versant Ventures: O; Vindico Medical Education: C,L; Visiogen: C,L,S; Vista Research: C; Vistakon Johnson & Johnson Vision Care: C,P,S. Soosan Jacob, MD, FRCS: Has a patent pending for modified versions of the glued capsular hook. Terry Kim, MD: Acucela: C; Acuity Advisors: C; Alcon: C,L,S; Allergan: C; Bausch + Lomb: C,L,S; CoDa Therapeutics: C; Foresight Biotherapeutics: C; Kala Pharmaceuticals: C; NovaBay Pharmaceuticals: C; Ocular Systems: C; Ocular Therapeutix: C,O; Oculeve: C; Omeros: C,L,O; PowerVision: C; Presbyopia Therapies: C; Shire: C; Stealth BioTherapeutics: C; TearLab: C; TearScience: C. Thomas Kohnen, MD, PhD, FEBO: Abbott Medical Optics: C,L,S; Alcon: C,L,S; Carl Zeiss Meditec: C,L,S; Geuder: C; Oculus: L; Rayner Intraocular Lenses: C,L,S; Schwind eye-tech-solutions: C,L,S; TearLab: C; Thieme Compliance: C; Zeimer: C. Stephen S. Lane, MD: Alcon: C,L; Bausch + Lomb: C,L; ClarVista: C; Kala Pharmaceutical: C; Lifecore: C; Ocular Therapeutix: C,O; Omeros: C,O; PowerVision: C; PRN: C; RPS: C,O; TearScience: C,O; VisionCare Ophthalmic Technologies: C; WaveTec: C,O. Richard L. Lindstrom, MD: Abbott Medical Optics: C; AcuFocus: C,O; Alcon: C; Alphaeon: C; ASCRS Foundation: C; Aviana Molecular Technologies: C; Bausch + Lomb: C,P; BioSyntrx: C,O; Calhoun Vision: C,O; Clarity: C; ClearSight: C,O; Confluence Acquisition Partners: I,O; Curveright: C; EBV Partners: C,O; EGG Basket Ventures: C,O; Encore: C,O; Equinox: C; Evision: C,O; Eyemaginations: C,O; ForSight Vision6: C; Foresight Venture Fund: C,O; FzioMed: C,O; Glaukos: C,O; Healthcare Transaction Services: O; Heaven Fund : O; High Performance Optics: C,O; Hoya: C; iiCayr: C; Imprimis Pharmaceuticals: C; InnerCity Tennis: C; Ista Pharmaceuticals: C; LensAR: C,O; Life Guard Health: C,O; Lindstrom Restoration: C,O; Lumineyes: C; Minnesota Eye Consultants: C,O; Minnesota Eye Foundation: C; NASA Vision for Mars: C; Novartis: C; NuLens: C,O; Ocular Optics: C,O; Ocular Surgery News: C; Ocular Therapeutix: C; Oculeve: C; Omega Ophthalmics: C,O; Omeros: C; PogoTec: C; Qwest: C,O,P; Refractec: C,O; ReVision Optics: O; TearLab: C; University of Minnesota Foundation: C; Vision Foundation: C. Susan M. MacDonald, MD: Alcon: L; MedEdCore: O; Transcend Medical: C; 3D Communications: C. Nick Mamalis, MD: ARC Laser: S; Aaren Scientific: S; Abbott Medical Optics: S; Alcon: S; Allergan: S; Anew Optics: C,S; Bausch + Lomb: S; Calhoun Vision: S; ClarVista: S; Genisphere: S; Hoya: S; LensGen: S; Medennium: C,S; Minosys: S; NuView Technologies: S; Omega Ophthalmics: S; PowerVision: S; Sharklet: S. Samuel Masket, MD: Accutome: S; Alcon: C,L; Haag-Streit: C,P; Morcher: P; MicroSurgical Technology: L; Ocular Theraputix: C,O; PowerVision: C; VisionCare Ophthalmic Technologies: C; WaveTec: C,S. Kevin M. Miller, MD: Alcon: C,L,S; Calhoun Vision: S. Thomas A. Oetting, MD: None. Randall J. Olson, MD: Jade Therapeutics: O; Mastel: P; XEnd: O,P. Robert H. Osher, MD: Abbott Medical Optics: C; Alcon: C; Bausch + Lomb: C; Beaver-Visitec International: C; Carl Zeiss Meditec: C; Clarity: C; MicroSurgical Technology: C; Omeros: C; Video Journal of Cataract & Refractive Surgery: O. Richard B. Packard, MD: Alcon: C,L. Michael E. Snyder, MD: Alcon: S; Bausch + Lomb: S; Haag-Streit: C; HumanOptics: C; Icon Bioscience: S; Xigen: S. Roger F. Steinert, MD: Abbott Medical Optics: C,S; Avedro: C,O; LensGen: O; ReVision Optics: C; Rhein Medical: P. Geoffrey C. Tabin, MD: None. Mitchell Weikert, MD: Zeimer Ophthalmic: C. Abhay Raghukant Vasavada, MBBS, FRCS: Alcon: S. Elizabeth Yeu, MD: Abbott Medical Optics: C,L; Alcon: C,L; Allergan: C,L; Bausch + Lomb: C; Bio-Tissue: L; RPS: O; TearLab: C; TearScience: C. Sonia H. Yoo, MD: Abbott Medical Optics: S; Alcon: C; Allergan: S; Avedro: S; Bausch + Lomb: C; Bioptigen: C; Carl Zeiss Meditec: S; Slack: L; Transcend Medical: C.
See the disclosure key at www.aao.org/eyenet/disclosures.
|