By Annie Stuart, Contributing Writer, interviewing Andrew G. Lee, MD, Marc H. Levin, MD, PhD, and Neil R. Miller, MD
Download PDF
Controversy persists about how best to manage patients with acute central retinal artery occlusion (CRAO) and how much can be done to recover their vision—especially given how quickly the retina experiences irreversible ischemia following a CRAO. Further, the importance of a systemic workup, specifically the urgency of a stroke evaluation, may not be recognized by some physicians. Three neuro-ophthalmologists explain what should be uppermost in their colleagues’ minds when these patients walk through the door.
Is It CRAO?
CRAO comes with challenges, but diagnosis is usually not one of them. The typical presentation is sudden, severe, painless, monocular, central vision loss, said Marc H. Levin, MD, PhD, at the Palo Alto Medical Foundation in Palo Alto, Calif. More than three-fourths of eyes have visual acuity of 20/400 or worse at presentation.1
The patient’s prognosis is better if there is sparing of the cilioretinal artery circulation feeding the central macula, said Dr. Levin. This can be seen in up to 30% of cases; and in 80% of these patients, visual acuity usually returns to 20/50 or better within a couple of weeks.2
Challenges. Although CRAO is the ocular equivalent of an ischemic stroke, patients don’t consider it as urgent as they would a cerebral stroke; thus, they rarely seek emergency medical help. That means presentation to the ophthalmologist—and treatment—is commonly delayed, said Dr. Levin. To complicate matters: If a patient awakens with vision loss, it is difficult to establish the timing of onset.
Telltale signs. At first, the fundus signs of intraretinal ischemia in CRAO are not always dramatic, said Dr. Levin. “But within hours after the event, a cherry-red spot and edematous retinal whitening and thickening can be seen.” Opacification resolves within 4 to 6 weeks.2
“The arteries may have evidence of emboli,” said Dr. Levin, “and boxcarring may appear in both arteries and veins, signifying poor perfusion.”
 |
|
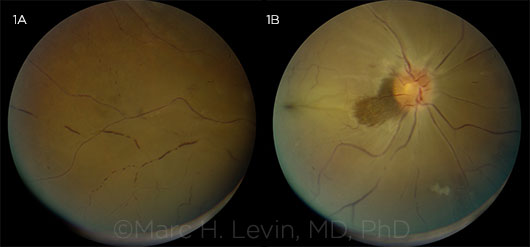
CRAO. This 54-year-old man awoke 6 hours prior to presentation with complete painless vision loss in the right eye. He had similar episodes 2 days and 1 week earlier that were transient. Note the (1A) boxcarring of vessels and (1B) retinal whitening with early cherry-red spot at the fovea, with a small darker retinal patch adjacent to the disc signifying a small region of preserved cilioretinal artery perfusion.
|
Pinpoint the Cause
The cause of the occlusion may affect workup and management.
Clots. Most of the time, an embolus (clot) causes CRAO, said Andrew G. Lee, MD, at Houston Methodist Hospital in Houston. Clots may originate in the eye (thrombus) or come from somewhere else (embolus). Emboli are commonly made up of cholesterol, fibrin-platelets, or calcium fragments, said Neil R. Miller, MD, at Johns Hopkins University in Baltimore.
If the cause of the occlusion is an embolus, said Dr. Lee, the embolic source can shower more emboli into the brain, so the workup should be fast and aggressive. Check for other common comorbid conditions and risk factors, he said, such as carotid occlusive disease, cardiac disease, diabetes mellitus, systemic hypertension, smoking, and hyperlipidemia. In addition, said Dr. Miller, patients should be assessed for systemic conditions—such as connective tissue disease, coagulopathy, atherosclerosis, or atrial fibrillation—that predispose to emboli. “Some patients will require anticoagulation,” said Dr Lee, “while others require only antiplatelet therapy with aspirin. The indications vary by etiology.”
Vasospasm. Previously called retinal migraine, vasospasm can also cause occlusion, said Dr. Miller. As with clotcaused occlusions, a cherry-red spot and choroidal whitening may appear; however, the patient is more likely to recover vision after vasospasm.
Giant cell arteritis. Another cause of CRAO is inflammation of the retinal artery due to giant cell arteritis, which occurs mainly in people over age 50, said Dr. Miller. “Giant cell arteritis can cause headache, ear pain, jaw pain, scalp tenderness, arthralgia, fatigue, fevers of unknown origin, chronic cough, or a combination of these symptoms.”
It is critical to rule out giant cell arteritis, which is a serious systemic condition that can cause vision loss in the other eye, stroke, heart attack, and death, said Dr. Miller. You can rule it out if you see a causative embolus, said Dr. Levin. “But when an embolus is not visible, proceed with expedited lab work, such as blood tests to check erythrocyte sedimentation rate and C-reactive protein. If giant cell arteritis is strongly suspected, emergent administration of systemic steroids is indicated prior to a confirmatory temporal artery biopsy.”
Acute Management: Timing & Treatment
How big is the window for treatment? Based on primate studies, the retina can generally survive for up to 100 minutes without circulation.3 But other factors may influence this number in humans.
Collateral blood circulation may enable the retina’s survival for up to 4 hours, said Dr. Levin. “A proximal embolus may also break free and travel further distally in the retinal vessels, allowing reperfusion and reoxygenation of tissues if occurring within a few hours of the occlusion.”
Too late, sealed fate? “Unfortunately, we often don’t see CRAO patients within the first few hours of symptoms,” said Dr. Levin. In fact, no more than 50% of CRAO patients reach the angiography suite within 8 hours.4 “Most patients will have isolated symptoms, and they will present first to the ophthalmologist, rather than the emergency room, because it’s a purely visual symptom.”
Given this all-too-common scenario, how should the ophthalmologist proceed?
Standard therapies. In certain cases, conservative therapy may aid reperfusion. “These methods cause no harm and don’t take much time,” said Dr. Miller. For example, said Dr. Lee, some ophthalmologists try to lower intraocular pressure (IOP) with drops or anterior chamber paracentesis. “The perfusion pressure of the retina equals the mean arterial pressure minus the intraocular pressure. Therefore, lowering the IOP might restore perfusion.”
A 2009 Cochrane review found these approaches no more effective than placebo.5 Still, there are anecdotal examples of success, said Dr. Lee, describing cases in which he used ocular massage to successfully move an embolus downstream and lowered IOP with drops. These measures might have been responsible for improving the visual outcome—or it might have been the natural history of CRAO.
Fibrinolysis. Paralleling an approach used for hemispheric stroke, intra-arterial fibrinolysis for CRAO involves catheterization of the ipsilateral internal carotid artery and infusion of thrombolytics into the ophthalmic artery, said Dr. Levin. This treatment has been used mostly in Europe.
Supporting this approach, said Dr. Miller, are several small case series, including one at Johns Hopkins, that suggest using intra-arterial—and possibly intravenous—tissue plasminogen activator (tPA) gives a better overall result than doing nothing or using conservative therapy. “However, a big prospective study, called the European Assessment Group for Lysis in the Eye (EAGLE) study, concluded that there was no benefit,” he said. Clinically significant visual improvement was equal between the 2 arms of the study.6 (See “EAGLE Eyes on Trial” below.)
In the EAGLE trial, intra-arterial tPA failed to help patients more than conservative therapy and it even harmed some patients, said Dr. Lee. Adverse reactions were nearly 9 times higher in the tPA arm, prompting the researchers to stop the study early.6 “Although tPA has worked relatively well for ischemic strokes in the brain, it didn’t work as well in the studies of CRAO, mainly because these patients come in too late to reverse the ischemia.” If patients came in time, said Dr. Lee, he would be more willing to treat them.
This trial suggests that there is no reason to do anything other than what’s been considered standard therapy (e.g., IOP drops, anterior chamber paracentesis, ocular massage) and to promptly refer the patient to a neurologist for a vascular evaluation, said Dr. Miller.
A surgical approach. Ophthalmologists in specialized centers have attempted surgery for CRAO, said Dr. Levin. Within 36 hours of symptom onset, the surgeon performs standard 3-port, 20-gauge pars plana vitrectomy and, using a 25-gauge microvitreoretinal blade, makes a longitudinal incision of the anterior artery wall near the embolus. Either the embolus spontaneously passes through the incision or the surgeon uses vitreoretinal forceps to express it from the vessel.7 The procedure lacks solid evidence, but case series have shown intriguing results, said Dr. Levin.
EAGLE Eyes on Trial
The multicenter randomized controlled EAGLE study in Europe failed to demonstrate improvement in vision using fibrinolysis for CRAO patients. However, questions remain about the conclusiveness of the study and whether tPA might still have the potential to outperform conservative treatment.
Wide treatment window. “The key criticism of this study,” said Dr. Levin, “was that it enrolled patients up to 20 hours after symptom onset, which is far beyond the traditional treatment window for fibrinolysis for either stroke or acute coronary syndrome.” The mean interval between the first symptom and treatment was nearly 11 hours for conservative therapy and nearly 13 hours for the intra-arterial tPA.1
The problem with this, said Dr. Lee, is that including these “latecomer” patients affected the final efficacy data because late treatment is known to be less effective or possibly ineffective. “Subgroup analysis suggested that treatment in an earlier time frame might have worked, but there were not enough patients to draw firm conclusions,” he said. “This study probably needs to be repeated with a larger sample size and a shorter treatment time interval.”
Potential confounder. Another question also emerged from this trial. “Unlike in the U.S.,” said Dr. Miller, “European neurologists routinely treat CRAO patients with several days of heparin. In the EAGLE trial, both groups received 5 days of heparin, whether in the conservative or tPA arm of the study. It is possible that the heparin equalized the results.”
___________________________
1 Schumacher M et al. Ophthalmology. 2010;117(7):1367-1375.
|
A Focus on Stroke
Since becoming a neuro-ophthalmologist several years ago, Dr. Levin said his attitude toward conditions such as CRAO has changed dramatically. “To me, the key is secondary prevention—minimizing the risk of hemispheric stroke.”
Take-home message. “Try your best with standard therapy,” said Dr. Miller, “but by all means don’t send the patient home.”
Instead, contact the local emergency room right away. “Tell them you have a patient with a CRAO who needs an assessment by a neurologist as soon as possible,” said Dr. Miller. Neurologists should assess the vast majority of CRAO patients, he said, because they’re knowledgeable about the type of vascular workup needed.
Dr. Levin agreed. “We’re not necessarily banking on interventions to restore vision, but properly and promptly referring for emergent evaluation.” This is especially true if symptoms are of recent onset—within hours, he said. “Young patients, regardless of symptom onset, should be evaluated very quickly,” said Dr. Levin.
It’s an emergency! Most ophthalmologists recognize that these patients should be evaluated for carotid artery and cardiac diseases, and probably should have a brain MRI, said Dr. Levin. But they may lack a sense of urgency and may wait for weeks to complete a workup. “That’s problematic because we know the highest window of stroke risk is within the first week.” The incident rate ratio for ischemic stroke peaks 1 to 7 days after CRAO (44.51; 95% CI, 27.07-73.20) and remains elevated for the first 30 days (14.0; 95% CI, 8.90-22.00).8
Get to the source. “An imaging workup should include noninvasive imaging of cervical vessels, such as carotid ultrasound, to look for stenosis or compromise of the internal carotid artery,” said Dr. Levin. Also, patients should undergo electrocardiography to check for an arrhythmia as soon as possible, and they should have an echocardiogram to evaluate for cardiac ischemia, he added.
Silent stroke. “Most patients with CRAO require a brain MRI with diffusion-weighted imaging within 24 hours, if the occlusion is detected acutely,” said Dr. Levin.
CRAO and other types of sudden monocular vision loss—such as branch retinal arterial occlusion or retinal transient ischemic attack—greatly increase the risk of silent brain infarctions. One study found that 89% of strokes following monocular visual loss were silent.9 Since these 3 conditions significantly increase the risk for subsequent clinical strokes, the researchers advised urgent and thorough evaluation on a stroke unit.
___________________________
1 Hayreh SS et al. Am J Ophthalmol. 2005;140(3):376-391.
2 Graham RH et al. Central Retinal Artery Occlusion. Medscape. Accessed Feb. 8, 2016.
3 Hayreh SS et al. Ophthalmology. 1980;87(1):75-78.
4 Framme C et al. Ophthalmologe. 2001;98(8):725-730.
5 Fraser SG, Adams W. Cochrane Database Syst Rev. 2009;1:CD001989.
6 Schumacher M et al. Ophthalmology. 2010;117(7):1367-1375.
7 Garcia-Arumi J et al. Br J Ophthalmol. 2006;90(10):1252-1255.
8 Park SJ et al. Ophthalmology. 2015;122(11):2336-2343.
9 Lauda F et al. Cerebrovasc Dis. 2015;40(3-4):151-156.
___________________________
Dr. Lee is chair of ophthalmology at the Blanton Eye Institute, Houston Methodist Hospital in Houston, and adjunct/clinical professor of ophthalmology at University of Texas Medical Branch (Galveston), Weill Cornell Medicine (New York, N.Y.), University of Texas MD Anderson Cancer Center (Houston), Baylor College of Medicine (Houston), and the University of Iowa Hospitals and Clinics (Iowa City). Relevant financial disclosures: None.
Dr. Levin was assistant professor of ophthalmology at the University of California, San Francisco, at time of writing. He is now in the department of ophthalmology at the Palo Alto Medical Foundation in Palo Alto, Calif. Relevant financial disclosures: None.
Dr. Miller is professor of ophthalmology, neurology, and neurosurgery and the Frank B. Walsh Professor of Neuro-Ophthalmology at Johns Hopkins University School of Medicine in Baltimore, Md. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.