By Garrick Chak, MD, Sameh Mosaed, MD, and Donald S. Minckler, MD
Edited by Ingrid U. Scott, MD, MPH, and Sharon Fekrat, MD
Download PDF
Juvenile open-angle glaucoma (JOAG) is a subset of primary open-angle glaucoma (POAG). The two conditions are classified somewhat arbitrarily by age, with JOAG affecting those who are between 5 and 35 years old and adult-onset POAG affecting those older than 35 years.1 JOAG is, fortunately, a rare condition and is estimated to affect 1 in 50,000 individuals.1
Because JOAG is a primary disease, all secondary causes of optic neuropathy must be excluded. Similar to POAG, JOAG has an insidious onset; however, JOAG is usually detected late and often presents with advanced optic nerve damage and an intraocular pressure (IOP) greater than 40 mmHg.2 As a result, the management of JOAG tends to involve surgical intervention more often than does POAG.
Sample Case History
A 21-year-old woman presented with a severe headache and a pinhole visual acuity of 20/50 in her right eye and 20/25 in her left eye. IOP measurements were 43 mmHg in the right eye and 33 mmHg in the left. There was a right relative afferent pupillary defect and no conjunctival injection.
Gonioscopy showed that the angle was open to the ciliary body band bilaterally without synechiae. The anterior chamber and lens were clear bilaterally. The cup-to-disc ratio was 0.95 in the right eye (Fig. 1) and 0.2 in the left eye. The visual field revealed a central island of remaining vision in the right eye (Fig. 2) and a full visual field in the left eye. After the workup was completed, the patient was diagnosed with JOAG.
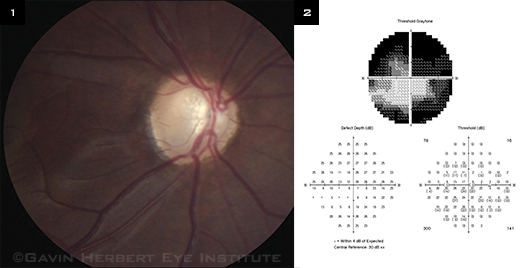 |
|
EVIDENCE. (1) This image is of the right optic nerve head of a patient with JOAG. (2) The visual field revealed a central island of remaining vision in that eye.
|
Genetics and Pathogenesis
JOAG is usually transmitted in an autosomal dominant fashion and most commonly involves the myocilin protein (previously known as trabecular meshwork inducible–glucocorticoid response protein, or TIGR). Based on human in situ studies, the myocilin protein is found in trabecular meshwork cells, trabecular beams, and juxtacanalicular connective tissue.
More than 70 identified mutations in the myocilin gene (MYOC) contribute to increased aqueous outflow resistance. Various aspects of trabecular meshwork cell function are compromised depending on the MYOC mutation. Research has shown glaucoma-associated risk stratification based on MYOC phenotype: mild risk with the Gln368Stop mutation, intermediate risk with the Thr377Met and Gly252Arg mutations, and severe risk with the Pro370Leu mutation.3 However, it is important to note that having an MYOC mutation does not necessarily mean that the patient has or will develop JOAG.1,3
Diagnosis
Because patients with JOAG frequently present with headache and optic neuropathy, it is important to exclude other potential etiologies, such as infectious, traumatic, and vascular causes that would place the patient’s life at risk and necessitate emergent systemic intervention. Concomitant focal neurologic signs, Horner syndrome, and cranial nerve palsies would not be expected in JOAG.
Imaging. Given that patients may present initially with severe unilateral headache out of proportion to the typical symptoms of chronic ocular hypertension, it may be prudent to order magnetic resonance imaging of the brain and orbits as well as a magnetic resonance angiogram of the brain in order to exclude intracranial and intraorbital pathology.
Differential diagnosis. During the history and ocular examination, the following should be ruled out.
- Steroid-response glaucoma. A detailed history of all present or past corticosteroid use should be obtained.
- Angle-recession glaucoma. The clinician should search for areas of widened ciliary body band on gonioscopy and conduct a detailed trauma history.
- Spontaneous closure of a cyclodialysis cleft. A history of trauma and incisional ocular surgery should be obtained. Gonioscopy may reveal a localized area of peripheral anterior synechiae that spontaneously closed the ciliary body disinsertion from the scleral spur. Associated exam findings may include iridodialysis and possibly distorted retinal architecture and choroidal fibrosis from chronicity of prior hypotony maculopathy, even if IOP is no longer reduced.
- Pseudoexfoliation glaucoma. Although this usually is found in older patients, it is worth looking for. Findings may include spoke-like fibrillar material on the anterior lens capsule, pupillary transillumination defects, phacodonesis, and asymmetric anterior chamber depth with zonular weakness.
- Pigmentary glaucoma. Findings include Krukenberg spindle endothelial pigment deposits, peripheral iris transillumination defects, myopia, and Sampaolesi’s line on gonioscopy.
- Uveitic glaucoma. Signs include anterior chamber reaction and evidence of chronic inflammation with synechiae in the angle or pupil.
- Neovascular glaucoma. NVG can be an open-angle process in its earlier stages when there is arborization of fine new vessels on the trabecular meshwork. If the patient is not diabetic and does not have ophthalmoscopic or angiographic evidence of a retinal vein occlusion, then one must consider ocular ischemic syndrome as a cause of NVG.
- Glaucomatocyclitic crisis. In this instance, a history of recurrent unilateral headache or spikes in IOP along with fine keratic precipitates may be present.
- Fuchs heterochromic iridocyclitis. The involved iris is hypopigmented and has stromal iris atrophy, stellate keratic precipitates, and fine vessels over the trabecular meshwork on gonioscopy.
- Etiologies sharing a mechanism of increased episcleral venous pressure. This category includes orbital congestion (carotid-cavernous fistula, dural arteriovenous shunting, thyroid-associated ophthalmopathy, or orbital tumor); scleritis; capillary vascular malformation from congenital anomalies (Sturge-Weber syndrome, type 1 neurofibromatosis, or nevus of Ota); and systemic high central venous pressure (which includes but is not limited to pulmonary hypertension, obstructive sleep apnea, or venous obstruction).
Management
Once the patient has been diagnosed with JOAG, management involves lowering IOP and assessing the rate of progression with serial optic nerve head imaging and static automated perimetry. In JOAG cases, the IOP is often refractory to maximal tolerated medical therapy, and incisional surgery is needed.
Surgery. Given the younger age range of JOAG patients and their life expectancy, it is worth considering an alternative to trabeculectomy with antifibrotics, which carries a risk of up to 1.2 percent per year of bleb-associated endophthalmitis.4 The Trabectome or a trabecular bypass shunt may be considered as a conjunctiva-sparing surgical option that is targeted to the disease, especially since JOAG patients may have a genetically maldeveloped trabecular meshwork. Outcomes with the Trabectome have been promising in a small case series.5
If IOP maintenance is refractory to the Trabectome, a glaucoma drainage device should be considered. We favor a Baerveldt 350 mm2 implant with scleral patch graft in either a one-stage approach, with an absorbable ligature, or in a planned two-stage procedure.
Repeat needling. Even after uncomplicated tube surgery, patients may still develop IOP spikes postoperatively despite having a patent, nonvalved tube in good position. Some eyes may benefit from repeat needling of the capsule that surrounds the plate of the implant to reduce aqueous outflow resistance. We recommend that the shortest interval for repeat capsular needling be one month.
Conclusion
It is important for clinicians to be familiar with JOAG, a chronic optic neuropathy that is usually bilateral and potentially blinding. Because the visual outcome can be poor if JOAG is missed, we recommend checking IOP as early as possible in children, especially those with a family history of glaucoma. If the child is not cooperative enough for Goldmann applanation tonometry, the clinician may use a TonoPen. Prompt diagnosis and early referral to a glaucoma specialist will give the patient the best chance of preserving vision.
___________________________
1 Turalba AV, Chen TC. Semin Ophthalmol. 2008;23(1):19-25.
2 Alward WLM et al. N Engl J Med. 1998;338(18):1022-1027.
3 Hewitt AW et al. Invest Ophthalmol Vis Sci. 2007;48(1):238-243.
4 Ang GS et al. Br J Ophthalmol. 2010;94(12):1571-1576.
5 Damji KF et al. Efficacy and safety of ab interno trabeculectomy with Trabectome in JOAG. Poster 21. Presented at the 24th annual meeting of the American Glaucoma Society; Feb. 28, 2013; San Francisco.
___________________________
Dr. Chak is a third-year ophthalmology resident, Dr. Minckler is director of ophthalmic pathology, and Dr. Mosaed is director of the glaucoma service; all are at the University of California, Irvine. Drs. Chak and Mosaed report no related financial interests. Dr. Minckler is a paid consultant to NeoMedix.