By Amelia M. Todd, MD, Niloofar Piri, MD, and Denis Jusufbegovic, MD
Edited By: Ingrid U. Scott, MD, MPH, and Sharon Fekrat, MD
Download PDF
Serpiginous choroiditis (SC) is a rare, bilateral, idiopathic inflammatory disorder that results in geographic destruction of the retinal pigment epithelium (RPE), retina, and choriocapillaris. It is a chronic, recurrent, and progressive disease that typically affects patients 30 to 60 years of age. There is a slight male predominance, but no racial predilection or consistent genetic factors have been associated with the disease.
Histopathologic studies have shown extensive loss of the RPE and destruction of the overlying retina. Diffuse and focal accumulation of lymphocytes has been observed in the choroid. Moreover, there is an increased frequency of HLA-B7 in patients with SC; these features are indicative of an inflammatory process.
The clinical course is characterized by multiple recurrences at intervals of months to years. Areas of reactivation are often seen adjacent to old scars. Unfortunately, patients are often asymptomatic until the fovea is involved, and they typically present with painless blurry vision and central or paracentral scotomas. Other ocular complications associated with SC include choroidal neovascularization (CNV), which occurs in up to 20% of cases, cystoid macular edema (CME), retinal vein occlusion, retinal vasculitis, and macular hole.1,2
There are two types of serpiginous choroiditis: classic and macular.
Classic SC. This type comprises 80% of cases and demonstrates the characteristic bilateral asymmetric serpiginous (snakelike) or geographic yellow-gray chorioretinal lesions that typically start at the peripapillary region and can extend into the macula (Fig. 1).
Macular SC. This variant involves the macula and spares the peripapillary region. It may initially be confused with geographic atrophy, as seen in macular degeneration, or with macular ischemia associated with various vasculopathies.
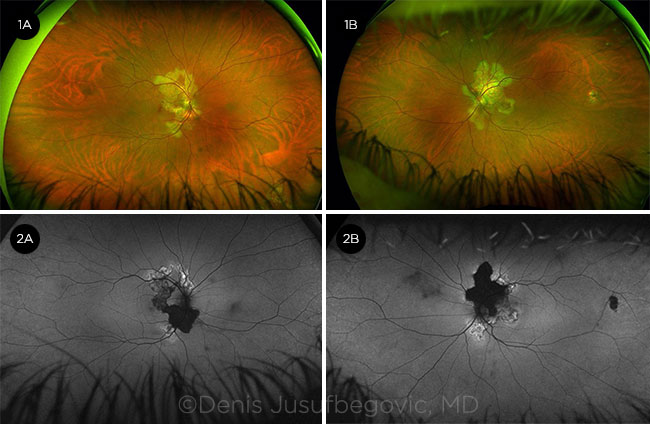 |
|
FUNDUS PHOTOS. Characteristic yellow-gray geographic lesions are visible in the right eye (1A) and left eye (1B). FAF. Hypoautofluorescent (inactive) and hyperautofluorescent (active) lesions are present in the right eye (2A) and left eye (2B).
|
Diagnosis
The diagnosis of SC is based primarily on clinical examination, imaging findings, and a thorough laboratory evaluation. Imaging modalities that are helpful in confirming the diagnosis include fundus autofluorescence (FAF), fluorescein angiography (FA), indocyanine green angiography (ICGA), optical coherence tomography (OCT), and OCT angiography (OCTA).
FAF (Fig. 2). FAF is a very sensitive, noninvasive modality that is used to differentiate active from inactive lesions, as new lesions often occur at the border of old lesions. Acute active lesions appear hyperautofluorescent, whereas old lesions are hypoautofluorescent. Red/green wavelength ultra-widefield FAF imaging is considered the best modality to monitor disease activity.
FA (Fig. 3). Acute lesions appear hypofluorescent, with irregular and poorly defined borders in early phases of the study. This appearance is likely due to hypoperfusion of the choriocapillaris and blocked fluorescence from edematous RPE and outer retina. In the later phases, hyperfluorescent borders are seen, representing leakage of fluorescein from the surrounding intact choriocapillaris. Inactive lesions are hypofluorescent with sharp borders in early phases of the study and become progressively hyperfluorescent with variable levels of staining.
ICGA (Fig. 4). Lesions appear hypofluorescent on ICGA throughout all phases of the study, representing disruption in choroidal perfusion. Early subclinical lesions may be better appreciated on ICGA than on funduscopic examination or FA, and they may appear as hypofluorescent spots resulting from isolated choriocapillaris involvement. ICGA may also be useful in differentiating active new lesions, which are hypofluorescent, from CNV, which appears hyperfluorescent during the middle to late phases of the study.
OCT (Fig. 5). Acute lesions primarily affect the outer retinal layers and choriocapillaris and appear as increased reflectivity localized to the outer retina and disruption of the photoreceptor bands. OCT of older lesions demonstrates outer retinal atrophy and RPE disruption.
OCTA. OCTA is a newer, noninvasive method of imaging retinal and choroidal vascular anatomy that can be useful in assessing SC progression. Choriocapillaris flow disruption is an early sign of the disease, preceding structural involvement of the outer retinal layers. Prompt treatment at this early stage may allow for resolution without chorioretinal scarring.3
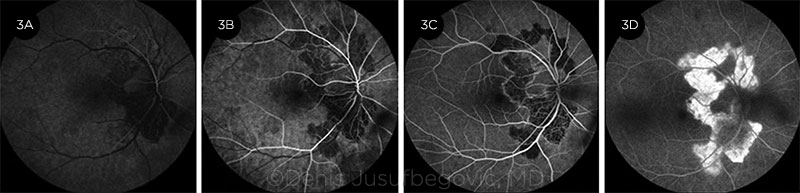 |
|
FA PHASES. Fluorescein angiography, right eye. (3A) Early arterial, (3B) arterial, (3C) arteriovenous, and (3D) late phases. (See text for description of findings.)
|
Masquerading Conditions
Before treatment for SC is initiated, an extensive workup is needed to rule out other inflammatory or infectious etiologies that may mimic the disease. The differential diagnosis for SC includes other inflammatory processes such as acute posterior multifocal placoid pigment epitheliopathy (APMPPE), ampiginous choroiditis, multifocal chorioretinitis, and persistent placoid maculopathy; infectious etiologies such as tuberculosis (TB), herpesvirus, toxoplasmosis, and syphilis; autoimmune diseases such as sarcoidosis; vascular diseases that cause retinal ischemia; and degenerative changes, such as age-related macular degeneration.
Laboratory testing for sarcoidosis, syphilis, herpes, toxoplasmosis, and TB should be part of the routine workup. If testing is negative and all diagnostic tests confirm idiopathic SC, treatment should be started promptly.1,2
Mycobacterium tuberculosis. This bacterium can cause serpiginous-like choroiditis (SLC). It is important to differentiate SLC from SC, as their treatments differ. Patients with SLC often present with multifocal lesions involving the periphery rather than peripapillary region. Anterior chamber cellular reaction and vitritis are also more common in SLC compared to SC. FAF may be helpful in distinguishing the two disease entities, as SC has a homogeneous hypoautofluorescent pattern, whereas SLC often demonstrates a more stippled appearance.
Both diseases are treated with oral steroids, but antitubercular medications are extremely important in treating SLC. Testing for TB with labs and a chest x-ray prior to initiating steroids is critical, as steroids may worsen SLC if used without concomitant antitubercular therapy.2
APMPPE and relentless placoid chorioretinitis (RPS). These two clinical entities can present a diagnostic challenge and may mimic SC or one another early in the course of disease.
APMPPE. Acute APMPPE lesions clinically appear similar to active SC or RPS lesions as deep yellow-gray patches at the level of the outer retina and inner choroid. They are typically multiple, discrete, and plaquelike in appearance and predominantly involve the posterior pole.
APMPPE lesions usually resolve on their own in a few weeks without treatment, with residual mild to moderate RPE changes. New lesions can develop over several subsequent weeks. Recurrent disease is uncommon and usually appears as new multifocal patches not associated with previously healed lesions. CNV is very rare in APMPPE.
RPS (also known as ampiginous choroiditis). The clinical appearance of acute RPS lesions is similar to APMPPE. However, RPS results in recurrent and progressive destruction of the choroid and retina. Lesions are usually widespread and may involve both the posterior pole and periphery. The clinical course and multifocal appearance of the RPS lesions distinguish it from SC, whereas the relentless progression of the disease differentiates it from APMPPE.2 Treatment of RPS is similar to that of SC.
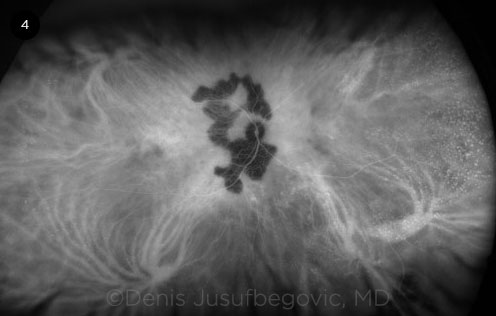 |
|
ICGA. Late-phase ICGA of the right eye shows persistent hypofluorescence of the lesions.
|
Management
Initial therapy for SC includes systemic corticosteroids to treat active lesions as well as concurrent immunosuppressive therapy to prevent recurrences. Oral corticosteroids are the mainstay of treatment for acute disease, but some studies have also shown that immediate intravitreal steroids may be beneficial in patients with foveal lesions.2
Triple therapy, including prednisone, cyclosporine, and azathioprine, has been found effective in controlling SC.4 Monotherapy with mycophenolate mofetil, azathioprine, or cyclophosphamide has shown some success, as described in several case reports.2
Biologic therapy with a tumor necrosis factor α (TNF-α) inhibitor such as adalimumab has been found effective in patients who progressed despite therapy with other immunosuppressants.5 Now, adalimumab is also frequently used as a first-line agent for SC. It is imperative to rule out TB before starting a TNF-α agent.
A recent study found that treatment with chlorambucil over six to nine months was effective in preventing recurrence and maintaining vision in 17 patients with SC. Of these, 12 patients had an average of 45 months of drug-free remission, and 14 maintained visual acuity within two Snellen lines.6
Complications of SC should also be addressed promptly (e.g., with anti-VEGF agents for CNV or intravitreal corticosteroids for CME).
Prognosis. Overall prognosis for this disease is poor despite treatment. Final visual acuity is <20/200 in about 25% of treated cases.7
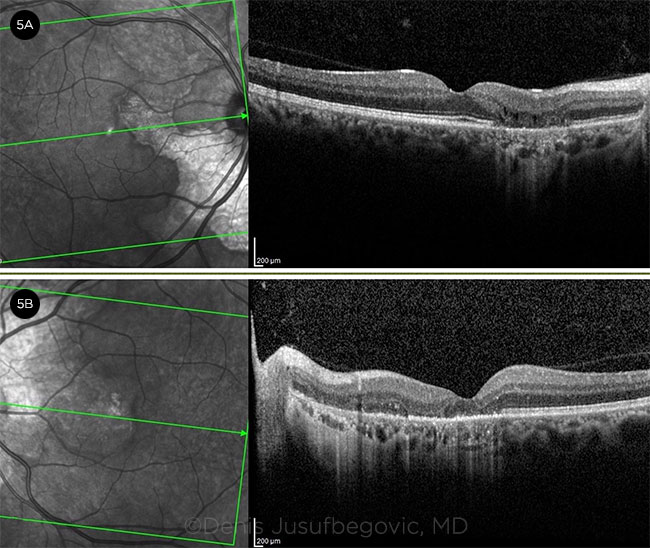 |
|
OCT. Imaging of the right eye (5A) and left eye (5B) shows complete RPE and outer retinal atrophy with subfoveal involvement in the left eye.
|
Key Points
Serpiginous choroiditis is a rare bilateral, idiopathic inflammatory disorder that causes geographic destruction of the retina and choroid in healthy middle-aged individuals. This chronic, recurrent, progressive disease has a poor visual prognosis if the fovea is involved. Symptoms include blurred vision and central and paracentral scotomas. A thorough workup is necessary prior to initiating treatment to exclude other inflammatory or infectious etiologies that may mimic SC. Treatment includes corticosteroids and immunosuppressive therapy. Patients should be monitored closely for disease progression and complications, including CNV and CME.
Web Extra: Case Report
A 41-year-old man presented with a history of a central blurry spot in the vision of his left eye for three months. He had no significant past medical or ocular history. His best-corrected visual acuity was 20/20 in the right eye and 20/400 in the left. Anterior segment examination was unremarkable, without anterior chamber inflammation or anterior vitritis.
Funduscopic examination revealed fingerlike projections of yellow-gray chorioretinal lesions extending from the optic disc to the macula, with sparing of the fovea in the right eye. There was a chorioretinal scar in the inferonasal periphery (see Fig. 1A). He had similar chorioretinal lesions in the left eye, but with involvement of the fovea, and a small scar temporal to the macula (see Fig. 1B).
Initial workup included a chest x-ray, complete blood count, comprehensive metabolic panel, T-Spot.TB test, angiotensin-converting enzyme/lysozyme, rapid plasma reagin, syphilis antibody test, and herpes simplex virus 1 and 2 antibodies. All tests were within normal limits.
Imaging. FAF revealed hypoautofluorescent lesions surrounding the optic nerve head, with hyperautofluorescent areas at the lesion edges in both eyes (see Fig. 2). FA demonstrated hypofluorescent lesions during the early arterial, arteriovenous, and venous phases of the study, with hyperfluorescent lesions in the late phases (see Fig. 3). ICGA revealed late-phase hypofluorescence (see Fig. 4). Spectral domain OCT showed complete RPE and outer retinal atrophy (see Fig. 5).
Diagnosis. The patient was diagnosed with idiopathic SC with foveal involvement in the left eye, and lesions encroaching on the fovea in the right eye. He was started on oral prednisone 60 mg/day. The active lesions showed marked improvement within two weeks. A slow steroid taper was started. We referred him to rheumatology to start immunosuppressive therapy. However, he lost his insurance coverage before treatment was initiated and was lost to follow-up.
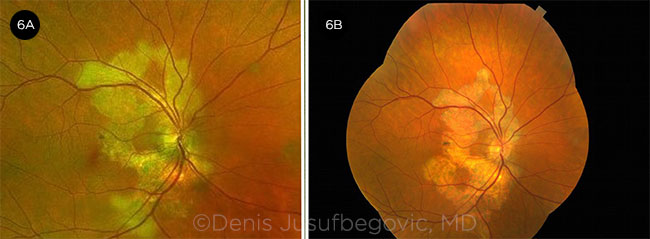
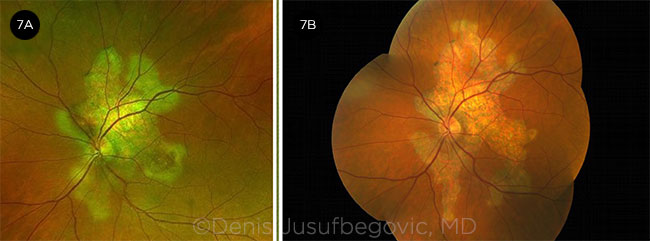
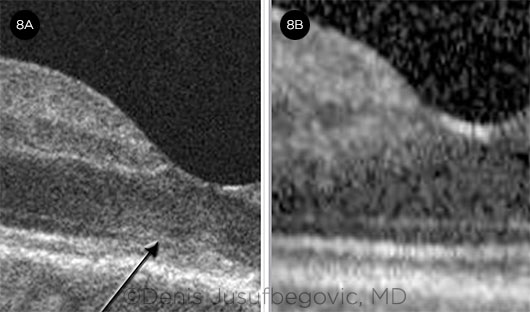
Disease course. He returned six months later. Vision in the right eye had decreased from 20/20 to 20/70. Examination showed significant disease progression in both eyes (Figs. 6 and 7). OCT showed increased reflectivity of the outer retinal layers in the subfoveal region of the right eye (Fig. 8A).
Management. He was promptly treated with intravitreal triamcinolone 2 mg/0.05 mL in the right eye and was started on oral chlorambucil therapy soon after. Active lesions resolved over the next two to three weeks. Subfoveal outer retinal hyperreflectivity resolved (Fig. 8B), and visual acuity improved to 20/25 in the right eye. Although there was no further progression in the left eye, its visual acuity remained the same as at presentation (20/400), as the fovea was already involved.
He has been on chlorambucil therapy for three months with complete resolution of active lesions in both eyes. The goal of therapy is to maintain white blood cell count (WBC) between 3,000 and 4,000 for six to nine months to attempt to induce a sustained remission.
 |
|
OD OVER TIME. Fundus photos of right eye at presentation (6A) and six months later (6B).
|
 |
|
OS OVER TIME. Fundus photos of left eye at presentation (7A) and six months later (7B).
|
 |
|
BEFORE AND AFTER. OCT of the right eye shows increased reflectivity and disruption of the outer retinal bands (arrow) in the subfoveal region (8A) and its resolution (8B) following intravitreal triamcinolone injection.
|
___________________________
1 Mirza RM, Lee MJ. White spot syndromes and related diseases. In: Schachat AP et al., eds. Ryan’s Retina. Vol 1. 6th ed. Philadelphia: Elsevier; 2017:1516-1561.
2 Khanamiri N, Rao NA. Surv Ophthalmol. 2013;58(3):203-232.
3 Pakzad-Vaezi K et al. Ophthalmol Retina. 2018;2(7):712-719.
4 Hooper PL, Kaplan HJ. Ophthalmology. 1991;98(6):944-951.
5 Chinchurreta Capote A et al. Ocul Immunol Inflamm. 2014;22(5):405-408.
6 Ebrahimiadib N et al. Ocul Immunol Inflamm. 2018;26(2):228-238.
7 Christmas NJ et al. Retina. 2002;22(5):550-556.
___________________________
Dr. Todd is a second-year ophthalmology resident, and Dr. Jusufbegovic is an assistant professor of clinical ophthalmology; both are at the Eugene and Marilyn Glick Eye Institute, Indiana University School of Medicine, Indianapolis. Dr. Piri is a third-year ophthalmology resident at the Department of Ophthalmology and Visual Sciences, University of Louisville, Kentucky. Financial disclosures: None.