By James A. David, MD, Albert Y. Cheung, MD, Robert J. Granadier, MD, Agatha Y. Obertynski, MD, M. Mazen Al-Hakim, MD, and Mark A. Rolain, MD
Edited by Steven J. Gedde, MD
Download PDF
Jennifer Kelmendi* was a 39-year-old woman who presented for evaluation of a 2-day history of horizontal binocular diplopia and concurrent right eye pain. The pain was concentrated in the superomedial aspect of the right eye and was severe enough to wake her at night. There was no pain with eye movements. Ms. Kelmendi told us that she had undergone a rhinoseptoplasty 2.5 months ago. She also had received superficial corticosteroid injections into her nose 6 days before the start of her vision problems.
Her visual acuity was 20/20 bilaterally. Ocular movements were full; however, there was a 16–prism diopter (PD) esotropia that decreased to 6 PD in left gaze. The exam was otherwise unremarkable.
She was sent to the emergency room (ER) for neuroimaging in the setting of a possible sixth cranial nerve palsy. Computed tomography (CT) of the head and magnetic resonance imaging (MRI) of the brain/orbit/face/neck were read as normal. She was evaluated by neurology and worked up for both thyroid disease and myasthenia gravis; in the assessment of the latter, there was no response to a trial of Mestinon (pyridostigmine bromide).
We See Her Again
Ten days later, Ms. Kelmendi returned and told us that her binocular diplopia had worsened over the previous 2 days. The diplopia was now vertical/oblique in nature and accompanied by ptosis, blurry vision, and increased right-sided orbital pain. Vision without correction remained 20/20 bilaterally.
In the right eye, the pupil measured 3.5 mm in dark conditions and constricted to 2.5 mm in light; in the left eye, the measurements were 4.5 mm and 3.0 mm, respectively. There was no relative afferent pupillary defect.
The motility examination revealed 12 PD of esotropia and 8 PD of hypertropia in primary gaze at 6 m (4 PD of left hypertropia at 1/3 m). This worsened in right gaze without significant change with left gaze, vertical gaze, or head tilt (minimal improvement on left head tilt).
The right eye had a 10% reduction in supraduction and 30% reduction in abduction. Left eye ductions were full.
Her margin reflex distance-1 (MRD-1) was 1 mm in the right eye and 4 mm in the left, while her MRD-2 measurements were equal in both eyes. Hertel measurement revealed a 3-mm proptosis in the right eye. Intraocular pressure, orbicularis function, levator function, color plate testing, and slit-lamp examination were within normal limits. Fundus examination was normal with visible spontaneous venous pulsations. There was decreased sensation in the V1 distribution on the right side; other cranial nerves were normal unless specified above. Anhidrosis was difficult to evaluate.
The Differential Diagnosis
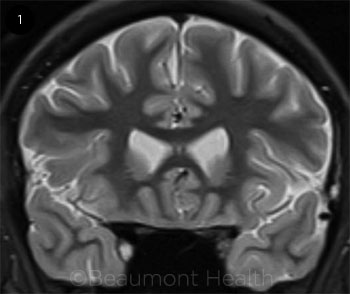
In our patient presenting with painful diplopia accompanied by a Horner syndrome and deficits in CN III (partial), CN V1, and CN VI, the pathology localized to the cavernous sinus. This was supported by reviewing the neuroimaging from the prior ER visit, which showed enhancement in the right anterior cavernous sinus (Fig. 1). The differential included infectious etiology (including fungal infection, given the recent corticosteroids), granulomatous disease (tuberculosis, sarcoidosis), cavernous sinus inflammation, neoplastic disease (e.g., lymphoma, meningioma), or vascular disease.
 |
|
NEUROIMAGING. The short tau inversion recovery (STIR) coronal MRI imaging (above) from the first visit was originally read as normal.
|
The Workup and Diagnosis
CT of the chest, abdomen, and pelvis revealed no acute intrathoracic or intra-abdominal process. Lumbar puncture demonstrated an opening pressure of 21 cm H2O and normal fluid analysis, which was negative for the following infectious and autoimmune assays: herpes simplex virus (HSV), varicella-zoster virus, cryptococcus, Lyme disease, Venereal Disease Research Laboratory (VDRL), angiotensin-converting enzyme (ACE), enterovirus, and oligoclonal bands. We also learned that her serum laboratory values were within normal limits, including HSV, VDRL, ACE, complete blood count, comprehensive metabolic panel, acetylcholine-receptor antibodies, erythrocyte sedimentation rate, C-reactive protein, thyroid-stimulating hormone, hemoglobin A1c, Lyme antibody, antineutrophil cytoplasmic antibody, antinuclear antibodies, fluorescent treponemal antibody, and lysozyme.
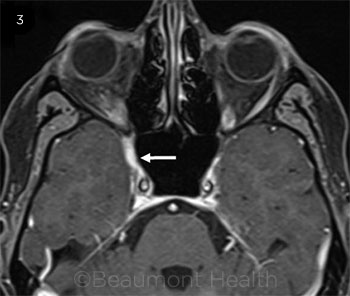
Repeat MRI of the brain and orbits exhibited asymmetry, with thickening and enhancement observed in the area medial to the orbital apex and the internal carotid artery at the junction of the cavernous segment with the supraclinoid segment (Fig. 2). The medial aspect of the middle cranial fossa also demonstrated enhancement (Web Extra, Fig. 3).
Based on the negative laboratory findings as well as the characteristic findings seen on imaging, Tolosa-Hunt syndrome (THS) with signs of idiopathic hypertrophic pachymeningitis was suspected. Therapy consisted of intravenous methylprednisolone (Solu-Medrol) 500 mg daily for 3 days under the guidance of neurology, followed by an oral prednisone taper.
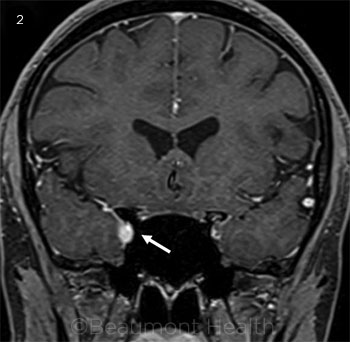 |
MRI REVISITED. During the patient’s second visit, we realized that areas of enhancement had been overlooked in the initial MRI ( see Fig. 1). In a second round of neuroimaging, the T1 fat-suppressed (2) coronal image demonstrates right-sided thickening and enhancement adjacent to the orbital apex and internal carotid artery at the junction of the cavernous segment with the supraclinoid segment.
|
Discussion
Tolosa-Hunt syndrome is largely a diagnosis of exclusion, characterized by an inflammatory, granulomatous process of undetermined etiology. Originally described by Tolosa in 1954 and Hunt in 1961, few major modifications have been adopted in the diagnosis of THS, with the requirement of specific MRI or biopsy findings being the most noteworthy addition in 2004.1
THS is a relatively rare condition, accounting for only 3% of cases of painful ophthalmoplegia.1
Diagnosis. Currently, the diagnosis of THS is based on the International Classification of Headache Disorder-2 (ICHD) from 2004 and includes the following 5 criteria: (1) 1 or more episodes of unilateral orbital pain persisting for weeks if untreated, (2) paresis of 1 or more of the third, fourth, and/or sixth cranial nerves and/or demonstration of granuloma by MRI or biopsy, (3) paresis coinciding with the onset of pain or following it within 2 weeks, (4) pain and paresis resolving within 72 hours with adequate treatment with corticosteroids, and (5) other causes having been excluded.2,3
For the diagnosis of THS to be made, the granulomatous material must be localized to the cavernous sinus or superior orbital fissure, or extend to the orbit without direct involvement of the associated nerves or muscles.3 Due to the location of the inflammation, there can also be associated involvement of the trigeminal nerve (often the first division, as seen in our patient), optic nerve, facial nerve, or acoustic nerve. When the granulomatous tissue is found only in the orbit, the diagnosis is more appropriately termed orbital pseudotumor, a condition thought to be distinguished from THS only on the basis of location.3 Due to the technical limitations of obtaining a biopsy in the cavernous sinus, most diagnoses are supported by MRI rather than biopsy. However, MRI findings are not always present in THS, calling into question one of the ICHD criteria. In one study, 8% of THS cases did not have any MRI findings.3 A larger study of 124 cases found that 35% had inflammation on MRI or biopsy-proven granuloma (termed “inflammatory THS,” by the authors), 31% had a specific lesion (“symptomatic THS”), and 33% had normal neuroimaging (“benign THS”).
Although most authors agree that improvement of pain within 72 hours of corticosteroid treatment is important for diagnosis, some argue that full resolution of pain and paresis in this time frame is too optimistic. In a study by Zhang et al., all 40 patients had improved pain at 72 hours, but 40% and 77.5% of patients reported complete pain relief within 72 hours and 1 week, respectively.2 In the same study, paresis was found to take longer to resolve, with only 1 patient (2.5%) having full resolution of cranial nerve paresis at 1 week, and 4 patients having no relief.2 In a review by Colnaghi et al., only 2% (of 62 patients) had full resolution of signs within 72 hours; rather, patients receiving a lower corticosteroid dose (initial dose of approximately 1 mg/kg/day; steroid used was unspecified) took a mean of 104 days for full resolution of paresis, and those on a higher corticosteroid dose (initial dose of 500-1,000 mg/day) fully recovered in an average of 35.5 days.3
Treatment. Although clinical criteria for THS include the phrase “adequate treatment with corticosteroids,” there currently is no standard treatment regimen for THS. Zhang et al. found that corticosteroid regimens were highly variable, ranging from 30 mg/day of prednisone to 1,000 mg/day of methylprednisolone.2 Additionally, Colnaghi et al. found that with use of high-dose instead of low-dose corticosteroids, pain resolution within 72 hours was statistically more likely, and recurrence decreased from 44% to 9% of patients.3 A proposed initial regimen includes intravenous corticosteroids (e.g., 125-1,000 mg of methylprednisolone or 4-10 mg of dexamethasone), as appropriate given the general health of the patient, followed by a prolonged oral corticosteroid taper. Following adequate treatment and clinical response, many experts propose that it is important to confirm resolution of MRI findings by repeat MRI, although imaging findings may not fully resolve for months in some cases.1
Prognosis. Most acute cases will resolve with corticosteroid treatment. Recurrences have been found to be more likely in patients who were younger (31.7 ± 13.5 years old vs. 47.8 ± 15.2 years old, p = .001) at the time of their first attack.2 One study showed that the duration and extent of symptom relief decreased with each recurrence, requiring higher doses (from 1.0 mg/kg per day of prednisone to 1,000 mg/day of methylprednisolone) to achieve the same clinical response. Acetaminophen and ibuprofen have been shown to be ineffective in pain management in THS.2
 |
|
The T1 fat-suppressed axial image reveals enhancement of the medial aspect of the middle cranial fossa.
|
Our Patient’s Progress
After 1 week of corticosteroid therapy, our patient’s pain had resolved; however, ptosis had worsened, and there was now an adduction deficit in the right eye. Fortunately, neurological deficits had improved by the next follow-up 2 weeks later, and the corticosteroids were slowly tapered. Interestingly, the earlier nasal corticosteroid injections were most likely a “red herring.”
___________________________
*Patient name is fictitious.
___________________________
1 Cakirer S. Eur J Radiol. 2003;45(2):83-90.
2 Zhang X et al. J Neurol Sci. 2014;341(1-2):13-16.
3 Colnaghi S et al. Cephalalgia. 2008;28(6):577-584.
___________________________
Dr. David is starting his residency in ophthalmology at Louisiana State University/Ochsner Health System; Dr. Cheung is a third-year resident; Drs. Granadier, Obertynski, and Rolain are in the Department of Ophthalmology; and Dr. Al-Hakim is in the Department of Neurology; all are at Oakland University William Beaumont School of Medicine, Royal Oak, Mich. Relevant financial disclosures: None.