By Maxwell Elia, MD, and J. Javier Servat, MD
Edited by Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Surgical techniques for managing thyroid eye disease have improved significantly in the recent past. A century ago, single-wall decompressions were first employed. In the 1950s, this technique was expanded by otolaryngologists to include transantral 2-wall decompression of the inferior and medial walls. Current techniques include transnasal endoscopic approaches with intranasal endoscopes.1
Oculoplastic surgeons can now achieve significant functional and cosmetic improvements through decompression of the orbital floor in addition to the medial and lateral orbital walls. However, orbital decompression for the treatment of Graves orbitopathy remains a challenging procedure because of the potential for damaging the important neural and vascular structures of the orbit.2
A 3-D Navigational Tool
To aid in navigating these delicate areas, intraoperative image guidance—first used in neurosurgery—has emerged as a tool that provides a 3-dimensional map of the position of the surgeon’s instruments. Using high-resolution CT images obtained preoperatively, electromagnetic image guidance is based on technology that detects the position and direction of surgical instruments by means of spatial variations in the strength and direction of a magnetic field produced by a field generator. After a brief presurgical calibration, image guidance software displays real-time coronal, axial, and sagittal views of instrument location on a computer monitor viewed by the surgeon. This technology is especially versatile because it uses electromagnetic waves, which do not require a direct line of sight to determine instrument position.
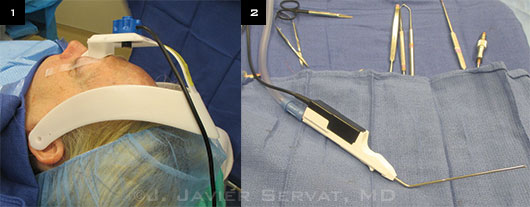 |
|
Patient Preparation and Instruments. (1) A head-mounted marker strip is secured above the patient’s brow. (2) Trackable suction tip device in foreground.
|
Indications
Intraoperative image guidance is most helpful in delineating anatomic location during the most complex decompression cases, particularly those in which maximal 3-wall decompression is desired. Additionally, this technique is especially valuable in guiding reoperations after subtotal decompression or in cases where preoperative imaging reveals anomalous orbital anatomy.
Presurgical Calibration
Accurate calibration of the image guidance equipment requires the patient to undergo preoperative contiguous noncontrast CT from the superior aspect of the frontal sinuses to the inferior aspect of the maxillary sinuses and lower teeth. This information is loaded into navigation software to create a virtual 3-dimensional map.
A head-mounted marker strip is secured above the patient’s brow (Fig. 1), and a registration probe is used to trace horizontally across the brow and vertically down the patient’s nose. This information is registered by a field-generating magnet mounted on the operating room table. This table-mounted setup generates the field necessary to provide spatial tracking.
After this initial calibration, the surgeon has access to a series of track-able instruments, including probes and suction tips (Fig. 2). The standard instruments include a registration probe as well as straight suction, 70-degree curved suction, and 90-degree curved suction tips. Up to 3 instruments can be tracked at a time with the current software; and as they are moved within the orbit, the position is displayed in coronal, axial, and sagittal CT image planes (Fig. 3).
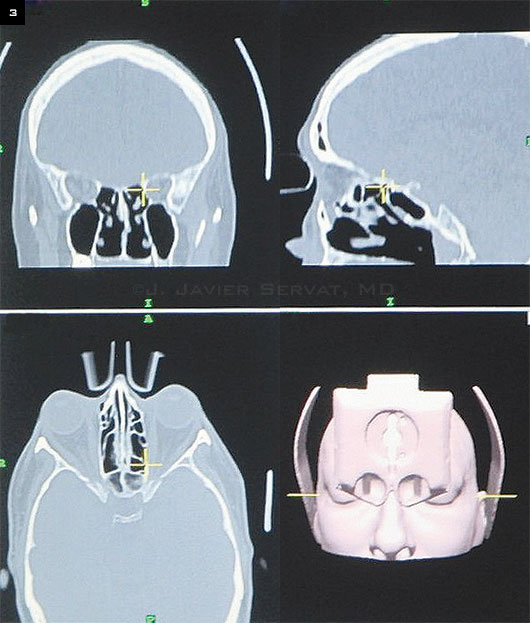 |
|
Intraoperative Viewing. The position of the instrument (circled) in the medial wall is displayed in coronal, sagittal, and axial CT image planes.
|
Technique
The surgical approach for orbital decompression utilizes standard techniques, beginning with transnasal medial wall decompression to facilitate entry to the orbit
Patient preparation. Intranasal vasoconstriction and anesthesia are achieved by packing the nasal cavity between the middle turbinate and the lateral nasal mucosa with ¼-inch neuropads soaked in a 4% cocaine solution (oxymetazoline can be used as an alternative). This packing is removed after 5 minutes. Local anesthetic is prepared in a 50:50 mixture of 2% lidocaine with 1:100,000 epinephrine and 0.5% bupivacaine with 1:200,000 epinephrine.
Under direct visualization of nasal mucosa with a 0-degree rigid endoscope, this anesthetic mixture is administered to the submucosa of the anterior middle turbinate and lateral nasal wall. The nasal cavity is repacked in the same fashion for an additional 5 minutes. The patient is then prepped and draped in the usual sterile manner for ophthalmic plastic surgery.
Procedure: Step 1. The endoscope is brought into the field. Under direct visualization, the uncinate process is incised and removed. While removing the lamina papyracea, the surgeon should preserve the junction between the medial wall and orbital floor (orbital ethmoidal strut) to reduce the risk of postoperative diplopia. During the medial wall decompression, use of the image guidance system to confirm location allows the surgeon to perform ethmoidectomy safely close to the orbital apex.3 A frontal dissector is used to remove the bone and periorbita from the sphenoid anteriorly, starting superiorly, closest to the ethmoid roof to begin the decompression.
Step 2. Canthotomy and cantholysis are performed to release the lateral canthal tendon. An inferior transconjunctival incision is carried out toward the inferior orbital rim. Fat decompression is performed by clamping each of the 3 fat pads and cauterizing them with both bipolar and monopolar cautery. After the fat decompression, the periosteum is incised with monopolar cautery and is reflected off of the orbital floor.
Step 3. The infraorbital bundle is identified, and a mosquito forceps is used to fenestrate the orbital floor medially, taking care not to damage the infraorbital nerve. Long Kerrison rongeurs (2-3 mm) are used to extend the fenestration anteriorly, medially, laterally, and posteriorly, leaving 6 to 7 mm of the anterior orbital floor intact. An image-guided straight suction probe can be used to localize the posterior extent of the decompression.
Step 4. Next, a #4 cutting burr is used to create a fenestration lateral to the infraorbital bundle. The extension of the lateral floor decompression is achieved with a combination of the burr and Kerrison rongeurs. The posterior extension of the lateral floor can be inspected without electromagnetic guidance.
Step 5. Finally, attention is directed to the lateral orbital wall. The lateral orbital periosteum is incised and reflected posteromedially. Using the #4 cutting burr, the lateral orbital wall is decompressed, utilizing the straight suction tip to precisely localize the superior and posterior extensions of the decompression. If maximal decompression is desired, the greater wing of the sphenoid can be removed, exposing underlying dura mater.
Postoperative Considerations
The eye should never be patched after orbital decompression, as patching can delay the detection of possible hemorrhage. Visual acuity should be measured 3 times in the immediate postop period to ensure that no visual compromise has occurred as a result of optic nerve damage during surgery.
Given the direct communication of the orbit and sinuses, patients should be treated prophylactically with oral antibiotics; we typically use cephalexin or amoxicillin/clavulanic acid. Oral steroids in the postoperative period are useful in limiting inflammation.
Pain is best treated with oral narcotics after these complex procedures. All patients will experience some hypoesthesia in the distribution of the infraorbital nerve, but most will recover in the first few postoperative weeks.
Summary
By providing intraoperative confirmation of instrument position, electromagnetic image guidance is particularly helpful in reducing surgical risks in maximal orbital decompressions for Graves orbitopathy. This technology may also be applicable to other orbital surgeries that require close proximity to sensitive structures. Calibration of this equipment can be done quickly and does not add significantly more time to the procedure. The CPT code for the use of this navigation modality is 61782.
___________________________
1 Lee KY et al. Ophthal Plast Reconstr Surg. 2009;25(4):300-302.
2 Millar MJ, Maloof AJ. Eye. 2009;23(7):1565-1571.
3 Servat JJ et al. Orbit. 2014;33(6):433-436.
___________________________
Dr. Elia is a resident in ophthalmology at Yale University School of Medicine. Dr. Servat is in practice at Oculofacial Plastic Surgeons of Georgia and is an assistant clinical professor at the University of Georgia. Relevant financial disclosures: None.