Download PDF
In a major advance in the field of diabetic retinopathy, researchers at the Joslin Diabetes Center in Boston have found an association between the presence and extent of predominantly peripheral lesions, as seen on ultra-widefield (UWF) imaging, and an increased risk of diabetic retinopathy (DR) progression over 4 years. This association is independent of baseline DR severity and hemoglobin A1c levels.1
The findings have far-reaching implications in terms of clinical care and future research. Moreover, the process of their discovery reflects how current technology can validate decades-old hypotheses (see “When Knowledge Predates Technology”), while setting the stage for expansion in how a disease is detected and treated.
“Several observations in the past have indicated that the retinal periphery plays a key role in predicting the risk of diabetic retinopathy progression,” noted lead author Paolo Sandico Silva, MD, at Joslin’s Beetham Eye Institute. “The advent of UWF imaging, with its ability to visualize and quantify these peripheral lesions, now allows clinicians to incorporate this information into their treatment decision-making process.”
Coauthor Lloyd Paul Aiello, MD, PhD, said, “If these findings hold true in larger ongoing studies, then the quantitative evaluation of changes in the retinal periphery may become very important in grading diabetic retinopathy severity and identifying patients at increased risk for disease progression.” Dr. Aiello is also at the Beetham Eye Institute.
How UWF Works
“UWF imaging devices capture up to 200 degrees in a single image, representing approximately 82% of the retinal area, with a resolution of 14 µm, in only 0.25 seconds,” said Dr. Silva. In contrast, under the current gold standard—the Early Treatment Diabetic Retinopathy Study (ETDRS) classification system—retinal photography uses 7 standard 30-degree overlapping photographic fields and covers approximately 30% of the retinal surface.
The advent of 200-degree images has revealed extensive areas of the retina that are not visible in the standard ETDRS protocol. The expanded field allows evaluation of both the posterior pole and the periphery of the retina in 1 image—giving researchers the opportunity to corroborate earlier hypotheses involving the role of peripheral lesions in DR.
Step 1: Image Validation
The Joslin researchers started 4 years ago with a prospective validation study, Dr. Silva said. “Our objective was to assess diabetic retinopathy by comparing lesions identified using nonmydriatic and mydriatic UWF imaging with images from ETDRS 7-standard field film photographs.”2 The patients also underwent a dilated retinal examination performed by a retina specialist.
The validation study included 103 diabetic patients (206 eyes) who had varying levels of DR, thus capturing nearly the full spectrum of the disease. The researchers found that the UWF images were in substantial agreement with ETDRS film photographs and the results of the retinal exam. This observation has been confirmed by other research groups.3
Intriguing findings. The UWF images obtained during the validation study also demonstrated that diabetic retinal lesions occurred in the retinal periphery, beyond the ETDRS fields, in up to 40% of eyes. Moreover, in 10% of eyes, lesions observed outside of the ETDRS fields suggested a more severe grade of DR—and this finding caught the researchers’ attention, Dr. Silva said.
What remained a mystery was whether the extent of additional peripheral lesions placed a patient at increased risk of retinopathy progression. “This became the impetus for our current study,” he said.
|
Web Extra: Clinical Update
|
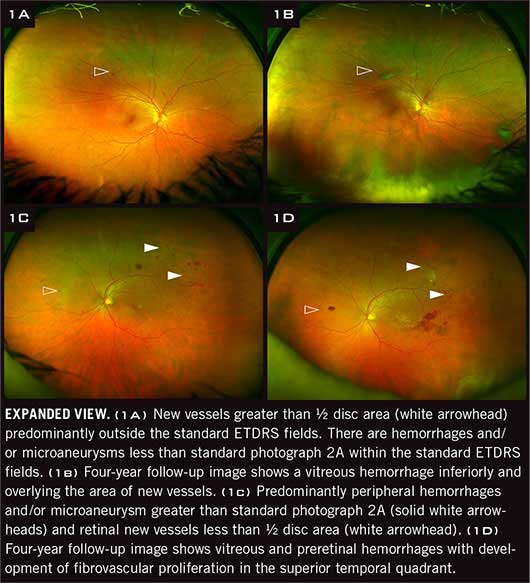 |
Step 2: Predicting Progression
Research objectives. In their most recent study, the Joslin researchers set out to determine whether the predominance of peripheral retinal lesions in any field peripheral to the area visualized by ETDRS 7-standard field photography was predictive for an increased risk of DR progression over 4 years.
The researchers invited the patients from the original validation study to participate in this new study, in which follow-up ETDRS photographs were taken in 80% (n = 146) of eligible eyes approximately 4 years after the baseline UWF images and ETDRS photographs from the initial study.
Unexpected results. As they evaluated the baseline UWF images, the investigators identified what they referred to as predominantly peripheral lesions, or PPL—that is, DR lesions with a greater extent outside versus inside the standard ETDRS fields. They found that eyes with PPL at baseline had a 3.2-fold increased risk of 2-step or greater DR progression and a 4.7-fold increased risk of progression to proliferative DR. These findings remained statistically significant after adjusting for gender, diabetes type, diabetes duration, hemoglobin A1c levels, and baseline DR severity.
“The association between peripheral lesions and the risk of DR progression was totally unexpected,” said Dr. Aiello. “If these findings are confirmed and hold up in broader national studies, we may have to revise our current ETDRS grading algorithms. Indeed, evaluation of the peripheral retina may become a crucial component of accurately characterizing DR severity.” In a remarkable twist of history, Dr. Aiello’s grandfather, William P. Beetham, MD, was among those who recognized more than 4 decades ago that the pathology in the periphery could be significant (see “When Knowledge Predates Technology”).
When Knowledge Predates Technology
The effort to formally classify retinal lesions observed in patients with diabetes began in September 1968, when a 12-member committee developed the Airlie House classification system. The committee sought to create a simple method that could be used to grade the presence and severity of diabetic retinal lesions identified via ophthalmoscopy or retinal photography.
Limits of technology. Peripheral lesions were not included in the Airlie House system. This omission was based not on the lack of knowledge but rather on the limits of available technology. “In creating this system, the committee studied 30-degree retinal images from 7 standard defined retinal fields per eye, which encompassed approximately 30% of the entire retinal surface,” said Dr. Aiello. “Yet the transcripts from this meeting indicate that the Airlie House participants knew there was often substantial disease in the peripheral retina that could not be easily detected, and that these lesions had important implications.”
Dr. Silva provided quotes from the transcripts to demonstrate how these retinal pioneers understood the importance of peripheral lesions but were constrained by the lack of technology. For instance, said participant William P. Beetham, MD, “…you can get out very much further toward the periphery [and there is a lot of pathology there] than you can get with your fundus photograph. Is that not true?”
And committee member Harold Spalter, MD, said, “the more areas of the retina we sample, the truer picture of the retinal pathology at any one time exists. We tried going out to the equator with about 25 photographs. To make a montage of all that the fundus camera could see at one sitting took about 12 hours.”
Today’s benchmark. Another milestone occurred in 1991 with the introduction of the extended modified Airlie House classification used in the ETDRS.1 This system is the current gold standard for determining the severity of DR based on the location and extent of retinal lesions visible in ETDRS photography of the posterior retina.
The ETDRS classification system is used to guide the eye care and treatment of patients with diabetes worldwide, and each severity level is closely linked to an estimated rate of DR progression over time. However, at the time the ETDRS was conducted, DR lesions in the peripheral retina could not be readily photographed and thus were not included in the development of the classification system.
___________________________
1 Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):786-806.
|
Reality Check: In the Clinic
Thomas W. Gardner, MD, MS, has been using UWF imaging technology for several years. “In the beginning, the cameras provided poor images, but the newer devices are easier to use, the colors from the images are better, and we are beginning to see lesions on the peripheral retina that were previously difficult to detect,” said Dr. Gardner, at the University of Michigan.
Given that UWF images have been shown to be comparable to those obtained by ETDRS protocol—and that the benefits of UWF include a reduction in the both the number of images needed and total imaging time—it would appear that UWF imaging would be ideal for uses in teleophthalmology for diabetes patients and others.
But is it practical? Not quite. At present, UWF imaging platforms are large and expensive, issues that may impact the technology’s current use in teleophthalmology, Dr. Aiello said. Dr. Gardner agreed. “The device has a fairly large footprint and is not portable. At this point, it is more of an office-based type of device.” Dr. Aiello added, “A smaller tabletop version is being manufactured, but it is still expensive, and we have yet to determine how accurately it performs.”
Looking Forward
Despite its cost and size, Dr. Gardner frequently utilizes UWF imaging and suggests that it may one day supplant what is now used for research studies. “This isn’t the final version of imaging technology, but it takes us one step further in identifying these lesions. Our next challenge is to see whether information gathered from the device can help with our patients’ longterm prognosis.”
DRCR.net study. Drs. Silva and Aiello cautioned that their study has its limits, and they noted that a larger multisite trial is currently under way by the Diabetic Retinopathy Clinical Research Network (DRCR.net). This study will involve more than 700 eyes and more than 4 years of follow-up. “Once we gather and analyze that data, we can make an informed decision as to whether or not to refine the current ETDRS grading system,” Dr. Silva said.
Significant potential. In the interim, researchers are “excited for the potential of this technology from a clinical and research perspective,” Dr. Silva said. For instance, he said, “UWF imaging allows us to readily assess the severity of peripheral nonperfusion, which is likely the driving force behind the peripheral lesions.” He has found that the presence of predominantly peripheral DR lesions and retinopathy severity is associated with peripheral nonperfusion on UWF fluorescein angiography.4
“We have the technology now to image most of the retinal periphery in a quarter of a second,” said Dr. Aiello. “Now we need to determine how this information may improve the assessment of diabetic retinopathy so that ophthalmologists might incorporate it into their decisionmaking process.”
___________________________
1 Silva PS et al. Ophthalmology. 2015;122(5):949-956.
2 Silva PS et al. Ophthalmology. 2013;120(12):2587-2595.
3 Price LD et al. Clin Ophthalmol. 2015;9:527-531.
4 Silva PS et al. Paper 2018 presented at: Association for Research in Vision and Ophthalmology Annual Meeting; May 4, 2015; Denver.
___________________________
Lloyd Paul Aiello, MD, PhD, is professor of ophthalmology at Harvard Medical School; director and vice president of ophthalmology at the Joslin Diabetes Center’s Beetham Eye Institute in Boston, and co-head of Joslin’s Section of Vascular Cell Biology. He is also vice chair for Centers of Excellence at Harvard’s Department of Ophthalmology and founding chair of the NEI’s Diabetic Retinopathy Clinical Research Network. Relevant financial disclosures: Optos: S.
Thomas W. Gardner, MD, MS, is professor of ophthalmology and visual sciences; professor of molecular & integrative physiology; the Healthy Eyes Taubman Scholar; and associate chair of research at the Kellogg Eye Center at the University of Michigan in Ann Arbor. He also serves as associate editor of Diabetes. Relevant financial disclosures: None.
Paolo Sandico Silva, MD, is assistant professor of ophthalmology at Harvard Medical School and a staff ophthalmologist and assistant chief of telemedicine at the Joslin Diabetes Center’s Beetham Eye Institute in Boston. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
