By Nieraj Jain, MD, and M. Tariq Bhatti, MD
Edited by Ingrid U. Scott, MD, MPH, and Sharon Fekrat, MD
This article is from April 2012 and may contain outdated material.
Fingolimod, the first FDA-approved oral agent for the management of relapsing forms of multiple sclerosis, offers a new route of administration for therapy to slow the potentially devastating neurological complications of multiple sclerosis (MS). All other FDA-approved disease-modifying drugs for MS require parenteral administration.
Fingolimod (Gilenya) hinders the trafficking of lymphocytes involved in the immune-mediated process of MS. Despite its generally favorable safety profile, fingolimod may cause macular edema in a subset of patients. As a consequence, ophthalmologists will be increasingly involved in the care of MS patients to perform baseline and surveillance ophthalmic examinations for the detection of fingolimod-associated macular edema (FAME).
Clinical Trials
Two large phase 3 clinical trials have shown the beneficial effect of fingolimod in relapsing-remitting MS. In the FREEDOMS trial, 1,272 patients were randomized to treatment with 0.5 mg/day fingolimod, 1.25 mg/day fingolimod or placebo for 24 months.1 The annualized relapse rate was 0.18, 0.16 and 0.40, respectively.
In the TRANSFORMS trial, 1,292 patients were randomized to treatment with 0.5 mg/day fingolimod, 1.25 mg/day fingolimod, or once-a-week intramuscular interferon-ß1a (Avonex) for 12 months.2 The annualized relapse rate was 0.16, 0.20 and 0.33, respectively. In both studies, the MRI findings supported the beneficial effects of fingolimod in reducing disease activity in the brain.
 |
|
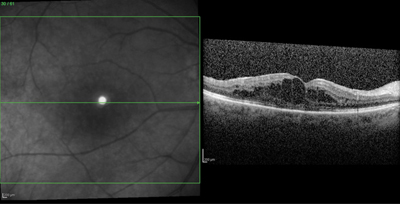
RAPID ONSET. A 56-year-old female with a history of MS and bilateral uveitis recently switched from natalizumab to fingolimod. Within one week of starting fingolimod, she reported blurred vision in her right eye. The ophthalmic examination revealed a visual acuity of 20/40, with macular edema confirmed by OCT.
|
Fingolimod-Associated Macular Edema
Pathophysiology. Fingolimod phosphate, the active metabolite of fingolimod, causes lymphocyte sequestration in the peripheral lymph nodes. Fingolimod phosphate is a structural analogue of sphingosine-1-phosphate (S1P) and binds to the S1P receptor, which directs the egress of lymphocytes from peripheral lymph nodes into the systemic circulation. By selectively modulating lymphocyte trafficking, fingolimod reduces inflammatory activity in the central nervous system. A second putative mechanism of action is through direct action on the CNS cells via neural S1P receptors.3
Fingolimod has been shown to have secondary effects on vascular endothelial barrier function, thereby potentially compromising the blood-retina barrier. Laboratory studies have demonstrated that the endothelial cell S1P receptor modulates intercellular junctions as well as the interplay between the cellular cytoskeleton and the extracellular matrix. Inhibition of the S1P signaling pathway in cultured pulmonary endothelial cells causes increased permeability.4 In vivo studies of acute lung injury in animal models have shown that infusion of S1P decreases acute pulmonary edema.5
Incidence. FAME was first identified in renal transplant recipients undergoing immunosuppression trials. Fingolimod, at a dosage of 2.5 mg/day or 5.0 mg/day, caused macular edema in 1.3 percent and 2.2 percent of patients, respectively.6 Based on these findings, both the FREEDOMS and TRANSFORMS trials incorporated routine baseline and surveillance ophthalmic examinations for FAME. Pooled data from these two studies found a 0.2 percent incidence of FAME at the lower FDA-approved dosage of 0.5 mg/day.
Surveillance for FAME is continuing in the extension studies for both trials. In addition, an ongoing phase 3 study (FREEDOMS II) is exploring FAME in further detail with serial optical coherence tomography scans in the first 300 participants.
Cormorbidity. FAME is more common in patients with uveitis and diabetes, although neither condition is a contraindication to the use of fingolimod. A history of uveitis was present in 25 percent of patients who developed FAME, in contrast to a 1 percent overall prevalence in the phase 2 and 3 MS studies.7 Uveitis, particularly the intermediate uveitis subtype, is associated with MS. Although diabetes was an exclusion criterion in the MS studies, earlier fingolimod studies in renal transplant recipients revealed an increased risk of FAME in the subset of patients with diabetes. These studies, which assessed fingolimod at a five- to 10-fold higher dosage than the FDA-approved dosage, demonstrated an incidence of FAME of 4 percent in nondiabetics versus 30 percent in diabetics.
Onset. In most cases, FAME develops within four months of treatment initiation. Only 1 of 13 cases of FAME reported in the phase 3 MS trials occurred after the initial four months of therapy (subsequent review of this case indicated that the macular edema was the result of a branch retinal vein occlusion). The ongoing extension phases of these trials have revealed six additional cases of FAME, with two cases at the 0.5 mg/day dosage. Including data from these extension studies, 68 percent of cases of FAME occurred within the first four months of treatment. The timing of onset of FAME is particularly important in guiding ophthalmic surveillance strategies.7
Visual symptoms. Some patients with FAME may experience minimal or no visual symptoms. Overall, 68 percent of patients with FAME were symptomatic in the phase 3 and extension MS studies. Symptoms included blurred vision and metamorphopsia, as is typical in cases of macular edema, and FAME was bilateral in 25 percent of patients.7 On examination, signs of FAME can be quite subtle, with a slight decrease in visual acuity and variable degrees of macular thickening. OCT reveals intraretinal cysts characteristic of cystoid macular edema (Fig. 1). Unlike in some other types of drug-induced macular edema, fluorescein angiography (FA) identifies leakage within the macula.
In patients with MS, clinicians must differentiate the ocular symptoms of macular edema from those of optic neuritis. In contrast to FAME, optic neuritis is associated with eye pain exacerbated by eye movement, a relative afferent pupillary defect, dyschromatopsia, visual field changes, disc edema (in one-third of patients) and absence of macular thickening. OCT, FA and MRI can supplement the clinical examination.
Screening guidelines. The initial evaluation of patients taking fingolimod should include a complete ophthalmic examination, including dilated fundus examination. A macular contact lens may assist in the assessment of macular thickening. The use of OCT as a screening tool is not mandatory. In our practice, we are more likely to obtain a baseline OCT in patients with uveitis, diabetic retinopathy, recent intraocular surgery or optic nerve pallor. Also, we perform Amsler grid testing and teach patients how to perform the test at home. We occasionally will perform FA, particularly in patients with diabetic retinopathy and possible macular edema.
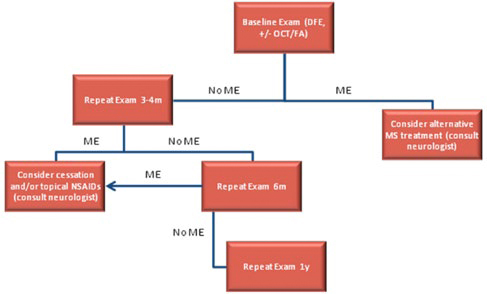
Repeat ophthalmic examination should be performed at three to four months, as most cases of FAME develop within this time period. Patients with visual symptoms, abnormal Amsler grid testing, decreased best-corrected visual acuity or macular thickening on clinical examination should undergo OCT and/or FA. We perform follow-up surveillance at six months and annually thereafter. We advise our patients to return sooner if they experience any visual symptoms.
 |
|
SCREENING GUIDELINES FOR FAME. Patients with visual symptoms at any time during treatment should have an additional eye examination, particularly during the initial months of treatment. DFE = dilated fundus exam; FA = fluorescein angiography; ME = macular edema; MS = multiple sclerosis; OCT = optical coherence tomography.
|
Management. The FREEDOMS and TRANSFORMS study designs mandated drug discontinuation in any participant who developed FAME. In most cases, FAME resolved when fingolimod was stopped.
In clinical practice, the ophthalmologist should discuss the possibility of discontinuing therapy with the patient and his or her neurologist. In patients with severe demyelinating disease who had a suboptimal response to prior alternative treatments or who are poor candidates for other therapies, fingolimod may be the best option available. In such cases, continuation of treatment requires a discussion with the patient about the potential for visual loss due to macular edema.
Limited data exist on the efficacy of pharmacologic treatments for FAME. In the FREEDOMS and TRANSFORMS studies, topical ophthalmic NSAIDs were occasionally prescribed by the patients’ community ophthalmologists. Pharmacologic management of the condition will be difficult to study given its relatively low incidence.
___________________________
Last October, the North American Neuro-Ophthalmology Society (NANOS) and the AAO Ophthalmic News and Education (ONE) Network Neuro-Ophthalmology Committee disseminated this report on fingolimod.
___________________________
1 Kappos L et al. N Engl J Med. 2010;362(5):387-401.
2 Cohen JA et al. N Engl J Med. 2010;362(5):402-415.
3 Cohen JA, Chun J. Ann Neurol. 2011;69(5):759-777.
4 Tauseef M et al. Circ Res. 2008;103(10):1164-1172.
5 Peng X et al. Am J Respir Crit Care Med. 2004;169(11):1245-1251.
6 Tedesco-Silva H et al. Transplantation. 2006;82(12):1689-1697.
7 Zarbin M et al. Poster 244, presented at the American Academy of Ophthalmology Annual Meeting, 2011, Orlando, Fla.
___________________________
Dr. Jain is chief resident in ophthalmology, and Dr. Bhatti is associate professor of ophthalmology and neurology; both are at Duke University. Dr. Jain reports no related financial interests. Dr. Bhatti has received consultancy fees, speaker fees or honoraria from Novartis.