Download PDF
This month, News in Review Highlights selected papers from the original papers sessions at AAO 2016. Each was chosen by the session chair because it presents important news or illustrates a trend in the field. Only 4 subspecialties are included here; papers sessions will also be held in 6 other fields. See the Meeting Program, which you’ll find in your meeting bag, or the Mobile Meeting Guide for more information.
In late July 2016, following release of results from the 2-year COMPASS trial,1 the FDA approved the CyPass Micro-Stent (Alcon). This minimally invasive glaucoma surgery (MIGS) device is designed to reduce intraocular pressure (IOP) in patients with mild to moderate glaucoma who are undergoing cataract surgery. It is placed through the phaco incision into the supraciliary space.
Now there are 2. Another MIGS device, the iStent (Glaukos), which is placed in the trabecular meshwork, was approved 4 years ago for the same indication. Although use of the iStent is increasing rapidly, it is being implanted in only a fraction of eligible patients, said Reay H. Brown, MD, a COMPASS investigator. He predicted that approval of CyPass should increase awareness among surgeons that MIGS devices are safe and effective, which may, in turn, lead to an increase in the number of MIGS implantations, he said.
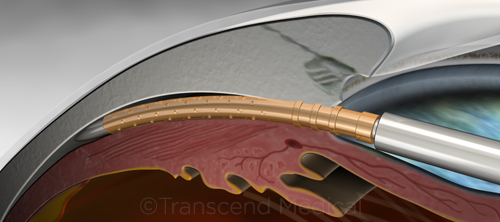 |
CYPASS. Stent is placed in the supraciliary space during cataract surgery.
|
Trial results. The multicenter COMPASS trial included 505 primary open-angle glaucoma patients who qualified for cataract surgery. Of those, 374 were randomized to receive phacoemulsification plus a microstent, while 131 controls underwent phaco only.
“CyPass patients achieved a reduction in IOP that was greater than in patients who had cataract surgery alone,” said Dr. Brown. “The COMPASS trial had 2 years of follow-up, and the IOP-lowering effect persisted for the full 2 years.”
The primary efficacy outcome measure was the proportion of eyes with unmedicated diurnal IOP reduction of 20% or more at 24 months after surgery compared with their unmedicated baseline IOP.
Baseline mean IOP was similar in the microstent and control groups: 24.4 mm Hg and 24.5 mm Hg, respectively. But mean IOP reduction was more robust in the stent group than in controls: 7.4 mm Hg versus 5.4 mm Hg, respectively. And a greater percentage of the stent group (72.5%) achieved IOP reduction of 20% or more compared with controls (58.0%).
Microstenting also reduced long-term glaucoma medication use to one-third of that required by control subjects, with a mean decrease of 1.2 medications at 24 months. At that point, 85% of microstent patients were medication free, versus 59% of controls.
Looking ahead. Dr. Brown said that CyPass approval should spur the market for MIGS devices. “There will definitely be competition between the iStent and CyPass, but both seem to be safe and effective.”
—Miriam Karmel
| Minimally Invasive Supraciliary Microstent for IOP Control in Combined Primary Open-Angle Glaucoma–Cataract Surgery: Two-Year COMPASS Randomized Controlled Trial Results. When: Monday, Oct. 17, 3:52-3:59 p.m., during the glaucoma original papers session (2:00-5:15 p.m.). Where: S405. Access: Free. |
___________________________
1 Vold S et al. Ophthalmology. Published online Aug. 6, 2016. doi: 10.1016/j.ophtha.2016.06.032.
___________________________
Relevant financial disclosures: Dr. Brown—Alcon: C; Allergan: C; Glaukos: P; Rhein: P; Transcend: S.
See the disclosure key at www.aao.org/eyenet/disclosures.
More from this month’s News in Review