By Miriam Karmel, Contributing Writer, interviewing Larry Benowitz, PhD, David J. Calkins, PhD, Joseph Caprioli, MD, and M. Bruce Shields, MD
Download PDF
The patient kept saying that her vision was getting better, recalled Joseph Caprioli, MD. “But I didn’t really believe her.” Dr. Caprioli blamed his doubt on what he called a common mindset in the glaucoma community—an “ingrained clinical belief” that glaucomatous visual field (VF) loss is irreversible.
Recently, however, Dr. Caprioli reported that lowering intraocular pressure (IOP) with trabeculectomy slowed the rate of perimetric decay and provided evidence of sustained, long-term improvement of visual function in glaucoma, as noted on VF testing.1 That improvement “is not just a little bump after surgery. Some patients continue to get better and better over years,” said Dr. Caprioli, at the Stein Eye Institute in Los Angeles.
Looking for Improvement
Reports of reversible field damage have appeared in the literature since the early 1970s. Then, in 1983, George L. Spaeth, MD, suggested a new paradigm that would essentially turn the clinical approach on its head. Instead of monitoring and waiting for a patient to get worse, he said, clinicians should be looking for evidence that treatment has led to improvement.
Detection. But detecting improvement isn’t easy, said M. Bruce Shields, MD, at Yale University. Indeed, Dr. Shields has tried: In a 2001 retrospective study, he looked for VF improvement following IOP reduction by focusing on regions of the field with different levels of sensitivity.2 The results suggested that detecting functional improvementafter pressure reduction could be enhanced by focusing on portions of the VF that correlate with regions of retinal ganglion cell (RGC) damage, particularly those test points with the lowest sensitivity. “We need a diagnostic measure that’s sufficiently sensitive to show that the treatment is adequate and the patient improved,” Dr. Shields said.
Documentation. Most VF analyzers, Dr. Caprioli said, “parse people into stable or worsening. They’re not even looking at improvement.” To overcome that limitation, his group has developed a software program that analyzes standard output from automated perimetry. With that, he has been able to identify improved visual function after trabeculectomy. And that gets us back to his patient, the one who insists her vision is improving. “I just saw her,” he said, “and now I really believe her.” Over years of VF testing, measured sensitivities at the majority of the abnormal VF locations have steadily improved, Dr. Caprioli said.
 |
|
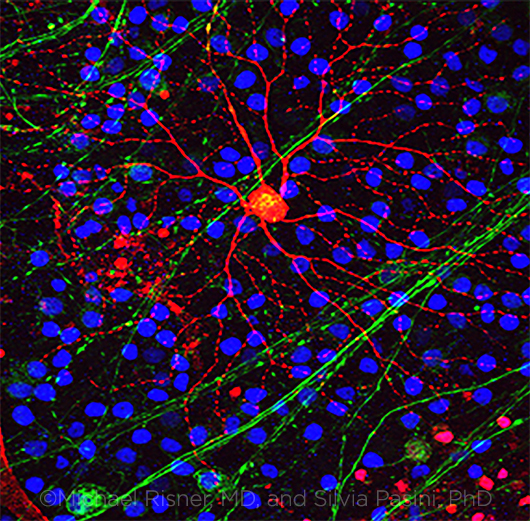
PRIME QUESTION. What is the process that leads from elevated IOP to axonal damage to the subsequent death of the RGC? (Note: RGC axons = green, RGC = red, other cells = blue.)
|
What Causes Improvement?
One theory holds that RGCs, which send information from the eye to the brain and are the primary cells damaged by glaucoma, go through a stage in which they are injured but not dead.
Not dead yet. “These cells that are still there are not firing because they’re sick. But they’re not dead,” Dr. Caprioli explained. “If we can treat them [as he did with trabeculectomy], they can begin to function again.” In other words, they are capable of being rescued, which gives us the term neurorescue. (This is not to be confused with neuroregeneration, which is to grow new cells.)
IOP and beyond. At this point, reducing IOP “is clinically all we have” for rescuing RGCs, said Dr. Caprioli. “But that may not be the best way.”
Dr. Shields agreed. “The only factor that we know with certainty causes the damage and ultimate death [of RCGs] is a certain level of pressure in the eye. But we also have reason to believe that there are many other factors involved with the damage,” he said. “If we can discover these other processes [involved in glaucomatous progression] and discover ways to treat that part of the process, then we will have other ways to protect the nerve in addition to lowering pressure.”
The Life and Death of RGCs
“We know some of the events that take place in the eye after the optic nerve is injured and cause the RGCs to die,” said Larry Benowitz, PhD. “In glaucoma, the initial insult is at the point where axons are exiting the eye,” he said. The question is, “How do you go from elevated IOP to the insult to the axons to the subsequent death of RGCs?”
Signaling cascade. One line of inquiry is looking at protein kinases, which set up a signaling cascade early in the process, said Dr. Benowitz, at Harvard Medical School. If you block an early step in the cascade, the RGCs will survive, although they can’t regenerate their axons. “This is the signal to the cell that the axon has been injured. In the absence of that signal, the cell shows a long delay in initiating the death program.” Thus, this approach only delays, rather than prevents, cell death. However, he pointed to research showing that blocking another downstream step in the death pathway enables RGCs to survive long-term, albeit in an atrophied and dysfunctional state.3
Ultimately, there’s a question of causal sequence. “Is there a choke point where you can stop the whole death process?” Dr. Benowitz asked. He predicted that a treatment to stop the entire cascade will eventually be found.
Multiple cascades, multiple actions. David J. Calkins, PhD, identifies molecular cascades in the retina and optic nerve that contribute to neurodegeneration. “There are myriad cascades,” he said. “Labs tend to pick their favorite molecular cascade to focus on.” Dr. Calkins’ laboratory at Vanderbilt University is working on a handful of potentially protective cascades.
Some cascades contribute to a prodegenerative state; others contribute to a protective state. He called this “a big leap in understanding,” because for years, research has focused mainly on death cascades. “The problem with that is that evolution has come up with about 50 different ways to get to death of neuronal tissue through these proapoptotic pathways.”
A tipping point? More recently, some researchers, including Dr. Calkins, have embraced the idea that retinal and optic nerve cells express intrinsic biochemical cascades that could be exploited to protect the system. He is looking for a tipping point, some point very early in glaucomatous progression where these prodegenerative and protective cascades are vying for dominance.
Beyond Lowering IOP
Here’s a brief look at some areas of glaucoma research.
Block inflammation. Work by Dr. Benowitz and others points to changes in glial (nonneuronal) cells and inflammatory cells at the point where the nerve fibers from RGCs exit the eye. This appears to be an important link between elevated IOP and RGC death. It is possible, Dr. Benowitz said, that blocking events in these latter cell types could be as protective to RGCs in humans as they have been in animal studies.
Use stress proteins. Dr. Caprioli and Natik I. Piri, PhD, have been investigating stress proteins, which are part of the cell’s own defense mechanisms. Under adverse conditions—in this case, glaucomatous neurodegeneration—stress proteins increase to repair and restore cells to normal function. In animal models, Drs. Caprioli and Piri have shown that some mechanisms used to induce these protective proteins can be harnessed to keep RGCs from being damaged by glaucoma. They foresee some pharmacological therapeutic intervention that might rescue RGCs and restore them to health.
Use existing drugs. Dr. Calkins hopes to harness existing drugs designed to either reduce oxidative stress or dilatory inflammation or to boost bioenergetics. The latter, he said, refers to how neurons and other cells in the optic nerve use energy and “whether these pathways can be bootstrapped to be protective.” He is also collaborating with biotechnology companies to test whether compounds designed for Alzheimer’s might also affect glaucoma. “My goal,” he said, “is to make ocular pressure irrelevant.”
|
Structural Persistence
In 2010, Dr. Calkins’ laboratory demonstrated in animal models of glaucoma that loss of communication between the optic nerve and brain is one of the earliest signs of neurodegeneration.4 In this early period, RGCs are challenged by glaucoma, yet the important structures that allow them to function remain in place. “The retinal nerve fibers persist, as do the synapses from those fibers to the brain projection sites,” Dr. Calkins said.
Next, in animal research, his laboratory showed that during this “window of opportunity,” when RGCs are challenged but still functioning, the optic nerve projection upregulates brain-derived neurotrophic factor. It does so only in the region where ganglion cell function is challenged.
“All of this suggests that the retina, the optic nerve, and the optic nerve projection to the brain react actively to stress in glaucoma to try to maintain the structure between the retina and brain for as long as possible,” Dr. Calkins said.
If a similar window exists in human glaucoma patients who have not progressed too far, it could explain Dr. Caprioli’s findings. “When Dr. Caprioli intervened and removed the stress due to ocular pressure, the system had a chance to reboot,” Dr. Calkins said.
The key point, he continued, is that if IOP stress is reduced in a timely manner, it’s possible to rescue those parts of the retina and optic nerve that remain functional. “The challenge for us,” he said, “is to understand the threshold at which you move from the need for neurorescue therapy to the need for a neuroregenerative therapy.” In truth, he added, we need both.
In the meantime, how to rescue the cells “is the million-dollar question,” Dr. Calkins said. “Intellectually, the pieces are all in place. In the lab, we have hundreds of ways. In humans, we need a well-defined trial with targets that the FDA would support. And we have to have buy-in from the NEI [National Eye Institute]. With enough money, it could happen in 5 years. We’re moving in that direction.”
Clinical Implications
“We don’t yet know how to replace the cells that are lost,” Dr. Benowitz said, but it’s reasonable to imagine that within a few years, researchers will find a way to replace those RGCs that have died—and, perhaps even sooner, rescue RGCs that have suffered damage to their axons—with a pharmacological or biotechnological treatment or even a prosthetic device. (For one approach, see “Progress in Axon Regeneration,” in News in Review, EyeNet, September 2016.)
For now, in light of his study, Dr. Caprioli said he is “more aggressive about getting the pressure robustly lower.” Although he is now more likely to do a trabeculectomy, he doesn’t advocate a rush to surgery. “You have to make that judgment call. I would be most aggressive about a patient [who is] deteriorating relatively rapidly.”
Finally, harking back to Dr. Spaeth, he said, “I am actively looking for signs of improvement as evidence of satisfying our treatment goals.”
That’s something every clinician can do. “You have to be willing to open your mind to the possibility of improvement before you can find it. You have to look for it,” Dr. Caprioli said. “The main thing is the realization that this can occur, and it’s not a rare event.”
___________________________
1 Caprioli J et al. Ophthalmology. 2016;123(1):117-128.
2 Salim S et al. Am J Ophthalmol. 2001;132(4):496-500.
3 Janssen KT et al. Invest Ophthalmol Vis Sci. 2013;54(3):1805-1815.
4 Crish SD et al. Proc Nat Acad Sci U S A. 2010;107(11):5196-6201.
___________________________
Dr. Benowitz is professor of neurosurgery and ophthalmology at Harvard Medical School. Relevant financial disclosures: None.
Dr. Calkins is the Denis M. O’Day Professor of Ophthalmology and Visual Sciences at Vanderbilt University in Nashville, Tenn. Relevant financial disclosures: None.
Dr. Caprioli is the David May II Professor of Ophthalmology at the Stein Eye Institute at the University of California, Los Angeles. Relevant financial disclosures: Alcon: S; Allergan: S; National Eye Institute: S; New World Medical: S; Research to Prevent Blindness: S.
Dr. Shields is the Marvin L. Sears Professor Emeritus of Ophthalmology and Visual Science at Yale University School of Medicine. Relevant financial disclosures: None.
See the disclosure key at www.aao.org/eyenet/disclosures.
More at the Meeting
Is It Progression? Is It Glaucoma? When: Saturday, 8:06-9:05 a.m. Where: Grand Ballroom S100ab. Access: Subspecialty Day badge that is valid for Saturday.
Glaucoma—It’s Not Just About IOP When: Saturday, 10:43-11:43 a.m. Where: Grand Ballroom S100ab. Access: Subspecialty Day badge that is valid for Saturday.
Computerized Scanning Imaging of the Optic Nerve and Retinal Nerve Fiber Layer (Lab119A). When: Monday, 9:00-11:00 a.m. Where: Room N231. Access: Ticket required.
|