Download PDF
Physician encouragement doesn’t go very far toward convincing many glaucoma patients to use their prescribed hypotensive medications, laments glaucoma specialist James D. Brandt, MD.
“Patients will come in and say, ‘Oh, yeah, I’m still taking that drop every night.’ Then you look at their pharmacy data in the EMR, and it’s really quite shocking. You see that they haven’t refilled their medication in 6 months,” Dr. Brandt said.
Countering noncompliance. A topical insert intended to ameliorate this problem by bathing the ocular surface in bimatoprost continuously for up to 6 months is expected to begin a phase 3 efficacy trial later this year, said Dr. Brandt. Results of the phase 2 trial were recently published.1
The preservative-free ocular ring is 1 mm thick and 24 to 29 mm in diameter. It incorporates 13 mg of bimatoprost into a silicone matrix, with a polypropylene core for support. The ring is inserted into the conjunctival fornices, where the drug diffuses passively into the tear film.
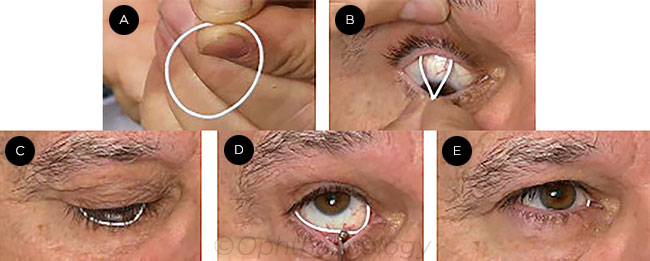 |
DEVICE INSERTION. The ring (A) is inserted first in the upper conjunctival fornix (B), then in the lower (C), where a scleral depressor may be used to ease insertion (D). After placement, the ring is barely visible (E).
|
Phase 2 results. Dr. Brandt, who is a professor of ophthalmology and visual science at the University of California, Davis, was the principal investigator in the phase 2 study. Using subjects who had open-angle glaucoma (OAG) or ocular hypertension (OHT), the masked trial compared outcomes over 6 months between 64 patients treated with the medicated insert and sham drops (artificial tears) and 66 controls treated with 0.5% timolol drops plus a sham insert.
The researchers reported that intraocular pressure (IOP) fell by 3.2 to 6.4 mm Hg (about 20%) with the bimatoprost ring, compared with 4.2 to 6.4 mm Hg in the timolol group. The study size was too small to determine noninferiority compared with the timolol, but that will be tested in phase 3, according to the researchers.
Clinical implications. “In the Ocular Hypertension Treatment Study, we demonstrated that lowering IOP by just 20% among ocular hypertensives reduced the rate of conversion to OAG itself,” Dr. Brandt said. “The bimatoprost insert is targeted at the millions of patients treated for OHT or early OAG who simply won’t, don’t, or can’t take their medications.”
But the real potential for this approach to hypotensive therapy lies beyond monotherapy, Dr. Brandt added. “To me, the biggest advantage is the versatility of this technological platform,” he said. “The device has so much volume that I could easily conceive of having 2 or even 3 medicines in the ring.”
—Linda Roach
___________________________
1 Brandt JD et al. Ophthalmology. Published online May 5, 2016.
___________________________
Relevant financial disclosures—Dr. Brandt: ForSight VISION5: S,C; Glaukos: C,O.
For full disclosures and disclosure key, see below.
More from this month’s News in Review