By Michael A. Klufas, MD, Jasmine H. Francis, MD, and David H. Abramson, MD
Edited by Ingrid U. Scott, MD, MPH, and Sharon Fekrat, MD
Download PDF
Treatment of intraocular retinoblastoma has improved substantially over the past century. This most common primary intraocular tumor of childhood, once considered to be a deadly pediatric cancer, is an oncologic success story, with a greater than 99 percent survival rate in developed countries. While the focus of treatment for advanced intraocular retinoblastoma remains preservation of life, current therapies have made it possible to also aim for globe retention and maintenance of visual potential. One of the most fascinating new treatment modalities is superselective intra-arterial chemotherapy, also known as ophthalmic artery chemosurgery (OAC).
 |
|
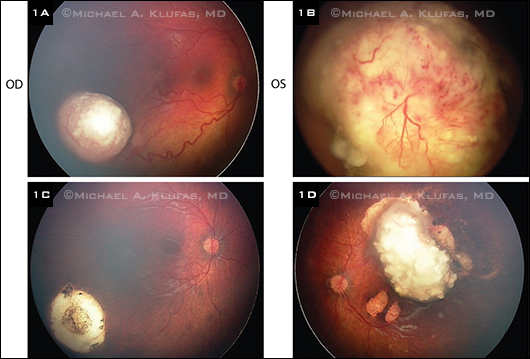
Clinical Response to OAC. (1A, B) Advanced bilateral retinoblastoma before primary treatment with OAC. (1C, D) After treatment with two cycles in the right eye and four cycles in the left eye, with adjuvant transpupillary thermotherapy laser in both eyes, there was regression of the tumor with no need for additional therapy or enucleation.
|
Intra-Arterial Technique
Intra-arterial chemotherapy for the treatment of retinoblastoma was first performed in 1954 by Algernon B. Reese, MD, with direct internal carotid artery injection of a nitrogen mustard analogue.1 Dr. Reese’s goal was to use chemotherapy to reduce the total dose of external beam radiation therapy (EBRT) needed to treat retinoblastoma.
Since 1988, a type of intra-arterial chemotherapy for the treatment of retinoblastoma has been performed in Japan and is described by Kaneko and colleagues as “selective ophthalmic artery infusion” (SOAI).2 A micro-balloon catheter is positioned using a transfemoral artery approach at the cervical segment of the internal carotid artery (ICA) just distal to the orifice of the ophthalmic artery. The balloon catheter is then inflated, thereby directing flow into the ophthalmic artery as chemotherapy is infused. This infusion method is not truly selective, however, because there are proximal branches of the ICA into which the drug can flow before reaching the ophthalmic artery. The goal of the Japanese clinicians was not to reduce the dose of EBRT with their technique but rather to offer a treatment option for families who wanted to avoid curative enucleation at all costs.
In 2006, the department of ophthalmic oncology at Memorial Sloan-Kettering Cancer Center (MSKCC) and interventional radiologist Y. Pierre Gobin, MD, of Weill Cornell/New York-Presbyterian Hospital pioneered a direct intra-arterial (through the ophthalmic artery) infusion technique also known as OAC. In brief, patients are placed under general anesthesia and intubated. The interventional radiologist precisely positions a microcatheter via a transfemoral artery approach under direct fluoroscopic visualization at the ostium of the ophthalmic artery (but not more distal), which allows selective delivery of chemotherapeutics to the eye via its main arterial supply. (View a video of the procedure below.) Patients are observed for four to six hours after the procedure and discharged the same day. This technique is now performed in more than 30 countries.
Indications and Management
One impetus for refining the intraarterial technique was to deliver a higher concentration of chemotherapy without increasing systemic toxicity, with the hope of successfully treating advanced disease (Reese-Ellsworth [RE] groups IV/V, International Classification of Retinoblastoma [ICRB] groups D/E), which has been plagued by dismal results using systemic chemoreduction3,4 and EBRT.5 Studies have demonstrated that OAC can be used successfully as primary therapy.6 It has been used to treat less-advanced disease7 and advanced disease,8,9 as well as cases with retinal detachment.10 Additionally, OAC can be used in bilateral cases (treating both eyes, “tandem therapy”)11 or in combination with sequential intravenous chemotherapy (“bridge chemotherapy”) in infants too young or small for OAC at presentation.12 Studies have also shown the efficacy of OAC as a salvage therapy in eyes that have previously failed conventional management including EBRT and systemic chemotherapy.8,13 Each case should have an individualized treatment plan developed in consultation with multiple ocular oncologists. All acceptable treatment options, including enucleation, EBRT, systemic chemoreduction with focal laser or cryotherapy, plaque brachytherapy, and OAC, should be considered.
 |
|
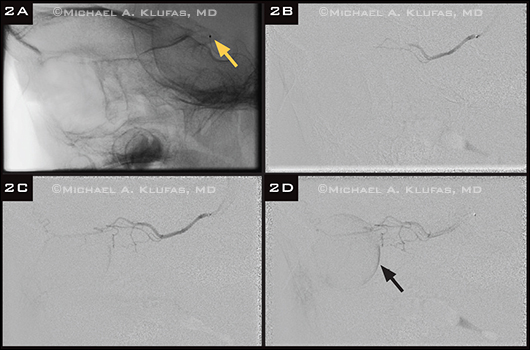
OAC Procedure. Angiography demonstrating infusion of chemotherapy via the ophthalmic artery. (2A) Unsubtracted lateral angiogram showing the catheter tip (arrowhead) at the ostium of the ophthalmic artery. (2B-D) Sequential imaging showing flow of contrast from the catheter tip through the ophthalmic artery to the eye, resulting in an excellent choroidal blush (2D, arrow).
|
Tips and Tricks
It’s surgery. Be prepared. OAC is most appropriately considered surgery rather than a minor procedure, and the preferred term is now chemosurgery rather than the previously used term intra-arterial chemotherapy. Just as any ophthalmic surgery is a serious clinical decision, the stakes are no less with OAC. As with any surgery, meticulous preparation is necessary. For instance, many interventional radiologists may be comfortable gaining vascular access in a child, but how far should the catheter be advanced into the ophthalmic artery and how exactly should the chemotherapy be infused? As with any surgical procedure, a detailed plan should be in place before this modality is offered as a treatment option.
Don’t fly solo. Teamwork is key. Teamwork is required before, during, and after the procedure. Close coordination between an ocular oncologist and interventional radiologist is only the first step to ensure success. Other important members of the team include staff from nursing, pediatric anesthesiology, pharmacy, social work, genetic medicine, and pediatric oncology. For example, a skilled pharmacist experienced with chemotherapeutics is needed to advise on the stringent preparation, storage requirements, and shelf life of the chemotherapeutic agents used.
Call for help before the procedure! The importance of preparation is paramount and should not be understated. Do your homework beforehand. Know in advance of surgery how to dilute the drug and what the dose should be. Visit a center that offers the procedure, and observe and learn the technique. Don’t be afraid to ask the experts, starting with the basics first.
Know the complications. No procedure, including OAC, is perfect for the treatment of retinoblastoma. Although the dose of each chemotherapeutic is small with OAC therapy, when multiple chemotherapeutic agents are delivered (melphalan, topotecan, and carboplatin), especially with tandem therapy, the total dose of chemotherapy must be calculated carefully to avoid myelosuppression. Likewise, in children who have previously failed systemic chemotherapy, even low total doses of melphalan may cause myelosuppression. Therefore, monitoring blood counts and watching for neutropenic fever in the postoperative period are recommended. In addition, retinal vascular events have been reported after OAC, including clinically visible occlusive vasculopathy.14,15 Groups at Wills Eye Hospital and other institutions have reported that this adverse effect is most likely related to the method of drug infusion, such as “wedge flow,” which causes rapid alteration of flow and pressure in the cannulated vessel, resulting in retinal hemorrhages.16,17 Furthermore, many investigators initially utilizing OAC were concerned about the occurrence of clinically significant bronchospasm in up to 8 percent of children undergoing the procedure. It is now thought that this complication is related to the depth of anesthesia and can be treated effectively with intravenous epinephrine bitartrate.8
Report Your Results
OAC for retinoblastoma continues to evolve as new reports emerge from centers in the United States and abroad. Given that there is a learning curve with OAC, we encourage centers to share their experiences once they have achieved confidence with the technique. Collegial partnership will allow for ongoing fine-tuning of OAC.
The largest clinical series reporting experience with OAC for the treatment of retinoblastoma comes from Gobin and colleagues at MSKCC and Weill Cornell/New York-Presbyterian Hospital. In a prospective, nonrandomized series of 95 eyes (87 percent, RE group V) with retinoblastoma, they reported that the Kaplan-Meier estimates of eye survival until enucleation or EBRT at two years were 70.0 percent for all eyes, 81.7 percent for eyes that received OAC as a primary treatment, and 58.4 percent for eyes that previously received intravenous systemic chemotherapy and/or EBRT. For RE group V eyes, Kaplan-Meier estimates of ocular event-free survival at two years were 66.5 percent for all eyes, 80.5 percent for eyes that received OAC as a primary treatment, and 51.5 percent for eyes that previously received intravenous systemic chemotherapy and/or EBRT. Median follow-up was 13 months.8
More recently, Abramson and colleagues reported the results of OAC for less-advanced retinoblastoma.7 A five-year retrospective review of 17 eyes classified as RE I to III and 30 eyes classified as ICRB B or C reported ocular event-free survival of 100 percent and 96 percent, respectively (one ICRB C eye was enucleated due to technically impossible cannulation of the vascular supply to the eye). Median follow-up was 16 months.
___________________________
1 Reese AB et al. Arch Ophthalmol. 1954;53(4):505-513.
2 Yamane TA et al. Int J Clin Oncol. 2004;9(2):69-73.
3 Friedman DL et al. J Clin Oncol. 2000;18(1):12-17.
4 Shields CL et al. Am J Ophthalmol. 2002;133(5):657-664.
5 Abramson DH et al. Arch Ophthalmol. 2004;122(9):1316-1323.
6 Abramson DH et al. Ophthalmology. 2010;117(8):1623-1629.
7 Abramson DH et al. PLoS One. 2012;7(4):e34120.
8 Gobin YP et al. Arch Ophthalmol. 2011;129(6):732-737.
9 Abramson DH et al. Ophthalmology. 2008;115(8):1398-1404.e1.
10 Palioura S et al. Pediatr Blood Cancer. 2012;59(5):859-864.
11 Abramson DH et al. Arch Ophthalmol. 2010;128(3):370-372.
12 Gobin YP et al. PLoS One. 2012;7(9):e44322.
13 Vajzovic LM et al. Clin Ophthalmol. 2011;5:171-176.
14 Shields CL et al. Arch Ophthalmol. 2011;129(11):1407-1415.
15 Munier FL et al. Retina. 2011;31(3):566-573.
16 Shields CL et al. Eye (Lond). 2013;27(2):253-264.
17 Jabbour P et al. J Neurosurg Pediatr. 2012;10(3):175-181.
___________________________
Dr. Klufas is an ophthalmology resident, New York-Presbyterian Hospital/Weill Cornell Medical College, New York. Dr. Francis is an ophthalmic oncology fellow, and Dr. Abramson is chief of the ophthalmic oncology service. Both are at Memorial Sloan-Kettering Cancer Center, New York. The authors report no financial interests. The authors would like to thank Y. Pierre Gobin, MD, Brian P. Marr, MD, Ira J. Dunkel, MD,and Scott E. Brodie, MD, PhD, for their assistance with this manuscript and continued efforts with OAC therapy.
___________________________
Dr. Klufas wrote an EyeWiki article on this topic.