Author: Brian A. Francis, MD, MS
This survey provides a summary of the reported uses for anterior segment endoscopic ophthalmic techniques. The basic science and technology of endoscopic cyclophotocoagulation (ECP) as compared to transscleral cyclophotocoagulation is presented. This is followed by an analysis of patient selection for ECP, a description of surgical techniques, and clinical results in the literature. The ophthalmic endoscope has other uses in anterior segment surgeries, ranging from angle procedures to ciliary sulcus procedures. The techniques and literature for these endoscope-assisted surgeries are discussed.
Introduction
The endoscopic ophthalmic surgical system gives a unique view of the interior structures of the eye, and can be used for a variety of anterior and posterior segment surgeries.
- The system allows for visualization of structures that are not routinely accessible through standard viewing, such as the anterior chamber angle, the ciliary body and processes, the ciliary sulcus space, the anterior retina, and in cases of opaque media.
- Fibertech Co., Ltd (Tokyo, Japan) produces viewing-only ophthalmic endoscope systems, AS-611 and MS-611, the latter having a higher resolution.
- The Endognost Vitroptik Flex (Polydiagnost, Pfaffenhofen, Germany) has an endoscope and light source but no laser.
- The only systems currently FDA-approved for use in the United States are the E2 and E4 by Endooptiks, Inc (Little Silver, NJ), made available in 1991.
- The E4 is an endoscope only, without the diode laser.
- The E2 combines a diode laser with a light source, aiming beam, and fiberoptic camera for viewing.The most common indication for E2 use is the treatment of glaucoma with endoscopic cyclophotocoagulation (ECP).
Glaucoma is the most common cause of irreversible blindness worldwide. While the pathophysiology of glaucoma is multifactorial, the treatment strategy remains essentially one-dimensional: lowering of intraocular pressure (IOP).
- Medical management involves agents that either improve outflow or suppress aqueous inflow.
- When surgical intervention is required, the traditional treatment is improving outflow filtration.
- Procedures that reduce aqueous production have previously been reserved for refractory glaucoma, technological advancements and novel applications to complex surgeries have challenged this treatment paradigm.
The traditional “gold standard” for the surgical management of glaucoma has been trabeculectomy or guarded filtration surgery. Such surgery, however, is associated with postoperative complications including overfiltering blebs, hypotony, and cataract.
- Late complications, such as bleb infection or endophthalmitis, may occur years or even decades later.
- Aqueous drainage devices are a reasonable alternative to trabeculectomy, with less risk of infection or conjunctiva-related complications, but tube shunts also involve significant risk, including diplopia, hypotony, corneal decompensation, and tube exposure.
- To avoid these complications, angle-based glaucoma procedures have been introduced, such as Schlemm’s canal stenting or trabecular meshwork ablation by internal approach.
- ECP, however, remains the only approach to reducing aqueous production.
When cyclophotocoagulation was introduced in the 1970s, it was employed as a last resort method to lower IOP when all other efforts had failed. The procedure has evolved through much iteration, including both contact and noncontact transcleral delivery systems with various laser platforms.
- The transcleral approach to cyclophotocoagulation, while effective at lowering IOP, has an unacceptably high rate of chronic inflammation, hypotony, and phthisis, and therefore has limited use early in the disease process.
- Endoscopic cyclophotocoagulation (ECP) is a more targeted guided technique that allows delivery of the laser energy in a precise and efficient manner, expanding the indications for cyclophotocoagulation to include eyes with better visual potential.
- Given that the procedure may be performed through the same incision, many surgeons have employed ECP as an adjunct to cataract surgery.
- This inflow-reducing strategy may be more synergistic to the IOP-lowering effect of phacoemulsification than procedures that completely bypass the trabecular meshwork, such as trabeculectomy.
- ECP spares the sclera and conjunctiva so that trabeculectomy or aqueous drainage procedure can be performed later if needed.
ECP employs a diode laser with a wavelength of 810 nm, a 175-watt xenon light source, a helium-neon aiming beam, and video imaging integrated into a fiberoptic system delivered through a 19-20 gauge probe. The laser delivery system allows the cilio-ablation to be performed under direct visualization, which helps to titrate the treatment in contrast to the transcleral approach.
Basic Science and Tissue Effects of Cyclophotocoagulation
Several modalities exist to reduce aqueous secretion, including the traditional method in which the ciliary body is frozen (cyclocryotherapy), transcleral cyclophotocoagulation, and endoscopic cyclophotocoagulation.
- Cyclocryotherapy may induce severe pain and be associated with the development of hypotony and phthisis.
- Safer laser procedures have therefore been developed for the ablation of ciliary body tissue and the treatment of recalcitrant glaucoma.
The Neodymium: Yttrium Aluminum Garnet (Nd: YAG) laser was originally developed to pass a beam of light (l=1120 nm) from a slit lamp onto targeted ciliary body (CB) tissues by passing through the conjunctiva and sclera.
- The length of the beam’s path was shortened subsequently by placing a fiber optic probe in contact with the conjunctiva and sclera, and focusing the laser light onto the CB tissues.
- When used in ciliary body applications in continuous mode, the Nd:YAG laser causes a thermal disruption of structural molecules, such as protein denaturation that results in coagulation of the targeted tissues.
- The diode laser emits a beam of light, targeting chromophores in the CB tissues and generating sufficient thermal energy to coagulate them.
- The diode laser beam can be applied by either passing the laser light transsclerally (TCP) or directly onto the CB process by using the ECP.
The energy requirements for laser procedures differ widely due to the distance and pathway from the light source to the targeted tissues (ie, transcleral vs. direct photocoagulation), and the wavelength of light applied (ie, 1120 vs. 810 nm).
- Lesions produced in eyes undergoing laser ablation may also mirror these differences in the energy levels applied.
- Histopathological analyses have been performed on the effects of these procedures in vivo and post-mortem.
- The results range from contact and noncontact TCP to ECP diode in human and animal, living and enucleated eyes.
Normal Ciliary Body Architecture
The ciliary body and processes form the pars plicata and consist of the nonpigmented ciliary epithelium (NPCE) and pigmented ciliary epithelium (PCE), which rest on a loose connective tissue or stroma, that contains numerous capillaries and some fibrocytes and melanocytes.
The NPCE is the monolayer of cuboidal cells located internally facing the posterior chamber, and the PCE is the monolayer of cells containing numerous melanin granules, which is located more externally facing the ciliary body stroma.
- The two monolayers interact with each other along the apical surface, and contain basement membranes along their basal surface.
- The NPCE and PCE work in concert in the aqueous formation process.
Histopathology of Transcleral Cyclophotocoagulation
A study of 9 human eyes that had been treated with diode laser TCP in vivo and subsequently enucleatedwas reported by McKelvie and Walland.
- Damage to the pars plicata was evident in all cases, and consisted of the destruction of PCE and NPCE and capillaries in the ciliary processes.
- Pigment clumping, coagulative necrosis, and extensive destruction of the ciliary muscle with moderate decreased vascularity were noted.
- Unequal damage was seen around the circumference of treated areas, with some foci showing total loss of ciliary processes but other foci with some sparing anteriorly, posteriorly, and centrally.
- Although some cases of mild recent treatment showed proliferation of NPCE, this was disorganized with no reconstitution of normal ciliary process architecture.
- Pars plana injury was seen in 6 of 9 patients with loss of normal epithelium, pigment clumping and destruction of muscle and most vessels. The adjacent iris root was damaged in 3 patients, showing necrosis with loss of iris pigment, muscle, and vessels.
- The sclera in the path of the laser showed damage in 3 cases with scarring, increased fibroblast density, and vascularity.
A case report and subsequent analysis of a single subject treated with contact diode TCP 3 days prior to death was reported by Feldman et al.
- The patient had a blind painful eye with a diagnosis of chronic angle closure glaucoma and IOP of 44 mmHg.
- The laser treatment was applied for 270º in 16 shots for 2 seconds at 1,180 mW.
- Gross examination confirmed the treatment spots approximately 0.5 mm posterior to the surgical limbus with gray-white lesions on the pars plicata internally.
- Microscopic exam in the treated areas showed sparse inflammatory infiltrate in the episclera.
- The sclera and ciliary body revealed coagulative necrosis without inflammatory cells, and obliteration of the PCE and NPCE.
- There was a loss of cellular detail and external pigment disgorgement within the pars plicata.
- The untreated areas showed no changes.
Schlote et al evaluated changes in the vascular supply of the ciliary body histologically and by vascular casts after treating rabbit eyes in vivo with the diode TCP.
- Two weeks after treatment, marked destruction of the ciliary processes with severe pigment dispersion and near complete destruction of the ciliary epithelium was observed.
- At 6 weeks, atrophy of the ciliary body with flattening, fusion, and shortening of the processes was noted, with no regeneration seen at 6 or 12 weeks.
- In rabbits treated in the pars plana, similar effects were seen at 2 weeks with destruction of the ciliary epithelium and pigment dispersion.
- After 12 weeks, the pars plana was atrophic with no regeneration of the epithelium present. The adjacent untreated ciliary body showed no effects.
- The vascular casts showed rarefication, destruction, and obliteration of the vascular network with vessel break-up at the margin of the treatment zone.
- After 12 weeks, early neovascularization was seen at the margins of the laser treated areas. Similar obliteration of the vascular supply was seen in the pars plana treated areas.
A similar study by van der Zypen et al observed vascular changes in rabbit eyes treated in vivo with Nd:YAG laser TCP.
- Animals underwent vascular casting after laser treatment.
- These results were similar to those seen in Schlote’s study of diode laser TCP, with marked defects in the capillary networks of the ciliary processes and incomplete regeneration of the vasculature.
Schuman reported the effects of contact TCP with the diode laser in rabbits and found coagulation necrosis of the PCE, NPCE, stroma, and vasculature in the ciliary processes, and ciliary body in the acute phase. Long-term effects included focal atrophy of ciliary processes with fibrosis of the ciliary epithelium and stroma.
Brancato compared contact TCP with the Nd:YAG and diode lasers in a human eye, and found coagulative necrosis of the PCE, NPCE, and stroma with vascular congestion and thrombosis. It was concluded that the diode laser caused more damage of epithelium at corresponding energy levels and that the energy required was half that needed with the Nd:YAG to produce similar threshold lesions.
Histopathology of Endoscopic Cyclophotocoagulation (ECP) Diode
Figure 1. ECP Diode.
Pantcheva et al compared the histological effects of ECP to TCP in human autopsy eyes.The eye treated with TCP showed destruction of the PCE, NPCE, and capillaries in the ciliary processes, with pigment clumping, coagulative damage, and stromal destruction.
- In contrast, the ECP-treated eye showed loss of the “lacy appearance” of the ciliary stroma with destruction of the NPCE and clumping of the PCE, with little discernable effect outside the ciliary processes.
- Scanning electron microscopy showed shrinkage of the ciliary processes and effacement of the ciliary epithelium in the ECP eye.
Lin et al also compared ECP to TCP, studying histopathology and vascular effects in living rabbit eyes.
- Twenty eyes of rabbits were treated with each laser modality then immediately imaged (along with 5 untreated controls) by endoscopic fluorescein angiography (EFA) and again 1 day, 1 week, and 1 month after treatment.
- EFA was performed using a blue filter fitted onto the endoscope probe, and imaging the ciliary processes endoscopically after intravenous fluorescein injection.
- Normal untreated ciliary processes showed fluorescence within 10 seconds of injection.
- Both TCP and ECP showed severely reduced or lack of blood flow immediately and 1 day after treatment.
- At 1 month, the TCP eyes continued to show hypofluorescence, while the ECP eyes demonstrated partial reperfusion of the processes that was uniform but of a lesser magnitude than untreated eyes.
- The histopathologic analysis of the ECP treated eyes showed shrinkage, coagulative necrosis, and disorganization of the ciliary process architecture, with loss of epithelium and shrinkage and avascularity of the ciliary stroma at 1 day.
- After 1 month, patency of the deeper ciliary vessels was evident.
- At 1 day the TCP-treated eyes showed severe ciliary tissue damage, closure, of many both large and small vessels, and stasis in the remaining vessels.
- Anatomical structures adjacent to the ciliary processes, such as the pars plana, sclera, and iris showed disorganization and damage.
- After 1 month, the TCP eyes showed continued sclerosis of stromal tissue, disorganization of normal epithelial architecture, and exudative response.
Alvarado compared the histopathology of ECP and TCP performed in living human eyes that were subsequently enucleated for unrelated pathology.
- Two eyes treated with ECP 16 and 21 months prior to enucleation and 2 eyes treated with Nd:YAG TCP 2 years prior to enucleation were analyzed.
- The contact and noncontact Nd:YAG specimens showed obliteration of the ciliary muscle and replacement with collagenous scar tissue.
- The stroma was disrupted, and the 2 epithelial layers were absent at the lesion site, showing a paucity of blood vessels, fibrocytes, and melanocytes.
- The histologic findings of the ECP-treated eyes contrasted with those of both the noncontact and contact TCP procedures.
- By transmission electron microscopy the epithelial bilayer showed no discontinuities. The cells displayed a regular and uniform size, and an intact cell membrane.
- In the ciliary process stroma, closure of the small blood vessels was evident with preservation of larger vessels.
- There was an absence of melanin granules within the pigmented ciliary epithelium along the crest of each process. In untreated areas or at the boundary of treated/untreated sites, melanin granules were present with a variable distribution.
Discussion
Studies of TCP using both the Nd:YAG and diode lasers, show severe damage to treated tissues with coagulative necrosis, disorganization of normal ciliary process architecture, and replacement with fibrotic scar tissue.
- The vasculature is severely affected, with loss of capillaries in the stroma, and variable loss or closure of larger vessels.
- These changes are concentrated in the areas of the laser burns and may miss the ciliary processes altogether.
In contrast, the studies of animal and human eyes after undergoing ECP show substantially less disruption of the ciliary body stroma or adjacent tissues.
- While the architecture of the ciliary processes are modified with evidence of tissue shrinkage, a reduction in the number and extent of blood vessels, and disruption of the ciliary epithelium, there may be regeneration of the epithelial bilayer as evidenced in the human eyes treated in vivo.
- Both the animal and human studies indicate an initial loss of vascular perfusion, with partial reperfusion over time.
- The disruption of tissues is markedly different from other cyclodestructive procedures that exhibit the permanent loss of both ciliary epithelial layers, and extensive coagulative necrosis with permanent damage of some or all of the ciliary body structures, including its vasculature and musculature.
Endoscopic Cyclophotocoagulation: Patient Selection
Severity of Glaucoma Disease
Patients with mild to moderate glaucomatous damage, and preoperative IOP in the 18 to 30 mm Hg range on multiple glaucoma medications, are good candidates for ECP.
Target IOP should be in the mid-teen range, which is usually acceptable for patients with mild to moderate disease, but may not be adequate for those with advanced optic nerve damage.
ECP can be performed at the same time as or after newer angle-based glaucoma surgeries, such as canaloplasty (iScience, Menlo Park, CA), Trabectome (trabeculotomy internal approach, Neomedix, Tustin, CA), or iStent (canalicular bypass stent, Glaukos, Laguna Hills, CA).
When a target IOP is in the low teens and external filtration surgery is to be avoided, combining an internal filtering procedure and aqueous reduction procedure may achieve this goal.
Patients with advanced glaucomatous damage that have failed 1 or more external filtration surgeries are also candidates for ECP.
- These patients typically have a failed trabeculectomy, 1 or more aqueous tube shunts already in place, and are on maximally tolerated medical therapy.
- ECP can be performed to lower aqueous production, working in concert with prior outflow procedures to maximally lower IOP.
- In these cases, the pars plana approach (ECP plus) can be used.
Types of Glaucoma
Glaucoma patients with aphakia or pseudophakia are candidates for ECP. Phakic patients can also be treated with ECP, but use caution not to touch or violate the lens capsule.
Symptomatic cataract patients with uncontrolled glaucoma or controlled glaucoma with a desire to reduce medications are candidates for combined phacoemulsification cataract extraction and ECP (phaco-ECP). The potential population encompasses patients with primary or secondary, open or closed angle glaucoma.
Patients usually present with one of the following characteristics and are candidates for either ECP or phaco-ECP:
- ECP alone:
- Pseudophakic/aphakic patients with uncontrolled glaucoma
- Patients post-tube or filtration surgery
- Patients with severe conjunctival scarring (eg, post scleral-buckle, chemical burns) and failed filtration surgeries
- Patients who would otherwise be nonoptimal candidates for filtration surgeries, due to chronic ocular surface disease (eg, ocular rosacea, pemphigoid, refractory blepharitis, severe dry eyes) or high risk for complications (eg, aphakic eyes, vitrectomized eyes, history of suprachoroidal hemorrhage, history of trabeculectomy complications)
- Combined phacoemulsification and ECP:
- Cataract and uncontrolled glaucoma
- Cataract and controlled glaucoma, but with a desire to reduce medication use due to compliance, cost, or side effects
- Cataract and narrow angle glaucoma, especially plateau iris
ECP In Different Settings
Potential benefits of combined phaco-ECP include:
- Further reduction of IOP, while not violating the conjunctiva and sclera
- Management of patients with optic disc damage who would otherwise not be good candidates for filtration surgery due to advanced age, ocular surface disease or conjunctival scarring, or likelihood of complications related to hypotony
- Possibly increasing patient compliance by reducing the need for postoperative glaucoma medications
- Offering the patient an alternative procedure to standard filtration surgery, especially if there is a history of complications from filtration surgery
- Ability to avoid problems associated with discontinuing anticoagulants in high risk cardiovascular and stroke patients
Eyes with Narrow Angles or Shallow Anterior Chambers
Patients with short axial lengths and narrow angles pose special intraoperative and postoperative hazards. The trend is moving away from external filtration surgery due to the increased likelihood of aqueous misdirection (malignant glaucoma), choroidal effusions, and shallow anterior chambers.
Instead of filtration surgery in these eyes, phaco-ECP affords several potential benefits.
- The lens extraction helps to deepen the anterior chamber and reduce IOP by increasing outflow.
- ECP lowers IOP by reducing aqueous production.
- This procedure can also be employed in patients with chronic synechial angle closure, in whom angle-based procedures or aqueous tube shunts may be difficult.
- Recent evidence demonstrates that a variation of ECP helps open the angle in patients with plateau iris syndrome by shrinking the ciliary body and its processes away from the posterior iris and angle structures.
In Aphakic Eyes
Glaucoma in aphakic eyes can be refractory to traditional management, with a high rate of choroidal hemorrhage that may be exacerbated from prior vitrectomy.
ECP by an anterior approach with an anterior chamber maintainer, or by pars plana approach with infusion is a safer method of IOP reduction for these eyes.
- The procedure can be combined with a first stage aqueous tube shunt.
- Several weeks after surgery, after encapsulation of the plate, the tube may be inserted for better IOP control, negating the likelihood of hypotony associated with sudden opening of the ligature.
In Pseudophakic Eyes
Cataract surgery typically involves placement of a posterior chamber intraocular lens (PCIOL) in the capsular bag with a well-preserved posterior chamber. Excellent visibility of the ciliary body processes is achieved in these pseudophakic eyes, enabling thorough treatment.
- In eyes with advanced pseudoexfoliation, the white pseudoexfoliative material coating the zonules and ciliary processes creates difficulty in the uptake for the laser.
- Patients with advanced pseudoexfoliation glaucoma are not optimal candidates for ECP.
Uncontrolled Glaucoma in Eyes After Failed Drainage Implant or Filtration Surgery
Eyes with prior trabeculectomy and/or drainage implants are good candidates for ECP.
- In most cases, the conjunctiva is badly scarred and ECP is a good option to further lower IOP by reducing aqueous formation.
- This procedure can be performed either as a stand-alone surgery, or combined with cataract extraction via an anterior limbal or pars plana approach.
Eyes at High Risk for Complications Following Filtration Surgery
A distinct patient group are at high risk for complications after filtration surgery, and ECP offers a potential route to avoid complications.
Patients fitting the following descriptions may benefit from ECP to lower IOP:
- High myopia
- Very long or short axial length
- Prior vitrectomy
- Natural bleeding diatheses
- Using anticoagulants to prevent systemic thromboembolic disease
- Narrow-angle eyes prone to aqueous misdirection
- Severe conjunctival scarring after scleral buckling procedures
If the IOP is still not controlled, other procedures have not been precluded. Long-term disruption of the blood aqueous barrier is not present post-ECP and should not affect the efficacy of other types of future glaucoma procedures.
Contraindications and Cautions
Patients with advanced pseudoexfoliation are not good candidates for ECP due to the white fibrillar material coating the ciliary processes that limits laser energy uptake. Patients with uveitis or neovascular glaucoma must be approached carefully, as they have propensity for severe postoperative inflammation or hypotony.
- A less aggressive approach is recommended for these patients, treating for 270 or less instead of 360 degrees, and reducing the energy applied.
- Aggressive perioperative and postoperative treatment to reduce inflammation can be utilized, such as intraocular preservative free dexamethasone, intravenous steroids at the time of surgery, and oral prednisone taper after surgery.
Phakic patients can be treated if the anterior chamber is deep, but special care must be taken to avoid lens trauma.
- The pars plana approach cannot be employed in a phakic patient.
- Patients with severe anterior segment scarring are usually best treated with a pars plana approach.
Complications and Adverse Events
Inflammation is commonly seen following ECP and may range from mild anterior chamber cell to severe inflammation and fibrin formation.
- The amount seen is likely dependent on the amount of energy used and the glaucoma diagnosis and ocular comorbidities.
- Inflammation may also lead to transient hypotony from ciliary body shut down, cystoid macular edema, or corneal transplant graft rejection episode in patients with a prior penetrating keratoplasty.
Other rare complications can occur as in any intraocular surgery, such as infection, bleeding, retinal detachment, and cataract formation.
Surgical Techniques of ECP
Basic Considerations
Anesthesia for ECP can range from intracameral infusion to general anesthesia, depending on the particular patient requirements.
- The ciliary processes are sensitive to manipulation or treatment, and therefore topical anesthesia is not adequate.
- Intracameral injection of preservative-free lidocaine is popular, especially when combined with phacoemulsification cataract extraction. If more aggressive treatment is performed, peribulbar, sub-Tenon’s, or retrobulbar anesthesia with lidocaine and bupivicaine is preferred.
- Surgeons will typically sit at the superior or temporal position for the best surgical approach.
- Settings on the console should be a power of 0.25 to 0.4 watts, with a continuous (surgeon-controlled) ablation time, and an aiming beam setting of 20-40.
- The intensity of the light source is varied depending on how far away the endoscope is from the target tissue.
Anterior approach
- The incision is made adjacent to the limbus in clear cornea with a width of approximately 2 mm. The incision should be large enough to facilitate entry of the probe without significant resistance. A water-tight fit is not necessary, as the eye is typically filled with viscoelastic.
- The anterior chamber is filled with viscoelastic but not overfilled. Some viscoelastic products are not compatible with ECP as they form bubbles when the laser is activated. The authors recommend a cohesive viscoelastic such as sodium hyaluronate (Healon, Abbott Medical Optics, Santa Ana, CA), as it is does not form bubbles, and is easily removed at the end of the procedure.
- The ciliary sulcus space is then expanded with viscoelastic to create space between the lens and the iris. The thicker form of hyaluronate (Healon GV, Abbott Medical Optics, Santa Ana, CA) is preferred by some surgeons because of its ability to maintain the ciliary sulcus space more readily.
- In an aphakic and vitrectomized eye, an anterior chamber maintainer with balanced salt solution keeps the eye pressurized.
- The endoscope tip is wiped clean and the view is focused and rotated by adjusting the camera probe where it enters into the laser console. Any blood at the incision must be removed, as it may adhere to the camera and obscure the view.
- The tip is introduced into the anterior chamber and advanced until it reaches the pupillary margin.
- At this point, the surgeon looks away from the microscope and towards the monitor and orients toward the position within the eye.
- The probe is advanced towards the ciliary sulcus until approximately 6 to 8 ciliary processes are visible on the screen. This gives distance gives the optimal laser energy for tissue absorption.
- The aiming beam is centered over a process, and the laser is activated by depressing the foot pedal.
- The duration and location of treatment is determined by visual feedback of tissue reaction. The ciliary process will shrink and whiten when treated properly. Treat the entire visible ciliary process from anterior to posterior. The amount of energy delivered is determined by the duration of the treatment and also the proximity to the target tissue. To avoid over-treating, the power or amount of ablation time in one area should be decreased when the processes are closer to the endoscope.
- The aiming beam is then centered on the adjacent process and so on until the probe has reached its full range. At this point, re-treating as the probe is rotated back to the entry position is recommended, to ensure adequate energy absorption. This enables the surgeon to treat the areas of ciliary epithelium in between the processes.
- When the midpoint is again reached, a curved probe must be rotated 180 degrees, along with the camera, and the process is repeated in the other direction.
- Once 180 degrees has been treated, the probe is withdrawn and cleaned, and another incision is made to allow treatment of the remaining processes.
- At the end of treatment, care must be taken to fully remove viscoelastic either by automated or manual irrigation and aspiration, or by flushing the anterior chamber and sulcus with balanced salt solution while distorting the corneal incision to allow it to exit. If not adequately removed, retained viscoelastic is the primary cause of a post-operative IOP spike.
Combined with cataract extraction
ECP combined with phacoemulsification cataract surgery is one of the most common indications for the procedure. The steps are essentially the same as that described in the anterior approach, with a few modifications.
- The cataract extraction is performed first, and the viscoelastic is removed from the capsular bag with irrigation / aspiration following intraocular lens implantation.
- The anterior chamber and ciliary sulcus are expanded with viscoelastic and the procedure follows as above.
- Typically one can treat up to 270 degrees of ciliary processes from the temporal incision.
- To treat the remaining processes, the paracentesis can be pre-placed 90-degrees from the temporal incision and then enlarged to accommodate the ECP probe.
Pars Plana approach and “ECP plus”
For more aggressive treatment, the pars plana approach can be used.
- This approach is useful in eyes with anterior chamber pathology that precludes a clear pathway for the endoscope probe.
- It is appropriate in pseudophakic or aphakic patients but cannot be employed in phakic patients.
- This approach allows the best view of the ciliary processes and facilitates a more complete treatment.
- A conjunctival peritomy is made nasally and temporally, and a sclerotomy is made approximately 3 mm posterior to the limbus with a 20-gauge MVR blade.
The procedure can be performed with a standard 3-port or 2-port vitrectomy technique.
- A full or limited posterior vitrectomy is performed prior to the ECP procedure.
- In the 2-port approach, the infusion is placed as an anterior chamber maintainer, or in one sclerotomy and the ECP probe in the other. The vitrectomy can be performed under endoscopic guidance.
- In the 3-port approach, the infusion is left in the inferior sclerotomy and one port is plugged or used to hold the vitrector.
The ECP probe is placed through one of the sclerotomies and advanced across the globe until the ciliary processes are visible. They are then treated in the same fashion as in the anterior approach. The posterior approach allows the surgeon to treat the entire ciliary process, thus one must titrate the degrees treated to avoid hypotony, especially in neovascular and uveitic glaucoma.
In recalcitrant glaucoma, the ECP plus technique can be employed, in which the entire circumference of ciliary processes are treated, as well as 1 to 2 mm of laser extending into the pars plana. This treatment can result in profound IOP reductions and should be used with care to avoid hypotony.
Endoscopic Cilioplasty (ECPL) for Plateau Iris Syndrome
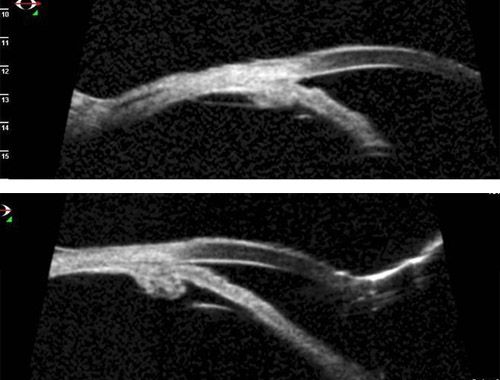
Figure 2. Plateau iris syndrome is characterized by a narrow peripheral anterior chamber angle caused by large and anteriorly rotated ciliary processes.
- In a minority of cases, peripheral iridotomy and laser iridoplasty are inadequate to open the angle.
- Cataract extraction with IOL can be combined with endoscopic laser treatment to change the shape and size of the ciliary processes and thus reconfigure the angle anatomy.
- In this procedure, the ciliary processes are treated in a similar fashion to ECP, but with less laser energy and no treatment of the spaces in between (unless IOP reduction is also desired).
- The processes are typically treated from posterior first, in order to cause the anterior portion of the processes to shrink posteriorly, away from the iris.
- Ultrasound biomicroscopy data presented by Ahmed et al show that the angle is opened widely after lens extraction and ECPL, but that the angle is still narrow in the quadrant untreated with ECPL in the same eye.
ECP with Opaque Media
An advantage of ECP is that it can be used to treat patients with anterior segment pathology that precludes the use of external filtering surgery due to severe conjunctival scarring.
- This presents challenges because of the coexistence of media opacity, which can affect the anterior approach.
- To inflate the ciliary sulcus, the surgeon should first inject viscoelastic into the anterior chamber and then inject it into the ciliary sulcus under endoscopic guidance.
- Once this space is expanded, the remainder of the procedure is performed with the endoscopic view.
Surgical Pearls
Scleral depression can be used to facilitate treatment of the ciliary processes via an anterior or posterior approach. This maneuver will splay out the processes and allow more complete treatment of the processes and the areas in between.
- Steroid use is helpful to prevent the most common complication of ECP: inflammation. To prevent significant inflammation, intensive topical steroids, subconjunctival injection, intracameral injection of preservative-free steroid, postoperative oral steroids, or perioperative intravenous steroids can be used alone or in combination.
- Steroid use can sometimes mask IOP lowering due to steroid response. Once inflammation is controlled, tapering of the steroid and reevaluation of IOP, if the desired lowering has not yet been achieved, is recommended.
- Significant anterior segment pathology may be encountered that makes ECP challenging. If severe, consider the pars plana approach.
- Anterior and posterior synechiae can generally be severed to enable access to the ciliary sulcus.
- In some cases, residual lens material or posterior iris synechiae are encountered. These can be removed if necessary, but often can be circumvented by manipulation of the probe. This generally results in the probe tip being in close proximity to the ciliary processes, and thus requires adjustment of the power.
Clinical Results of ECP: Efficacy and Safety
ECP can be used as a stand-alone procedure as an alternative or as an adjunct to standard external filtration surgery. ECP has demonstrated its clinical efficacy and safety in several studies, even for severe refractory and pediatric glaucoma.
In a series of 10 patients with neovascular glaucoma, Uram reported his success with the pars plana approach.
- Between 90 and 180 degrees of ciliary processes were treated.
- After almost 9 months of follow up, 9 out of 10 eyes were able to achieve IOP < 21 mmHg, despite a mean preoperative IOP of 43.6 mmHg.
- Uram also reported a case series of 10 patients where 180 degrees of ECP through a limbal incision was combined with phacoemulsification.
- After 19.2 months, the mean preoperative IOP of 31.4 mmHg had decreased to a mean of 13.5 mmHg, and all patients experienced a reduction in the number of glaucoma medications.
Chen et al described a retrospective case series of 68 ECP procedures in refractory glaucoma cases, including primary open angle, congenital, chronic angle closure, uveitic, exfoliative, neovascular, and angle recession glaucoma.
- The procedure was performed both with and without phacoemulsification surgery, through either a limbal or pars plana incision, in pediatric and adult populations.
- All patients had failed maximal medical therapy and prior filtration surgery, and some had failed prior transscleral cyclophotocoagulation.
- After a mean follow up of over one 1 year, IOP decreased from 27.7±10.3 mmHg to 17.0±6.7, with a reduction in glaucoma medications from 3.0±1.3 to 2.0±1.3.
- At 1 year, 94% of patients achieved IOP ≤ 21 mmHg, with 82% at 2 years, without any incidences of hypotony or phthisis bulbi.
In a retrospective study, Kahook et al compared the results between 15 patients treated with one-site corneal incision ECP (240-300 degrees) and 25 patients treated with two-site corneal incision ECP (360 degrees).
- Both procedures were combined with phacoemulsification and intraocular lens implant surgery.
- After 6 months of follow up, postoperative mean IOP was significantly lower in the group treated with two-site ECP (mean 13 mmHg) vs. the group treated with one-site ECP (mean 16 mmHg), without any higher incidence of complications related to the procedure.
- This indicates that it may be more effective to treat as much of the ciliary body as possible.
Gayton et al compared combined cataract surgery with ECP to cataract surgery with trabeculectomy in a randomized trial in which 58 patients were randomized to each procedure and the primary end point was at 6 months.
- Cataract surgery with ECP performed through a limbal cataract incision produced significantly less postoperative inflammation than cataract surgery combined with trabeculectomy.
- In patients followed for at least 6 months after treatment, 32% treated with ECP had IOP controlled (less than or equal to 21 mmHg) without medication and 45% with medications, as compared to 54% of patients treated with trabeculectomy without medications and 18% with medications.
- The IOP reduction was similar between the 2 groups: 24.6±6.2 at baseline with a reduction of 8.6±8.2 mmHg for trabeculectomy, and 24.8±8.6 at baseline with a reduction of 8.8±9.6 for ECP.
Lima et al studied the success of ECP when compared to the Ahmed drainage implant in refractory glaucoma patients.
- In this study, 68 Brazilian pseudophakic patients who had failed trabeculectomy with antimetabolite underwent either ECP or Ahmed drainage implant in alternating allocation.
- Patients were followed for an average of 20 months (range 2-24 months).
- Both procedures were effective and similar in lowering the IOP, though the Ahmed drainage implant resulted in statistically lower IOP in the first week after surgery.
- The mean preoperative IOP was quite high in both groups (41.2 mmHg Ahmed, 41.6 ECP), and was similar at the 2-year follow-up (14.7 and 14.1 respectively).
- The group that underwent Ahmed drainage implant surgery had a higher rate of decrease in visual acuity and early postoperative hypotony, requiring additional postoperative visits.
In cases of pediatric glaucoma, ECP has also been shown to be moderately effective. Neely and Plager reported a study of 36 pediatric patient eyes treated with single-incision limbal ECP over 6 years, with a minimum of 6 months of follow up.
- Ages ranged from 0.4 to 15 years, with a mean of 4.9 years, and the study included both phakic and aphakic eyes.
- 34% were successfully controlled after the procedure, with a mean follow up of 10 months.
- 66% were not successfully controlled after ECP and required further treatment.
- In 9 eyes on which repeat ECP was performed, 3 eyes demonstrated a favorable response, whereas 6 eyes did not receive further benefit from the procedure and required additional procedures, such as aqueous tube shunts, trabeculectomy, or cryotherapy.
In the pediatric population where anatomy is likely to be abnormal, Barkana et al demonstrated an advantage of ECP. In this case report, prior transscleral cyclophotocoagulation was unsuccessful, and at the time of endoscopic cyclophotocoagulation, it was discovered that the laser burns had been placed in the pars plana.
Similarly, Al-Haddad et al reported a case series of 12 eyes of 11 pediatric patients with corneal opacities (due to Peters anomaly, anterior segment dysgenesis, corneal scar, or micophthalmos) who were treated with ECP.
- Ten eyes had previously received transscleral cyclophotocoagulation and/or cyclocryotherapy, and 6 eyes had previous penetrating keratoplasties.
- Only 2 eyes (17%) had successful IOP control after the first ECP procedure, with a mean time to failure of 7.8 months.
ECP can also be performed after outflow procedures. Francis et al reported a prospective case series of 25 eyes of 25 patients with uncontrolled IOP after prior Baerveldt Glaucoma Implant 350 (AMO, Santa Ana, CA) aqueous tube shunt.
- The follow up was 6 months to 2 years, with the main outcome measures of IOP and medication reduction at 1 year.
- Success was defined as a reduction of IOP of 3 mmHg or greater or a discontinuation of oral carbonic anhydrase inhibitor medications, with IOP less than or equal to 21.
- Failure was defined as uncontrolled IOP, increase in glaucoma medications, loss of light perception, or additional glaucoma surgery.
- At 1 year, the mean IOP decreased from 24.0 to 15.4 mmHg, and medications from 3.2 to 1.5, with a success rate of 88%.
Summary
Endoscopic cyclophotocoagulation is a useful tool for glaucoma surgeons.
- It can be combined with a standard phacoemulsification and intraocular lens implant surgery.
- It has been shown to be comparable to other filtering or shunting procedures in control of IOP, and perhaps with fewer complications.
- It can be used to effectively treat refractory glaucomas, which have failed other forms of treatment.
- As an aqueous suppressant surgery, it can be added to outflow procedures.
- In pediatric patients, outcomes have been less favorable. This may be due to the degree of abnormality of the eye structures often present in congenital glaucomas, especially those associated with other anterior segment pathologies, or the increased ability for tissue regeneration in children.
Ophthalmic Endoscopy: Anterior Segment Surgery
The camera portion of the ophthalmic endoscope allows an excellent view of the anterior and posterior chambers of the eye as well as the anterior vitreous. It can be used to assist in a intraocular procedures that require a detailed view of the interior of the eye or have dense opacities blocking the view.
Identification and Treatment of Cyclodialysis Clefts
 Figure 3.
Figure 3. Cyclodialysis cleft, a disruption of the ciliary body muscle insertion from the scleral spur, may be due to blunt trauma or iatrogenic outcome from intraocular surgery.
- Diagnosis and repair of cyclodialysis cleft is critical to treat persistent hypotony and its sequelae.
- The operating microscope with a goniosurgical lens may provide a limited view in the shallow anterior chamber.
- Accurate appraisal of the size of the cleft determines management.
- If the cleft is larger than 2 clock hours, the endoscopic camera can aid in identifying the exact location and size of the cleft for surgical intervention by direct cyclopexy through a scleral window, or by passing the suture across the anterior chamber.
- If the cleft is less than 2 clock hours, it may be identified and closed with endoscopic laser to the cleft site.
Caronia et al have reported a novel technique employing an endoscopic laser to successfully visualize and treat a 2.5-hour cyclodialysis cleft in an 8 year-old patient with refractory hypotony following trabeculectomy and trabeculotomy procedures.
- The endoscope was used to identify the cleft and to apply laser to both the internal and external ciliary body surfaces within the depths of the cleft.
- Normalization of the IOP was noted 3 weeks after surgery.
Goniosynechialysis
 Figure 4.
Figure 4. Patients with angle closure glaucoma may benefit from goniosynechialysis (GSL), which removes the peripheral anterior synechiae (PAS) from the outflow structures. A two-handed technique involving use of the endoscope to visualize the chamber angle while using a goniosynechialysis spatula or forceps to peel the iris from the meshwork is a sophisticated method of reopening the chamber angle.
Fang et al have reported a novel technique of endoscopic GSL, which enabled better visualization of the angle without needing to reposition the patient’s head and use bridal sutures.
- They studied 12 eyes of 12 patients with synechial angle closure due to either acute angle-closure glaucoma with PAS or flat anterior chamber post-trabeculectomy.
- The procedure utilized the Endognost (Vitroptik Flex, Polydiagnost, Germany), which has an endoscope and light source but no laser.
- Viscoelastic was used to break the PAS in most cases, with some requiring blunt dissection with an iris repositor instrument.
- The synechiae were treated for their total extent, some for 360 degrees.
- A reduction of mean IOP from 42.9 ± 15.8 mmHg to 12.7 ±3.5 mmHg was reported.
Goniotomy in Pediatric Glaucoma
Bayrakar and Koseoglu described 12 eyes of 7 patients with congenital glaucoma treated with endoscopically guided goniotomy, using the Endooptiks system. A 20-gauge MVR blade incision was made in clear cornea, and an anterior chamber maintainer inserted. Two techniques are described: the 2-port and 3-port approaches.
 Figure 5.
Figure 5. Goniotomy blade.
- In the 2-port technique, a specially designed goniotomy blade (Endooptiks, Inc, Little Silver, NJ) is attached to the endoscope probe and advanced across the anterior chamber.
- The blade is then used under endoscopic guidance to perform a superficial cut to the trabecular meshwork until a whitish band is clearly seen by the endoscope.
- In the 3-port technique, another corneal incision is made, and a 20-gauge MVR blade is used to make the goniotomy incision while viewing through the endoscope.
- The inferior and superior angle is treated for at least 240 degrees.
- At a mean follow-up period of 14.2 ± 9.7 months, the mean IOP was decreased from 38.3 ± 6.9 mmHg to 17.6 ± 2.8, with a reduction in glaucoma medications from 2.1 ± 0.3 to 0.3 ± 0.5.
- Complete success was defined as IOP <17 mmHg without medications, qualified success as IOP between 18 and 21 without medications, and failure as IOP > 22 mmHg without medications.
- Using these criteria, there were 7 complete successes, 3 qualified successes, and 2 failures.
Kulkami et al reported a case series of 14 eyes of 8 patients with primary, congenital, or developmental glaucomas with opaque corneas treated with endosocopic goniotomy.
- The IOP was reduced 16.7± 16.7 mmHg from baseline, with a 43% success rate overall and 50% success rate of primary congenital glaucoma cases.
- Success was defined as IOP ≤ 21 mmHg with or without medications and no further surgical interventions.
Joos and Shen presented a case of endoscopic goniotomy in a 19-month old patient with congenital glaucoma with a poor view due to corneal haze.
- The procedure employed the same coaxial technique using the endoscope probe with goniotomy blade attachment.
- The IOP did not lower significantly after the procedure, but allowed clearing of the cornea for standard goniotomy.
Goniopuncture or Trabeculotomy in Adult Open Angle Glaucoma
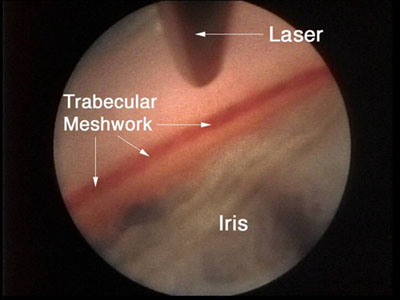 Figure 6.
Figure 6. Endoscopic aprproach for open-angle glaucoma.
Goniopuncture performed with the erbium:YAG laser via an endoscopic approach and combined with cataract extraction was evaluated by Feltgen et al.
- The procedure was performed with an endoscope erbium:YAG laser system (Sklerotom 2.9, Endognost, Schwing, Germany).
- The trabecular meshwork was treated for 180 degrees with 18 laser pulses.
- They compared the procedure prospectively to retrospective controls with combined cataract and trabeculectomy.
- In 24 eyes reaching 1-year follow up, they found a decrease in IOP from 23.4+/-3.7 mmHg to 16.3+/-6 mmHg and a decrease from 22.7+/-3.3 mmHg to 15.1+/-3.8 mmHg in controls.
- The number of glaucoma medications decreased in both groups.
- Postoperative complications were found more frequently in the control group, but postoperative visual acuity was lower in the control group.
Excimer laser trabeculotomy (ELT) is another angle-based glaucoma surgery in which small perforations are made through the trabecular meshwork to allow flow of aqueous into Schlemm’s canal. The procedure can be performed by viewing through a surgical goniolens or an ophthalmic endoscope.
Wilmsmeyer et al presented a prospective case series of 69 patients treated with endoscopic ELT, and 57 with combined ELT and phacoemulsification.
- At 1-year of follow up, IOP was reduced from a baseline of 24.1+/-0.7 mmHg to 18.8+/-0.8 (n= 37), and a success rate of 46% (IOP ≤ 21 mmHg and reduced 20%). 28% of the eyes needed repeat surgery due to insufficient IOP reduction.
- Combined phacoemulsification plus ELT reduced the IOP from 22.4 mmHg+/-0.6 to 16.4+/-0.4 (n= 35) with a success rate of 66%, and only 7% of the eyes requiring repeat surgery due to insufficient IOP reduction.
- The number of glaucoma medications was similar before and after surgery for both groups.
Hypotony Evaluation and Treatment
 Figure 7.
Figure 7. Ophthalmic endoscopic surgery has proven useful in evaluating the ciliary body in eyes with hypotony.
- The ciliary body and processes can be viewed with the endoscope to assess degree of ciliary atrophy, presence of ciliary body detachment or effusion, and presence of tractional circumferential bands of the ciliary body.
- The latter can be dissected under endoscopic viewing to release traction on the ciliary body and allow reattachment and resumption of aqueous production.
Using endoscopic evaluation and surgical dissection of the ciliary body in 14 eyes, Hammer and Grizzard visualized clinical findings associated with ocular hypotony, including “whitecaps,” or white surface changes with brush-like texture in ciliary processes and “traction elongations” of ciliary processes in the majority of the eyes.
- Membrane dissection from the ciliary body resulted in normalization of IOP in 78% of patients in the early postoperative period and 33% at last follow up.
- The return of hypotony may be due to the reformation of fibrous membranes over the ciliary body.
- These findings may provide early structural cues for management and pathophysiology of ocular hypotony.
Tube placement
Aqueous tube shunt placement is usually routine. It is critical for the long-term health of the eye for the tube to be in perfect position between the iris and cornea. It may be difficult to see the position of the tube when the cornea is cloudy and there is no view of the anterior segment anatomy.
- The ophthalmic endoscope may be inserted into the anterior or posterior chambers or the anterior vitreous, in order to assist in the exact positioning of the tube.
- The endoscope is positioned 180 away from the tube site and the 23-gauge needle is easily seen as it enters the anterior segment, verifying where the tube will go.
- The tube is then inserted, and its position verified with the endoscope.
In 19 consecutive eyes with uncontrolled chronic angle-closure glaucoma and corneal opacity or pupil fibrosis, Tarantola et al described combined endoscope-assisted pars plana vitrectomy and glaucoma tube shunt insertion.
- The mean IOP decreased from 31.3 mmHg ± 10.5 on 3.4 ± 1.0 glaucoma medications to 11.4 ± 2.9 mmHg on 1.3 ± 1.2 medications at last follow up.
- Based on the predetermined criteria, 14 of 19 achieved complete or qualified success with IOP £ 21 mmHg, while 5 of 19 were classified as failure due to phthisis, loss of light perception or requirement for additional intervention to control glaucoma.
Verification of IOL Position and Capsular Support
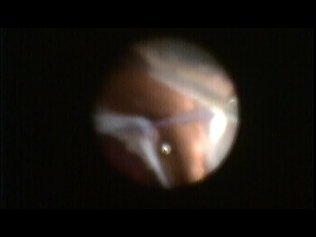 Figure 8.
Figure 8. Endoscopic view of dislocated IOL haptic.
The endoscope can be helpful during cataract surgery.
- It can be used to assess the amount of capsular support remaining to plan IOL placement during a complicated cataract extraction with capsular trauma.
- If unsure if one haptic might be out of the capsular bag, this is instantly identified with the endoscope.
- Endoscopy allows the surgeon to determine if a PCIOL placed in the capsule, a sulcus placed posterior chamber IOL, a scleral or iris suture fixated IOL, or if an anterior chamber IOL is most appropriate.
The endoscope is helpful to better understand anterior segment anatomy in eyes with malpositioned IOLs, especially haptic location.
- Usually the location of a subluxated IOL haptic can be visualized, along with the cause of the lens dislocation.
- It can be used to view the extreme periphery of the capsule, including zonular support, which may not be visible through the pupil.
Secondary IOL Implantation
 Figure 9.
Figure 9. Secondary IOL implantation may be guided by endoscopy. The capsule and zonular support can be assessed thoroughly. Placement of a posterior chamber scleral fixated IOL can be improved with endoscopic guidance.
In a retrospective case series of 74 eyes of 71 patients, Olsen and Pribila described a technique of pars plana vitrectomy and scleral suture fixation of a PCIOL.
- The preoperative diagnoses were trauma (42%), lack of capsular support with IOL subluxation (24%), uveitis (15%), congenital cataract (11%), Marfans or ectopia lentis (6%), and other (2%).
- The endoscope (GRIN solid rod endoscope, Insight Inc, Stuart, Florida) was used to visualize the ab interno suture pass into the ciliary sulcus and out through the sclera, thus avoiding hemorrhages from the ciliary body.
- The advantages of the technique were:
- Excellent visualization and haptic localization
- Optimal lens centration
- Buried knots
- Broad scleral imbrication
- Minimal vitreous hemorrhage complications
- The disadvantages were:
- Learning curve
- Increased operative time
- Long-term suture stability issues
- Long-term land limited endoscope availability
Uveitis Glaucoma Hyphema Syndrome (UGH)
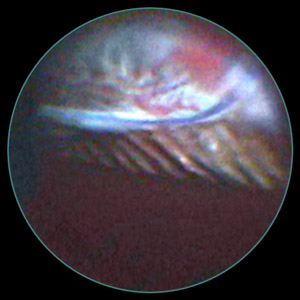 Figure 10.
Figure 10. UGH is a syndrome manifesting as chronic, intermittent, and recurrent episodes of hyphema, ocular inflammation, and often elevation of IOP. It is typically caused by the contact of an intraocular device, such as an IOL or tube, with sensitive uveal tissue, such as the iris or ciliary body.
- The endoscope can be used to search for the cause, and if abnormal blood vessels are seen, to photocoagulate them.
- In the example shown in the figure, a patient with recurrent uveitis, hyphema, and secondary glaucoma was examined intraoperatively with the endoscope.
- The IOL was not completely within the capsular bag, with one haptic in the sulcus.
- The endoscope enabled viewing of this malpositioned haptic, evidence of trauma to the posterior iris anterior to the haptic, and pigmented uveal tissue on the haptic surface.
- The IOL was left in place, but the haptic was amputated to prevent any further iris trauma.
Conclusion
Ciliary body ablation procedures remain an effective tool in glaucoma management. Methods of laser cycloablation include the transscleral route, which includes contact and noncontact approaches using either the Nd: YAG or semiconductor diode lasers, and the endoscopic route using the diode laser.
- While cycloablative surgeries were traditionally used for refractory glaucoma or as a surgery of ‘last resort,’ recent trends and technological advancements in endoscopic surgery have enabled it to be used earlier in the surgical management of glaucoma.
- ECP is an effective procedure with a favorable side effect profile compared to glaucoma filtration surgery.
- Further research is required to define better the long-term complications and outcomes of ECP, beyond the traditionally studied 1 to 2 year postoperative period.
- A comparative study between ECP and other surgical modalities in treating glaucoma will be edifying in guiding surgical management of glaucoma.