AAO Quality of Care Secretariat, Hoskins Center for Quality Eye Care
Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy
Retinal toxicity from chloroquine (CQ) and its analogue, hydroxychloroquine (HCQ), has been recognized for many years. Chloroquine toxicity remains a problem in many parts of the world, but is seen less frequently in the United States where the drug largely has been replaced by HCQ. Hydroxychloroquine is used widely for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis, and related inflammatory and dermatologic conditions. It is now being considered for new applications in diabetes mellitus, heart disease, and adjunct cancer therapy. Thus, it is important for ophthalmologists and other physicians to understand the prevalence and risk factors for retinopathy.
The American Academy of Ophthalmology recommendations for screening that were published in 20111 are revised in this article to account for new scientific data. The recent publication of a large demographic study has shown that toxicity is not rare among long-term users of the drug, and the risk is highly dependent on the daily dose by weight.2 These data showed that real weight was better than ideal weight for calculating dose, and lower risk was achieved with doses �5 mg/kg real weight. It also has been found that the classic “bull’s-eye” distribution of toxicity is infrequent in patients of Asian heritage,3,4 who typically show early damage in a more peripheral pattern.
Toxicity is of serious ophthalmologic concern because it is not treatable. Nonetheless, it has been demonstrated that central vision can be preserved if damage is recognized before there are changes in the retinal pigment epithelium (RPE).5 With proper screening, bull’s-eye retinopathy, as classically described with these drugs, no longer should be seen.
The goal of screening for retinopathy is not to stop valuable drugs at the first borderline abnormality, but to recognize definitive signs of toxicity at an early enough stage to prevent a loss of visual acuity. Ophthalmologists provide a valuable service not only by screening but also by advising medical colleagues and patients about risk, safe dosing, and appropriate screening procedures. Despite the existence of published guidelines, screening practices often have been inconsistent or deficient.6,7 The recommendations in this revision are more concise and practical than the prior version, to encourage wider compliance.
Hydroxychloroquine and Chloroquine Toxicity
The mechanism of CQ and HCQ toxicity is not well understood. High experimental doses have acute effects on the metabolism of retinal cells, but it is not clear how these short-term metabolic effects relate to the slow and chronic damage that characterizes the clinical state of toxicity. Binding to melanin in the RPE may serve to concentrate the agents and contribute to, or prolong, their toxic effects.
However, melanin binding also could serve as a mechanism for removing toxic agents from intracellular sites of damage. Inner and outer retina are damaged by CQ exposure in animal studies, but recent work suggests that inner retina is not damaged significantly as human HCQ toxicity develops.8,9 In clinical practice, the primary damage is to the photoreceptors, and as the outer nuclear layer degenerates, there is secondarily disruption of the RPE.10 No anatomic features of the retina and RPE are known to correlate specifically with the parafoveal or extramacular patterns of damage as CQ and HCQ toxicity develops. The macular localization of the disease suggests that light absorption or possibly cone metabolism may play a role, but that is speculation.
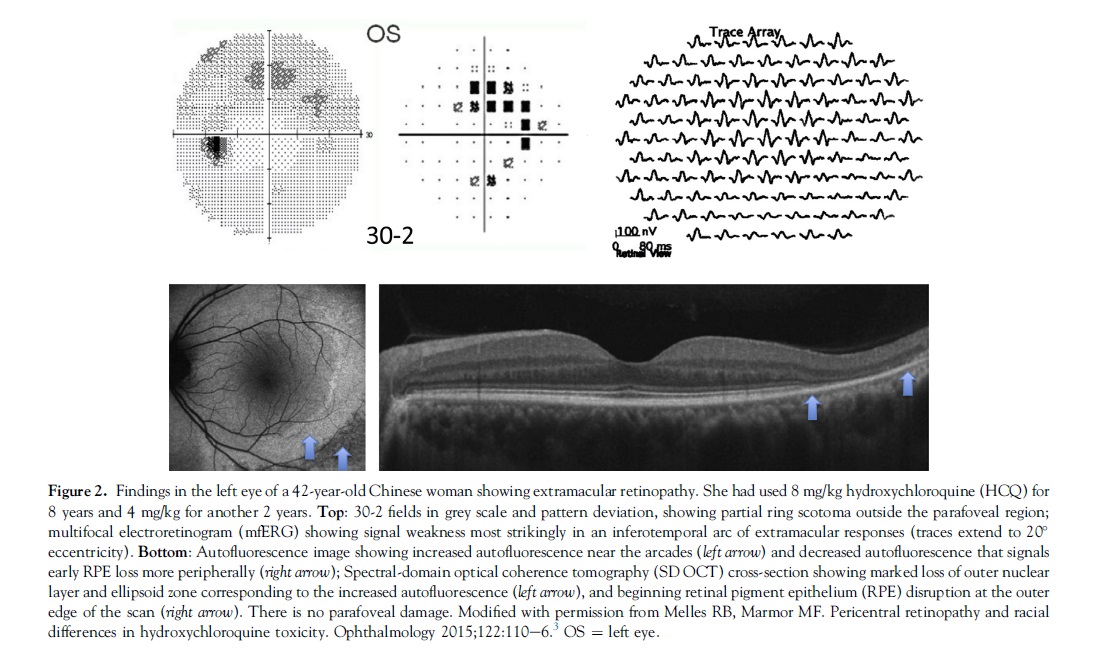
The clinical picture of HCQ and CQ toxicity had been characterized classically as a bilateral bull’s-eye maculopathy, an appearance caused by a ring of parafoveal RPE depigmentation that spares a foveal island. However, this “textbook” pattern should no longer be seen, because recommended screening tests will detect HCQ toxicity long before RPE damage is visible by imaging or fundus examination. Although most patients of European descent show initial photoreceptor damage in the classic parafoveal distribution (Fig 1), most patients of Asian descent will show initial damage in a more peripheral extramacular distribution near the arcades (Fig 2).3,4 African-Americans and Hispanics in that study3 showed predominately a parafoveal pattern of damage as in European subjects, but possibly a greater tendency toward extramacular involvement. The numbers of patients of other races were too small to draw conclusions.
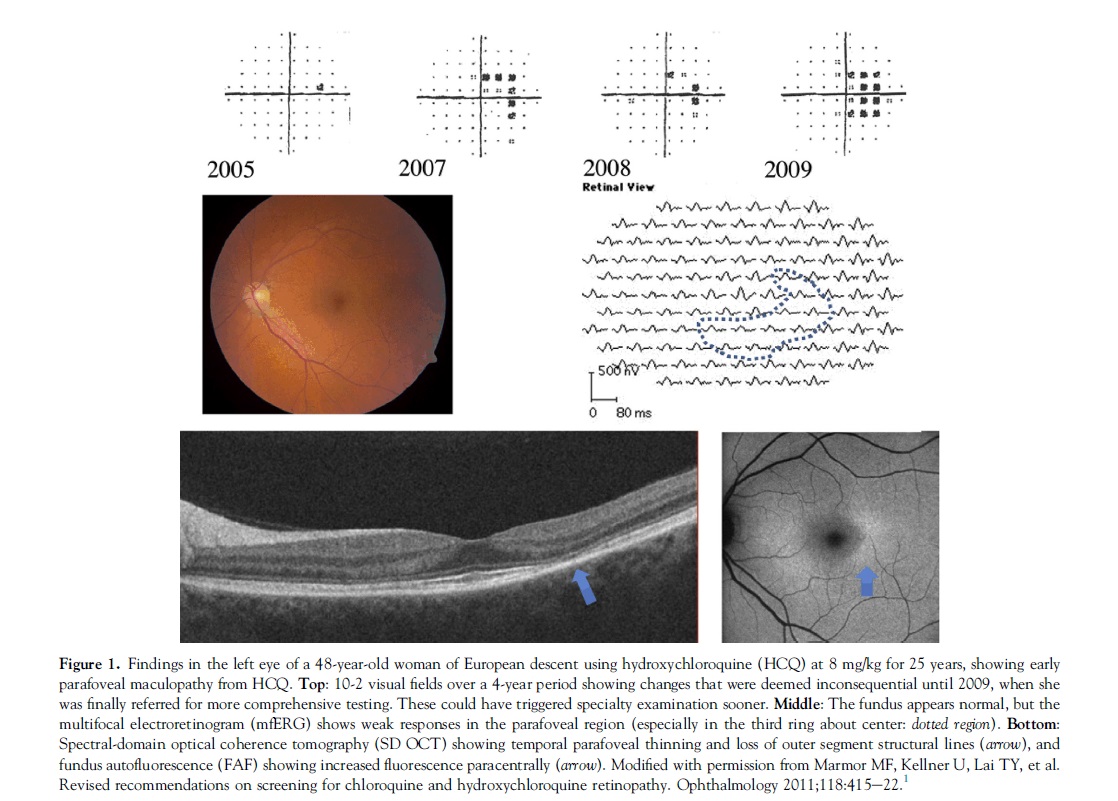
Visual acuity usually is excellent with either pattern until severe stages of damage, and most patients who develop HCQ toxicity have no visual symptoms at all. A few perceptive patients may notice paracentral scotomas while reading. If drug exposure continues, the area of functional disturbance expands, the RPE becomes involved, and the maculopathy can encroach on the foveal center with eventual loss of visual acuity (Fig 3).2,10 Cystoid macular edema sometimes may develop,11 and advanced cases show widespread RPE and retinal atrophy with loss of visual acuity, peripheral vision, and night vision.
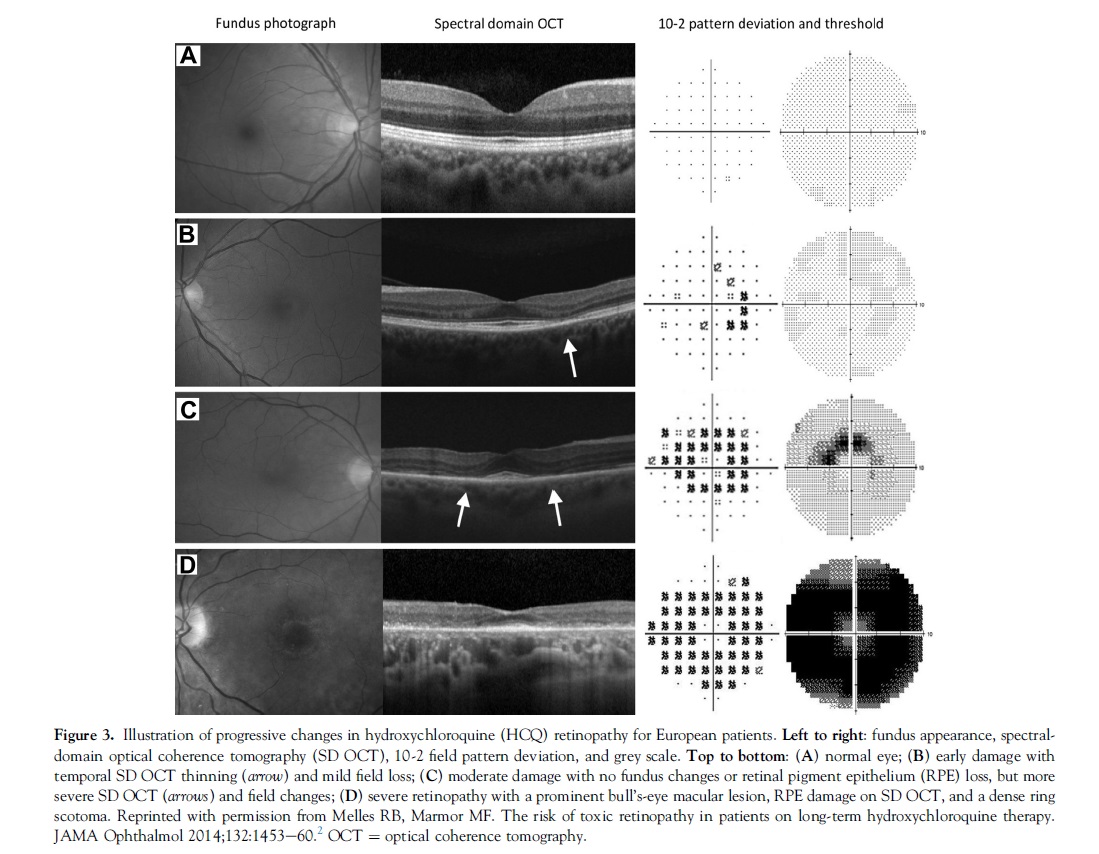
Hydroxychloroquine and CQ retinopathy can progress even after the drugs are stopped, although the amount of progression and the risk to vision are functions of the severity of retinopathy at the time it is detected.5,11 It seems doubtful that this late progression of damage after stopping the drug results from a continued reservoir of the drug, although clearance from the body does take many months.The late progression may represent a gradual decompensation of cells that were injured metabolically during the period of drug exposure.
Chloroquine, and less frequently HCQ, can cause whorllike intraepithelial deposits (verticillata) in the cornea. These corneal changes are not a direct marker for retinal damage, are not associated with visual loss, and in contrast to retinopathy are usually reversible.
Statistical Risk of Toxicity
Earlier literature on the prevalence of CQ or HCQ retinopathy included few patients on long-term therapy and only recognized severe toxicity (bull’s-eye changes). These reports have been superseded now by a large study of 2361 patients who used HCQ for more than 5 years and were evaluated with 10-2 visual fields or spectral-domain optical coherence tomography (SD OCT) so that toxicity could be recognized before there were any visible signs on fundus examination.2 The overall prevalence of toxicity in this study population was 7.5%, although it varied greatly with the daily dose and duration of use. Daily dose (more properly, daily use, as measured by actual pharmacy dispensing) was the most critical determinant of risk, and the risk was more closely correlated with real weight than ideal weight. Very thin patients in particular are at increased risk when dose is calculated by ideal weight (as previously recommended). Patients in this new study2 mostly had been prescribed routine doses of HCQ by prior standards, but the average use was approximately 5.0 mg/kg of real weight because of varying compliance and body habitus. Thus, 5.0 mg of HCQ/kg real weight corresponds with present medical prescription practices and should be therapeutically effective for most patients.
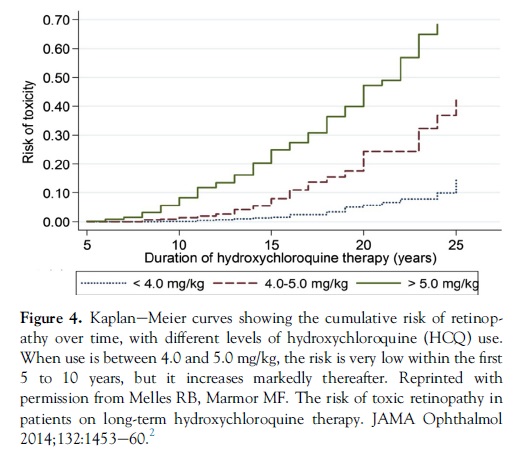
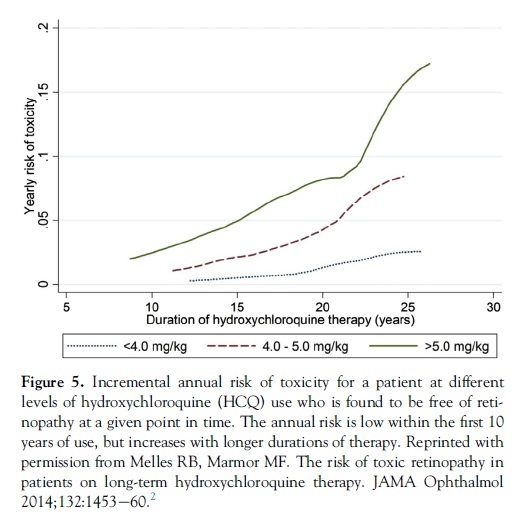
Population statistics from the new study showed that patients taking HCQ using 4.0 to 5.0 mg/kg real weight had markedly lower cumulative risk of toxicity than those using higher levels. Kaplane–Meier curves show that patients staying with �5.0 mg/kg have less than 1% risk in the first 5 years of therapy and less than 2% up to 10 years (Fig 4). Beyond this point, the risk increases sharply to approximately 20% after 20 years. The risk is much higher when the daily dose is higher. Although the risk is smaller with low doses, it is not clear that there is any truly “safe” dosage for long durations of use.
Smoothed response curves (Fig 5) show that the annual incremental risk (for a patient who shows no signs of toxicity) is less than 1% during the first 10 years of therapy if use is �5.0 mg/kg and increases to only approximately 4% after 20 years. Thus, this dosage recommendation is associated with a relatively acceptable risk of toxicity for patients being screened annually.
Dosage Recommendations
On the basis of the risk data described, we recommend that all patients using HCQ keep daily dosage <5.0 mg/kg real weight.2 There may be rare instances when higher doses are needed to manage life-threatening disease or a lower limit is advisable because of major risk factors (described later). Following this guideline will minimize the risk of retinopathy and allow long-term use of HCQ for most patients. Previous recommendations to use ideal body weight for the calculation of dose were based on the idea that these drugs were not retained in fat; however, the available laboratory studies show that these drugs store primarily in melanotic tissue, liver, and kidney, whereas concentrations are low in muscle, fat, and a variety of other organs.12,13 Ideal weight formulas result in overdosage in thin individuals, whereas the recommended formula using real weight accounts for risk evenly over a broad range of body habitus.2
There are no comparable demographic data for CQ use and toxicity. The mechanisms of action are presumed to be similar for both drugs, and older clinical literature on antimalarial toxicity approximately equated 3.0 mg of CQ with 6.5 mg of HCQ.14 With this estimation, the equivalent of 5.0 mg/kg HCQ would be 2.3 mg/kg CQ. Many reports suggest that CQ is somewhat more toxic than HCQ, but there are no good data on pharmacologic equivalence. The higher toxicity of CQ in clinical use may be an artifact of common prescription practices, which have been biased by the available CQ tablet size (250 mg). Almost any patient taking 1 tablet of CQ will receive more than 2.3 mg/kg.
Because HCQ is available in 200 mg tablets and CQ is available in 250 mg tablets, it may seem a challenge to prescribe intermediate doses. However, blood levels of these drugs only stabilize over many weeks, so that variable dosing will average out over time. Intermediate doses can be obtained easily by splitting tablets or by simply eliminating a tablet on certain days of the week. In theory, blood levels would seem an aid to dosing or to the evaluation of poor clearance of these drugs (see “Renal Disease”). However, literature on the measurement of HCQ blood level has shown it to be an unreliable indicator of medical effectiveness or toxicity.15–17 Hydroxychloroquine is metabolized by cytochrome P450 enzymes, which can be affected by a variety of drugs, and some of the variability in blood levels may relate to these metabolic pathways.18,19
Risk Factors for Toxicity
Major Factors
The most important risk factors are listed in Table 1.

Daily Dose and Duration of Use. The most critical risk factor for the development of HCQ toxicity is excessive daily dose by weight.2 Dosage >5.0 mg/kg dramatically increases both population risk and annual incremental risk, and extreme doses can be exceedingly dangerous. Two recent reports on patients receiving HCQ at 800 to 1000 mg/day (up to 20 mg/kg) for nonrheumatoid diseases showed a 25% to 40% incidence of retinopathy and signs of damage within 1 to 2 years.20,21
Duration of use is linked to dosage as a critical factor. Even patients using a recommended dose have significant risk after decades of use. Earlier literature had suggested that “cumulative dose” (which combines daily dose and duration) might be a simple indicator of risk,1 but this does not hold up well.2 Risk is most accurately assessed on the basis of duration of use relative to daily dose/weight, as charted in Figures 4 and 5.
Renal Disease. Hydroxychloroquine and CQ are cleared to a large degree by the kidney, so that renal disease effectively increases the circulating level of the drug and the risk of toxicity.2,22 Renal disease is not uncommon in SLE and related diseases, so that careful inquiry is important. Patients with renal disease can have unpredictably high blood drug levels, and both dosage and screening frequency may need to be adjusted.
Tamoxifen Use. An unexpected finding of the recent large study on HCQ use was that concomitant tamoxifen (a drug used for long-term treatment of breast cancer) increased the risk of toxicity approximately 5-fold.2 The reasons are unclear, although tamoxifen is a retinal toxin in its own right, and there may be adverse metabolic synergy. Newer estrogen analogs such as anastrozole have not shown an association with HCQ toxicity to date, but the number of patients studied has been limited. Patients taking tamoxifen need careful dosing and screening.
Retinal and Macular Disease. Patients with underlying retinal disease may be at higher risk for toxicity, although there are no specific data to confirm this. It seems reasonable not to add a potentially toxic agent to the retina on top of a retinal dystrophy or significant degeneration. The other major concern with maculopathy is that it may cause test abnormalities that interfere with the interpretation of screening procedures (visual fields, SD OCT, fundus autofluorescence [FAF], multifocal electroretinogram [mfERG]). Thus, significant central photoreceptor loss would be a contraindication, whereas isolated drusen (that leave good visual fields and intact photoreceptor structure) should not interfere with screening.
Lesser Factors
Age. Elderly patients might seem to be at higher risk, given that aged tissue could be less resistant to the toxic effects of a medication. However, the recent demographic study found no significant association between age and risk of toxicity.2
Liver Disease. The liver participates in the metabolism of these agents, but no clear association between liver disease and toxicity has been demonstrated.
Genetic Factors. There have been suggestions that some patients have a genetic predisposition to HCQ toxicity (e.g., from abnormalities in the ABCA4 gene),23 but a new report suggests that some nonpathogenic ABCA4 polymorphisms actually may be protective.24 Polymorphisms in the cytochrome P450 gene might influence blood concentration.18,19 Genetic factors may underlie the difference in disease presentation between European and Asian eyes.
Rationale for Screening
Hydroxychloroquine and CQ retinopathy are not reversible, and cellular damage may progress even after the drugs are stopped. When retinopathy is not recognized until a bull’seye appears, the disease can progress for years, often with foveal thinning and an eventual loss of visual acuity. However, when retinopathy is recognized early, before RPE damage, there is only mild and limited progression after discontinuing the medication, and the fovea is not threatened.5 Thus, screening may not “prevent” damage, but if conducted properly it enables the detection of toxicity before vision is significantly affected.
It is important to emphasize that HCQ and CQ are useful drugs and that they have fewer systemic side effects than many of the alternative medications used for immune or inflammatory diseases. Thus, screening can be viewed as a means of helping patients to continue HCQ or CQ (by not stopping the drugs for uncertain findings) as much as a means of preventing serious retinal damage (by the early recognition of definitive findings). Although it is important to be sensitive to signs of damage in a typical pattern, it is also important to verify such signs with more than 1 test or by repeat testing.
Screening Frequency
Next, we provide guidelines and recommendations for screening that we deem a fair balance of risk and cost, but the exact timing and extent of screening relative to risk and prevalence, and to cost and legal considerations are judgments that individual physicians and health plans must ultimately determine (Table 2).
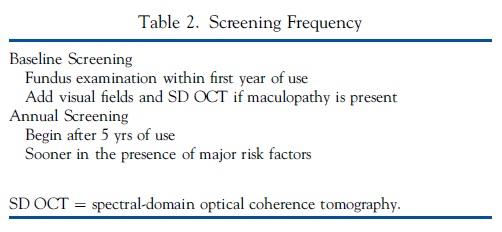
Baseline Examination. All patients beginning long-term HCQ or CQ therapy should have a baseline ophthalmologic examination within the first year of starting the drug to document any complicating ocular conditions and to establish a record of the fundus appearance and functional status. Most critical is fundus evaluation of the macula to rule out any underlying disease that might make use of these drugs unwise because of preexisting tissue damage or interference with the interpretation of screening tests. Although baseline visual fields and SD OCT are always useful, it is not critical to obtain them at baseline unless abnormalities are present (e.g., focal macular lesion, glaucoma) that might affect screening tests. The baseline examination also provides an opportunity for advising patients and prescribing physicians about proper dose levels (and the ability to adjust them) and the importance of regular screening if they continue the medication long-term.
Annual Screening. Given the initial low risk of HCQ or CQ retinopathy, with a proper dose and in the absence of major risk factors, annual screening can be deferred until there has been 5 years of exposure. Screening should begin sooner if the risk is high (Table 1). We consider annual examinations to be sufficient because toxicity develops slowly, and there is time to repeat tests or perform additional tests whenever results are suspicious but not definitive.25 More frequent screening may be considered for patients with major risk factors. It is important to check the dosage relative to weight at every visit and to ask about changes in systemic status, such as major weight loss, kidney disease, or tamoxifen use.
Clinical Examination Techniques
Screening techniques that are recommended or that should be avoided are listed in Table 3.
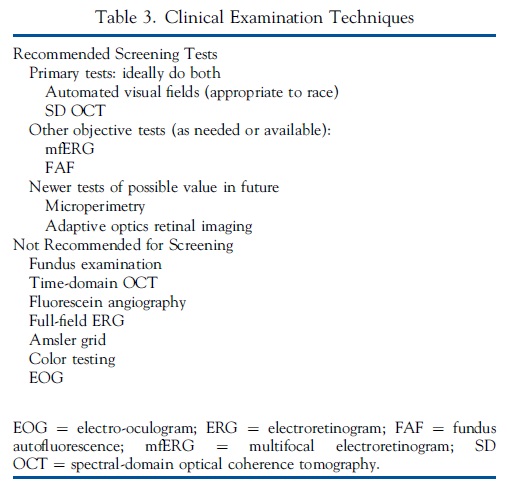
Recommended Screening Tests
We recommend the use of both automated visual fields and SD OCT for routine primary screening, because these are widely available. Fields potentially are more sensitive, but are subjective and patients differ in the reliability of their responses; SD OCT is objective, highly specific, and generally sensitive for levels of damage that might be visually significant. Unless toxic changes are advanced and obvious, at least 1 objective test should confirm subjective findings before toxicity is diagnosed.
Subjective, Functional: Automated Threshold Visual Fields. Central visual field testing is very sensitive in reliable patients. We recommend white SITA testing with pattern deviation plots, which distinguish regional loss from background sensitivity better than the grey scale.26 The 10-2 field pattern has high resolution within the macula and is excellent for non-Asian patients (Figs 1 and 5). However, wider test patterns (24-2 or 30-2) are needed for Asian patients in whom toxicity often manifests beyond the macula (Fig 2). These larger patterns have only 4 central test spots, and even a single central spot of reduced sensitivity should be taken seriously. Some practitioners prefer red targets that are perceptually dimmer, but this comes at a price of more “noise” and a lack of pattern deviation plots.27
Visual fields can vary markedly between visits, and some patients respond more reliably than others. Proper field interpretation requires a sensitive eye to the characteristic pattern of HCQ and CQ loss in both the parafoveal and extramacular regions. The most frequent regions of the retina showing early damage are inferotemporal, with a corresponding superonasal field defect, but this is not absolute. Uncertain visual field changes should trigger retesting (for consistency) or evaluation with other objective tests, such as mfERG (which measures the field electrically), SD OCT, and FAF.
Objective, Structural: Spectral-Domain Optical Coherence Tomography. As damage from HCQ or CQ develops, the SD OCT shows localized thinning of the photoreceptor layers in the parafoveal region (Figs 1 and 3) in non-Asian eyes or near the arcades in many Asian eyes (Fig 2). These localized areas of photoreceptor loss are strong indicators of toxicity. Initial damage sometimes can be recognized as distinct focal interruption of the photoreceptor outer segment structural lines.10,28,29 Outer retinal thickness remains normal until these focal signs develop, so that screening should aim at recognition of previously unrecognized areas of retinopathy, rather than expect gradual and chronic changes.24 Wider-angle scans or scans directed across the vascular arcade region are important for Asian eyes especially. As with fields, if very early changes seem tenuous, the test can be repeated or other tests performed for confirmation. Spectral-domain OCT may not be quite as sensitive as visual field or mfERG, but it is definitive when regional thinning is present in a typical pattern.
Additional Useful Screening Tests
Objective, Functional: Multifocal Electroretinogram. The mfERG generates local electroretinogram responses topographically across the posterior pole and can objectively document parafoveal or extramacular electroretinogram depression in early retinopathy (Figs 1 and 2). The mfERG is similar in sensitivity to visual fields and can provide objective confirmation of suspected field loss.30 Sensitivity can be enhanced by comparison of amplitudes between rings of responses about the center.31 The mfERG requires proper well-calibrated equipment and experienced personnel to perform and interpret well, and is available in large clinical centers and some specialty offices.
Objective, Structural: Fundus Autofluorescence. Autofluorescence imaging can reveal early parafoveal or extramacular photoreceptor damage as an area of increased autofluorescence that may precede thinning on SD OCT.10,32 Late RPE loss appears as a dark area of reduced autofluorescence (Figs 1 and 2). Fundus autofluorescence is especially valuable in giving a topographic view of damage across the posterior fundus, and wide-angle images can show extramacular patterns of damage in Asian eyes.3,4
Newer Screening Tests
Subjective, Functional: Microperimetry. This procedure localizes visual field test flashes accurately on the retina. In concept, it should be useful and more reliable than automated perimetry, but in practice it is similarly complicated by fatigue and long examination times, and has not been proven as yet to be more revealing. Different test patterns are needed for non-Asian and Asian eyes.
Objective, Structural: Adaptive Optics Retinal Imaging. Special cameras with enhanced optics to reduce wave-front distortion can image the cone array directly and show cone damage with early disease. However, distinguishing damage from artifact is still difficult with current instruments, and at the time of writing, this remains primarily a research tool.
Tests Not Recommended for Screening
Fundus Examination and Photography. Ophthalmoscopy is not a screening tool because photoreceptor damage is detectable with other techniques well before visible changes in the fundus. A bull’s-eye, by definition, implies RPE loss and an advanced stage of toxicity.
Time-Domain Optical Coherence Tomography. The resolution is not sufficient to detect early toxic changes.
Fluorescein Angiography. This can recognize RPE defects, but these are late changes.
Full-Field Electroretinogram. The full-field electroretinogram is a global test of retinal function and will show abnormalities in only very late CQ or HCQ toxicity. It may be useful to judge the extent of damage beyond the macula.
Amsler Grid. Amsler grid testing is not consistent enough for reliable screening of subtle scotomas.
Color Vision Testing. Color errors may occur but are not sensitive or specific.
Electro-oculogram. The electro-oculogram has not been validated as a reliable screening test.
Management of Eyes at Risk or with Retinopathy
No diet or medical therapy has proven effective as yet to prevent, treat, or reduce risk from HCQ or CQ retinopathy other than cessation of the drug. Even stoppage of the drug does not prevent progression of retinopathy, although this is typically mild if the toxicity is recognized before there is RPE damage. Patients with age-related maculopathy or macular dystrophies sometimes are advised to avoid excessive sun exposure and maintain intake of lutein and zeaxanthin (which are foveal protectants). However, the value of such recommendations for patients at risk fromHCQor CQ exposure, or after retinopathy is recognized and the drug is stopped, is unknown.
Once definitive signs of retinopathy are recognized, the decision to stop medication should be made in conjunction with the patient and the prescribing medical physician to ensure that medical risks are managed (e.g., a potential flareup of SLE). The patient can be advised about the risk of further visual loss depending on the severity of the retinopathy. This risk is minimal if the retinopathy was detected early, but significant if there is already a bull’s-eye lesion and some reduction in central foveal thickness, because damage can progress for a number of years.5
References
1. Marmor MF, Kellner U, Lai TY, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011;118:415–22.
2. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol 2014;132:1453–60.
3. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology 2015;122:110–6.
4. Lee DH, Melles RB, Joe SG, et al. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology 2015;122:1252–6.
5. Marmor MF, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol 2014;132:1105–12.
6. Browning DJ. Impact of the revised American Academy of Ophthalmology guidelines regarding hydroxychloroquine screening on actual practice. Am J Ophthalmol 2013;155: 418–28.
7. Nika M, Blachley TS, Edwards P, et al. Regular examinations for toxic maculopathy in long-term chloroquine or hydroxychloroquine users. JAMA Ophthalmol 2014;132:1199–208.
8. Lee MG, Kim SJ, Ham DI, et al. Macular retinal ganglion cellinner plexiform layer thickness in patients on hydroxychloroquine therapy. Invest Ophthalmol Vis Sci 2014;56: 396–402.
9. de Sisternes L, Hu J, Rubin DL, Marmor MF. Localization of damage in progressive hydroxychloroquine retinopathy on and
off the drug: inner versus outer retina, parafovea versus peripheral fovea. Invest Ophthalmol Vis Sci 2015;56:3415–26.
10. Marmor MF. Comparison of screening procedures in hydroxychloroquine toxicity. Arch Ophthalmol 2012;130:461–9.
11. Kellner S, Weinitz S, Farmand G, Kellner U. Cystoid macular oedema and epiretinal membrane formation during progression of chloroquine retinopathy after drug cessation. Br J Ophthalmol 2014;98:200–6.
12. McChesney EW, Shekosky JM, Hernandez PH. Metabolism of chloroquine-3-14C in the rhesus monkey. Biochem Pharm 1967;16:2444–7.
13. McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med 1983;75:11–8.
14. Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology 2002;109:1377–82.
15. Lee JY, Luc S, Greenblatt DJ, et al. Factors associated with blood hydroxychloroquine level in lupus patients: renal function could be important. Lupus 2013;22:541–2.
16. Carmichael SJ, Day RO, Tett SE. A cross-sectional study of hydroxychloroquine concentrations and effects in people with systemic lupus erythematosus. Intern Med J 2013;43:547–53.
17. Costedoat-Chalumeau N, Dunogué B, Leroux G, et al. A critical review of the effects of hydroxychloroquine and chloroquine on the eye. Clin Rev Allergy Immunol 2015;49:317–26.
18. Jallouli M, Galicier L, Zahr N, et al. Determinants of hydroxychloroquine blood concentration variations in systemic lupus erythematosus. Arthritis Rheumatol 2015;67:2176–84.
19. Lee Y, Vinayagamoorthy N, Han K, et al. Association of polymorphisms of cytochrome P450 2D6 with blood hydroxychloroquine levels in patients with systemic lupus erythematosus. Arthritis Rheumatol 2016;68:184–90.
20. Leung LS, Neal JW, Wakelee HA, et al. Rapid onset of retinal toxicity from high-dose hydroxychloroquine given for cancer therapy. Am J Ophthalmol 2015;160:799–805.
21. Navajas EV, Krema H, Hammoudi DS, et al. Retinal toxicity of high-dose hydroxychloroquine in patients with chronic graft-versus-host disease. Can J Ophthalmol 2015;50:442–50.
Ophthalmology 2016;123:1386-1394 2016 by the American Academy of Ophthalmology.