Author: Rober F. Steinert, MD
Figures and portions of the text were previously published in Steinert RF, Puliafito CA: The Nd:YAG laser in ophthalmology: principles and clinical applications of photodisruption, Philadelphia, 1985, WB Saunders.
Introduction
Photodisruption with the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser can effectively treat a number of disorders arising after cataract surgery and intraocular lens (IOL) implantation. The pressure wave generated by optical breakdown of an Nd:YAG laser pulse allows the surgeon to cut and manipulate intraocular structures in a variety of postoperative disorders.
Pupillary Block Glaucoma
Acute angle closure glaucoma in aphakia and pseudophakia may take several forms (Table 1). Corneal edema and haze, anterior chamber reaction, and iris congestion may make argon laser iridectomy impossible. Argon laser iridectomy may not relieve the glaucoma because of the role of the vitreous. The Nd:YAG laser better treats these conditions and is the treatment of first choice. Success of the Nd:YAG laser "anterior hyaloidotomy" in curing ciliovitreal block glaucoma, demonstrates the pathophysiologic role of the anterior hyaloid face in many cases of pupillary block glaucoma.
Table 1. Classification of acute aphakic and pseudophakic glaucoma
|
Pupillary block (iridovitreal block)
|
Aphakic malignant glaucoma (ciliovitreal block)
|
- Absent, imperforate, or secluded peripheral iridectomies
- Inflammatory adhesion of intact hyaloid face to iris
- Anterior chamber hemorrhage and exudate
|
- Posterior diversion of aqueous
|
Figure 1 illustrates a case of pupillary block in a patient who had an iridectomy and antibiotic therapy for endophthalmitis after complex extracapsular cataract extraction with an anterior chamber IOL (AC IOL). The surgical iridectomy became occluded postoperatively, but an argon laser iridotomy relieved the resultant iris bombé. The argon laser iridotomy closed within weeks, and the iris bombé recurred with an intraocular pressure (IOP) of 50 mm Hg. The Nd:YAG laser at 4 mJ created new iridotomies with permanent relief of the iris bombé and pressure elevation. The pupillary membrane was cleared by the Nd:YAG laser.

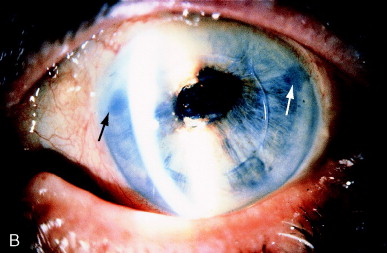
Figure 1. (A) Recurrent pseudophakic pupillary block with iris bombé after endophthalmitis and closure of argon laser iridectomy. (B) Nd:YAG laser at 4 mJ readily created several iridectomies (arrows) with permanent relief of the iris bombé. A pupillary membranectomy was also performed. From Steinert RF, Puliafito CA: The Nd:YAG laser in ophthalmology: principles and clinical application of photodisruption, Philadelphia, 1985, WB Saunders, p 106.
Pseudophakic block is most common after AC IOL placement, typically after complicated extracapsular cataract extraction or phacoemulsification. Risk of pupillary block rises when the surgeon fails to place a large surgical iridectomy. Further vitreous may prolapse and occlude the pupil against the optic of the AC IOL. Large surgical iridectomy may be occluded by prolapsing vitreous and by capsular and cortical remnants.
The role of the hyaloid face in aphakic malignant glaucoma is illustrated in Figure 2. Three months after cataract extraction and IOL removal in a patient with a large superior loss of iris, the chamber became shallow, and the pressure rose to 34 mm Hg over several days, with the onset of deep pain. A thin, intact hyaloid face or inflammatory membrane was present. The patient was treated with the Nd:YAG laser, focused and fired at 3 mJ on the hyaloid face through the mild corneal edema despite less than 1 mm of residual anterior chamber depth. The anterior chamber immediately deepened.
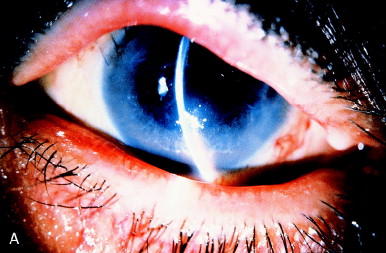
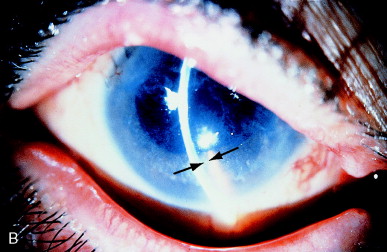
Figure 2. (A) Aphakic malignant glaucoma with apposition of inferior iris to edematous cornea. (B) Depth is restored to the anterior chamber immediately after Nd:YAG laser pulses have opened the anterior hyaloid face. Arrows show separation of iris and cornea, in comparison with part A. From Steinert RF, Puliafito CA: The Nd:YAG laser in ophthalmology: principles and clinical applications of photodisruption, Philadelphia, 1985, WB Saunders, p XII.
Technique for Aphakic and Pseudophakic Iridectomy and Anterior Hyaloid Vitreolysis
Preparation of the Patient
Application of a miotic agent such as pilocarpine 2% is advisable to place the iris on maximal stretch.
Procedure
Four to 8 mJ can usually perforate the iris in one pulse. Higher energy may be necessary for corneal edema or an anterior chamber reaction. At least 3 iridotomies should be made to
- Ensure full relief of aqueous entrapment
- Increase the chance of maintaining one long-term patent iridotomy. Iridotomies shrink as bombé is relieved, and the iris falls back. Inflammation may close iridotomies postoperatively.
The haptic of an AC IOL provides clearance from the cornea if the chamber is shallow or flat. Make first laser shots adjacent to haptic insertion to avoid corneal injury.
Nd:YAG laser should be fired into the anterior vitreous through the iridectomy or the pupil after completion of iridotomy or when a patent basal surgical iridectomy is present. The hyaloid face is then ruptured, relieving any malignant glaucoma component caused by the intact hyaloid face.
Postoperative Care
Intense topical steroid therapy (eg, prednisolone acetate 1%, dexamethasone 0.1%), 4 times daily, or more if required. Inflammation and synechia formation also require cycloplegia and mydriasis. Monitor and treat IOP appropriately.
Anterior Vitreolysis and Cystoid Macular Edema
Vitreous strands and bands to the wound may cause eccentric pupils and can be associated with cystoid macular edema (CME). Iliff reported visual improvement after surgical section of such vitreous bands to the wound. He coined the term "vitreous-tug syndrome," although no evidence was given that tugging on the vitreous body was present or responsible for the visual loss.
Katzen, Fleischman, and Trokel reported the use of the Nd:YAG laser to lyse strands of vitreous to cataract wounds. Vision improved in all 14 patients treated. The presence of CME, however, was judged clinically, and the results of fluorescein angiography before and after laser treatment were not reported for 13 of the eyes.
Because of the unpredictable natural history of aphakic CME, with erratic response to antiinflammatory agents and frequent spontaneous improvement, small and controlled series cannot unequivocally prove the efficacy of a given technique. Clinical experience, however, has confirmed a high rate of visual improvement after anterior segment vitreolysis, particularly in favorable cases. In their series of 29 patients, 22 had fluorescein angiographic confirmation of CME before laser treatment. The interval between cataract extraction and treatment averaged 10 months, with a range of 1 to 42 months; average follow-up after laser vitreolysis was 10 months, with a range of 3 to 27 months.
The change in best-corrected Snellen acuity is shown in Figure 3. No patient had loss of vision after laser vitreolysis. The visual acuity in 16 of the 29 patients (55%) improved by 2 or more lines, with stable acuity following treatments. Five (17%) patients' vision improved by at least 2 lines, but they experienced ongoing fluctuation of acuity.
Vision in 8 patients (28%) showed less than 2 lines of improvement. Of these 8 patients: 2 had progressive maculopathy in addition to the CME (1 had an epiretinal membrane and one had progressive diabetic maculopathy); 2 had severe glaucoma with loss of central vision in addition to the CME; and 2 had persistent CME.
Two patients were lost to follow-up without documentation of the basis for persistent unimproved acuity. Patients who did not respond to vitreolysis had the poorest pretreatment visual acuity measurements.
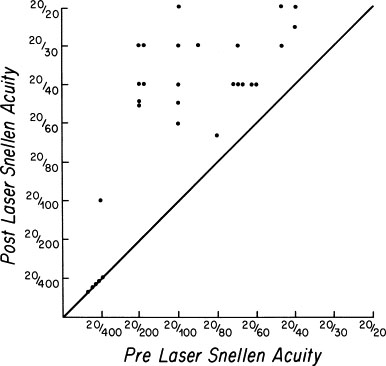
Figure 3. Scattergram of visual acuity level before and after Nd:YAG laser vitreolysis. Points above the diagonal line represent improvement. From Steinert RF, Wasson PJ: Neodymium:YAG laser anterior vitreolysis for Irvine-Gass cystoid macular edema, J Cataract Refract Surg 15:304-307, 1989.
Postlaser fluorescein angiography was performed on 9 eyes. Three eyes showed complete resolution of the CME; 2 showed improvement but persistent leakage; 1 showed an unimproved appearance of the fluorescein angiographic leakage but still experienced improved acuity; and 3 had persistent CME and less than 2 lines of visual improvement.
Techniques for Anterior Vitreolysis
Preoperative Assessment
A comprehensive examination including fluorescein angiography should be performed to establish a definitive diagnosis of CME.
Small vitreous strands may be missed on casual examination. The strand is usually best seen on slit lamp examination with a narrow slit beam in a darkened room. Gonioscopy may be necessary to visualize the strand, particularly if the vitreous enters the anterior chamber through the area of a peripheral iridectomy. The most favorable cases for Nd:YAG laser vitreolysis are those with relatively discrete strands under tension. Broad bands are most difficult to fully transect. Amorphous vitreous herniation is extremely difficult to cut with a laser approach. The larger the amount of vitreous involvement, the more consideration should be given to pars plana vitrectomy for definitive removal of all pathologic vitreous.
Pupillary distortion may be subtle. Figure 4A shows mild peaking of a pupil, indicating a vitreous strand coming around the pupil. After vitreolysis, less peaking is present, although some permanent change has occurred in the sphincter, preventing complete rounding of the pupil (Figure 4B).
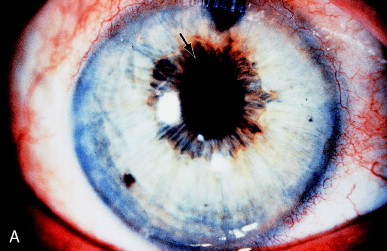
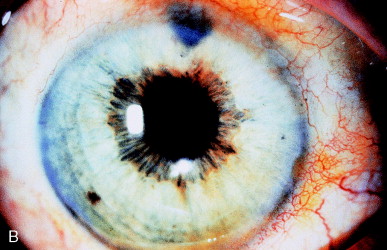
Figure 4. (A) Fine vitreous strand caused mild peaking of the pupil (arrow). Gonioscopy showed a fine vitreous strand to the wound. (B) After laser vitreolysis, less peaking is present, but chronic change in the sphincter prevents a completely normal pupillary contour. From Steinert RF, Puliafito CA: The Nd:YAG laser in ophthalmology: principles and clinical applications of photodisruption, Philadelphia, 1985, WB Saunders, p 120.
Permanent changes in the iris stroma are frequent in cases of long duration. Decreased but persistent oval shape of the pupil after lysis of a vitreous strand is shown in (Figure 5).
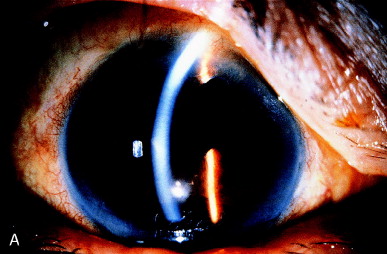
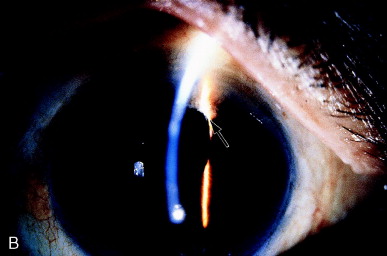
Figure 5. Eccentric pupil caused by a vitreous strand. B, After vitreolysis, depigmentation of the underlying iris stroma, present before the laser treatment, is more readily seen (arrow), and the pupil remains partially distorted. From Steinert RF, Puliafito CA: The Nd:YAG laser in ophthalmology: principles and clinical applications of photodisruption, Philadelphia, 1985, WB Saunders, p 120.
The iris stroma is partially depigmented locally, perhaps indicating chafing of the iris by the vitreous strand. The vitreous band may distort the pupil in several ways, depending on the angle, direction, and number of vitreous strands under tension. Figure 6 shows a "hammock" effect by two separate strands.
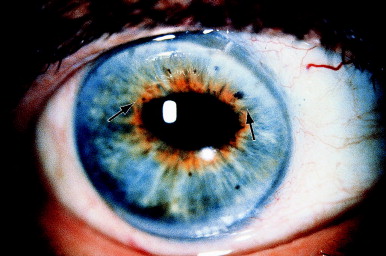
Figure 6. Pupillary distortion caused by two separate strands of vitreous to the wound (arrows). The pupil became round after treatment. Despite CME of 57 months' duration, vision improved from counting-fingers level to 20/50. From Steinert RF, Puliafito CA: The Nd:YAG laser in ophthalmology: principles and clinical applications of photodisruption, Philadelphia, 1985, WB Saunders, p 116.
Patient Preparation
Explain the procedure beforehand to the patient and obtain informed consent. Remind the patient the procedure often requires more than one session.
When the vitreous strand or band passes through the pupil, treatment is often facilitated by administration of pilocarpine 2% every 10 minutes for 3 or 4 drops preoperatively. Inducing stretch of the vitreous through miosis facilitates identification of the strand. When the laser transects the strand the release of tension is shown more definitively.
Procedure
Figure 7 illustrates the three most common configurations of vitreous to the wound:
- A small discrete strand
- A broad band
- A band with either adhesions to the iris or iris entrapment behind the band
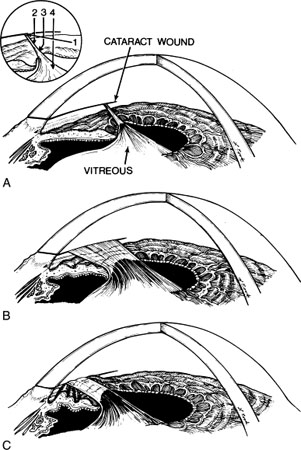
Figure 7. (A) A narrow vitreous strand to a cataract wound. The inset shows possible laser pathways for vitreolysis: (1) a gonioscopic approach, directed at the cataract wound—the location at which the vitreous strand is often the most discrete; (2) a direct approach near the limbus; (3) a direct approach in the region of the collarette; and (4) a direct approach at the pupil. This last approach is rarely successful. (B) A broad vitreous band at the wound. C, Iris pulled upward in a tentlike configuration and entrapped by the vitreous incarceration in the wound. From Steinert RF, Puliafito CA: The Nd:YAG laser in ophthalmology: principles and clinical applications of photodisruption, Philadelphia, 1985, WB Saunders, p 122.
The laser can be directed at a vitreous strand in 4 general areas (inset, Figure 7A):
Pathway 1. The cataract wound is the most reliable landmark during vitreolysis because the vitreous band or strand has to terminate at that location. The cataract wound is visualized with a gonioscopy lens (pathway 1 on figure), and the laser can be fired at the wound area with a reasonable chance of successful vitreolysis. The energy settings are usually in the 6- to 12-mJ range to obtain adequate cutting power. Disadvantages of this technique include the requirement of a gonioscopy lens, and the need to use a contact lens for the completion of the treatment session––even if a different approach is needed later in the session––because of the application of gonioscopy fluid to the cornea.
Pathway 2. If the cornea is clear near the limbus and a vitreous strand can be visualized with clearance from the iris stroma, direct cutting without a contact lens or with a peripheral button Abraham lens may be successful along pathway 2. Usually 4 to 8 mJ is required. In the course of dozens to hundreds of shots along this pathway, considerable pigment may be liberated from the underlying iris stroma, which will ultimately obscure the surgeon's view. Misfocused shots can cause local damage to the underlying or overlying stroma.
Pathway 3. If directed at the vitreous passing over the iris collarette, pathway 3 can be helpful, especially when the vitreous has formed adhesions to the collarette, pulling it forward in a tentlike formation. Close proximity of vitreous and iris makes damage to the underlying iris stroma likely, but this may be clinically tolerable.
Pathway 4. Pathway 4 is rarely successful if directed at the vitreous as it passes around the pupil. Vitreous traction components are poorly defined as they come around the pupil. The shock wave is ineffective at rupturing vitreous strands except directly at the laser focal point. Firing the laser immediately adjacent to the pupillary border causes low-grade capillary hemorrhage and release of pigment, obscuring visualization of the area.
Successful treatment releases the tension and converts a discrete strand or band to an amorphous gelatinous appearance. Observation of the change and any iris deformation is the best indicator of a successful release of tension. Hundreds of shots over several treatment sessions may be necessary to cut a large band.
Postoperative Care
Strong topical steroids (eg, prednisolone acetate 1% or dexamethasone 0.01%) are given 4 times daily until visual improvement occurs, typically in 2 to 3 months. Topical nonsteroidal antiinflammatory drugs may be of benefit alone or with topical steroids. Ketorolac or diclofenac drops are usually administered 4 times daily. The addition of a systemic nonsteroidal antiinflammatory drug can be considered in recalcitrant cases.
IOP elevation following vitreolysis has not been well documented. A beta-blocker or brimonidine drop at the time of treatment probably provides adequate prophylaxis.
Prophylactic Vitreolysis
With the availability of laser vitreolysis, the surgeon may treat vitreous strands to the wound in the absence of CME in an effort to prevent its later development. Only a large, long-term randomized treatment trial can determine the usefulness of this approach. Some patients with vitreous strands to the wound never acquire CME.
A baseline fluorescein angiogram is recommended to document the macular status in patients with vitreous to the wound and good visual acuity. If CME is detected, laser vitreolysis is probably indicated even in the presence of good acuity. If visual loss later develops with the new onset of CME, the baseline angiogram will be useful to further support therapeutic intervention.
Coreoplasty
The Nd:YAG laser can cut through iris stroma or the pupillary sphincter to open an occluded visual axis. Indications for coreoplasty include pupillary enlargement for restoration of vision or improvement of the fundus view for examination and treatment. Synechialysis can also affect a pupillary configuration.
Figure 8 illustrates a case in which the pupil became updrawn after intracapsular cataract extraction many years earlier. The upper lid covered the pupil. Unless the lid was elevated, vision was limited to counting fingers. The Nd:YAG laser cut through the sphincter with only a localized, self-limited hemorrhage. Vision improved to 20/70 and was limited only by preexisting maculopathy.
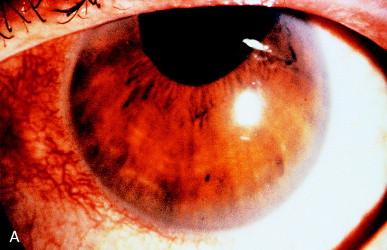
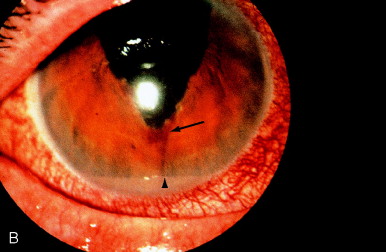
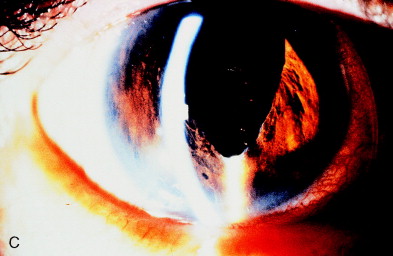
Figure 8. (A) Updrawn pupil after intracapsular cataract extraction. (B) Immediately after sphincterotomy, a candle wax–like trickle of blood was seen clotted at the inferior margin of the sphincterotomy (arrow). A light reflection from the lid margin gave an appearance similar to hypopyon (arrowhead), but no gross hemorrhage or inflammation occurred. (C) One week later, the clot had cleared, and the central cornea was in the optical axis. From Steinert RF, Puliafito CA: The Nd:YAG laser in ophthalmology: principles and clinical applications of photodisruption, Philadelphia, 1985, WB Saunders, p 108.
In Figure 9, postoperative inflammation caused nearly complete seclusion of the pupil over a posterior chamber IOL (PC IOL). Dense associated fibrosis was present with thickening of the iris stroma. Six mJ pulses directed through a central button Abraham lens successfully enlarged the pupil with a resultant improvement in acuity from 20/80 to 20/30.
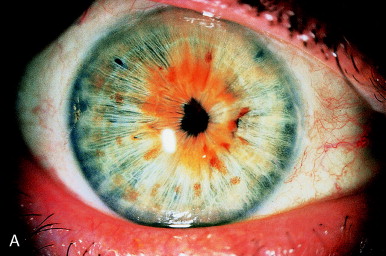
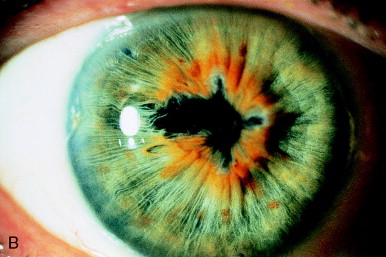
Figure 9. (A) Nearly total seclusion of the pupil secondary to postoperative inflammation after extracapsular cataract extraction with posterior chamber IOL implantation. (B) Coreoplasty with the Nd:YAG laser resulted in little improvement superiorly, inferiorly, and nasally (to the right). However, in the temporal zone, to the left, the sphincter was successfully transected, and a clear visual axis was restored.
Technique
Preparation of the Patient
In addition to a comprehensive eye examination and obtaining informed consent, the presence of bleeding abnormalities must be specifically elicited in the history. Clotting abnormalities increase the risk of a large hyphema.
Preparatory thermal iris photocoagulation with an argon laser is not usually necessary or helpful. Heavy and extensive iris coagulation is necessary to prevent bleeding when the pulsed Nd:YAG laser is subsequently used. The pressure wave after optical breakdown radiates over several millimeters with enough shearing force to cause a capillary oozing. It is difficult to eliminate bleeding without widespread intense preparatory coagulation. An exception is a visible blood vessel whose patch cannot be avoided where prior photocoagulation would be necessary. Patients in whom preparatory laser coagulation has been performed state photocoagulation is more painful than photodisruption.
Procedure
Six to 10 mJ is the usual setting. Numerous shots are required to cut across 3 to 5 mm of sphincter and stroma. The sphincter is the most difficult region to cut and, with the minor arterial circle, the most prone to hemorrhage. Gross hemorrhage and free red blood cells and fibrin in the anterior chamber may prevent completion of the treatment in one session if the surgeon starts at the pupil and cuts across the sphincter. Treatment should begin in the peripheral iris stroma and progress toward the sphincter and pupil to avoid significant hemorrhage until nearly the end of the session. Figure 10 illustrates a sequential sphincterotomy.
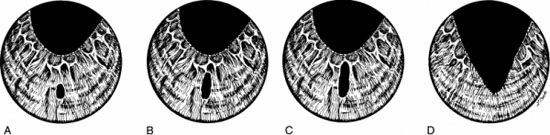
Figure 10. Technique for Nd:YAG laser sphincterotomy. (A) Treatment is begun in the peripheral iris stroma. (B and C) Progressive cutting is made toward the iris sphincter. (D) Sphincter is cut last so that bleeding is minimized for as long as possible to increase the chance of completing the treatment in one session. From Steinert RF, Puliafito CA: The Nd:YAG laser in ophthalmology: principles and clinical applications of photodisruption, Philadelphia, PA, WB Saunders, p 110.
If bleeding begins without clotting and does not cease rapidly, pressure should be applied to the globe through a contact lens, by a finger through the eyelids, or with a cotton-tipped swab applied to the globe. Pressure is maintained until effective iris intravascular coagulation has time to occur.
It is possible to perform sphincterotomy and coreoplasty over a PC IOL without damaging the underlying lens because the damage zone from optical breakdown ("plasma growth") occurs backward along the beam path toward the Nd:YAG laser source. It is not advisable to perform this procedure in a phakic patient, as the pressure wave generated can rupture the anterior capsule of the natural crystalline lens, inducing immediate cataract formation.
Postoperative Care
A strong topical steroid (typically prednisolone acetate 1% or dexamethasone 0.1%) is given 4 times daily initially, with the dosage tapered as inflammation subsides. Cycloplegia is usually unnecessary. IOP elevation is monitored and treated appropriately.
Synechialysis
Localized synechiae with associated pigment may be broken by photocoagulation with the argon laser. Nd:YAG laser is generally more successful than argon laser in synechialysis because pigmentation of the target is not required and forceful rupture of adhesions can be achieved.
The most common synechiae are to the anterior or posterior capsule, or both. Often a tag of anterior capsule adheres to the underside of the iris. As progressive fibrosis and adhesion between the anterior and posterior capsule occur, the iris adhesion is brought posteriorly. Sufficient force is exerted to bring the iris sphincter around the edge of a PC IOL, leading to iris capture of the IOL optic. In Figure 11A, the iridocapsular adhesion has exposed the superior edge of the PC IOL. This is more evident after dilation. Two-millijoule shots applied to the area of adhesion both freeze the adhesion and rupture the posterior capsule in that area. Following treatment (Figure 11C), the iris sphincter is back in proper position anterior to the PC IOL.
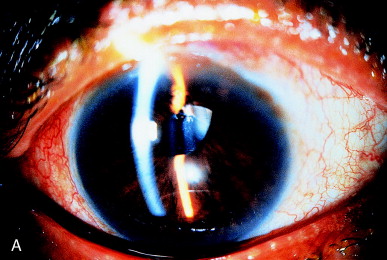
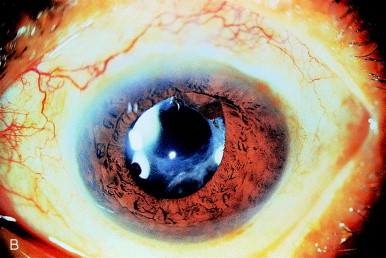
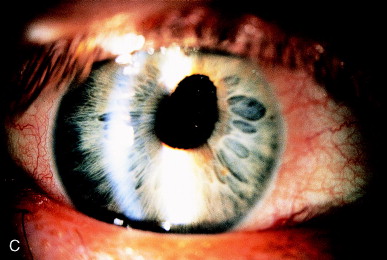
Figure 11. (A) Iris capture of a PC IOL secondary to iridocapsular adhesions in an undilated patient. (B) Adhesion of the iris to the anterior capsular edge is seen clearly after dilation. (C) IOL capture is released, and the pupil becomes round after Nd:YAG laser capsulotomy releases the iridocapsular adhesions.
A retained fragment of anterior capsule incarcerated in the cataract wound is another cause of iridocapsular adhesion. The appearance will simulate a vitreous strand to the wound (Figure 12A). Examination discloses a grayish membrane resulting from fibrosis of the epithelial cells on the anterior capsule, distinctly different from the appearance of a vitreous strand. The capsular origin can be seen after dilation (Figure 12B). The capsular fragment is disrupted with the laser at approximately 2 to 3 mJ if the laser is used and approximately 6 mJ if the laser is used gonioscopically. Extra energy is needed because of the thickened fibrotic nature of the capsule.
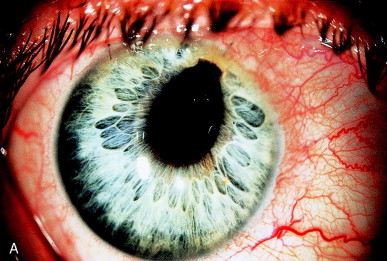
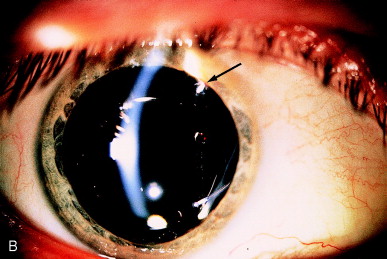

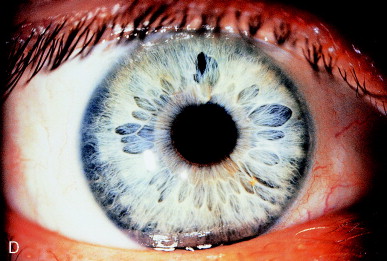
Figure 12. (A) Iridocapsular adhesion simulating pupillary distortion seen with vitreous incarceration in the wound. (B) After dilation, the iridocapsular adhesion is better seen (arrow). (C) After lysis of the anterior capsular fragment, improvement in the pupillary position is seen compared with the preoperative photo (A), but the iris distortion continues to expose the edge of the IOL optic, giving glare symptoms. (D) After gonioscopic application of Nd:YAG laser pulses to the iridocapsular adhesions posterior to the iris, the pupil becomes central, and the glare symptoms are resolved.
If the capsular fragment is not disrupted within several months postoperatively, additional adhesions may form because of the proliferation of the cells present on the anterior capsule fragment. The pupillary position has improved with photodisruption of the capsule strand incarcerated in the wound, but the pupil remains partially eccentric (Figure 12, C). The edge of the PC IOL is exposed and a source of functionally significant glare for the patient. This was addressed with further Nd:YAG laser pulses to the epithelial pearl adhesion underlying the iris superiorly. This freed the sphincter and resulted in a nearly round pupil (see Figure 12, D).
IOL Precipitates Removal
Inflammatory precipitates on the IOL surface are not usual. They may consist of both pigmented and nonpigmented, relatively round precipitates similar in appearance to keratic precipitates or the inflammation may cause the deposition of a more fibrinous gray sheet. These precipitates may respond to appropriate antiinflammatory therapy. Visually significant precipitates may remain after the inflammation is quiet. A mixture of localized dense inflammatory precipitates and a sheetlike precipitate remain after a severe postoperative inflammation (Figure 13A). Marked clearing of the IOL optics is evident following Nd:YAG laser therapy (Figure 13B).
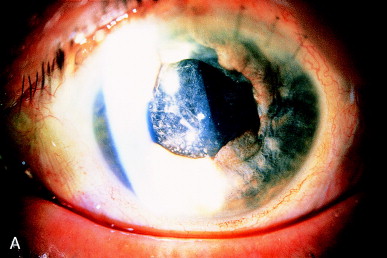
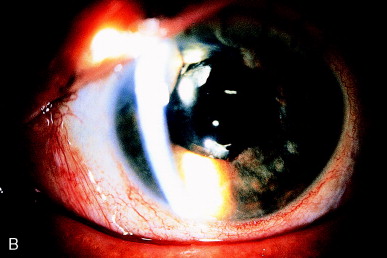
Figure 13. (A) After complicated extracapsular cataract extraction with PC IOL implantation and prolonged postoperative inflammation, fibrinous and cellular debris persist on the anterior IOL optic. (B) Nd:YAG laser photodisruptive pulses have cleared most of the debris from the IOL surface.
To remove IOL precipitates, the Nd:YAG laser is typically set at about 2 to 3 mJ, an energy level adequate to create optical breakdown in the aqueous. The Nd:YAG laser aiming beams are first focused on the anterior IOL surface. The laser is then slightly defocused by withdrawing the laser slightly toward the beam origin and away from the beam target. The abrupt pressure wave generated by the subsequent optical breakdown in front of the IOL precipitate liberates inflammatory debris into the aqueous humor, leaving a cleaned IOL.
Anterior Capsule
The lens anterior capsule becomes hazy postoperatively because of the epithelial cells present on the inner surface of the anterior capsule. Retained anterior capsule in the pupillary zone will become opaque. Retained capsule does not become strongly adherent to the underlying IOL.
A large remnant of anterior capsule remained after implantation of a PC IOL and significantly opacified (Figure 14A). The pupillary zone was cleared with Nd:YAG laser photodisruptive pulses of 2 to 3 mJ. These pulses were applied beginning in the upper left corner and then in the direction of the lower right corner (Figure 14 B). The capsular membrane was not fully liberated but was left adherent at the lower right to curl up on itself. Damage to the underlying PC IOL is avoided by focusing on the anterior capsule and then withdrawing the laser focus slightly anterior to the target. The propagation of the laser plasma is toward the laser source. Damage to the underlying IOL can be avoided as long as the photodisruptive optical breakdown is not focused within the IOL itself.
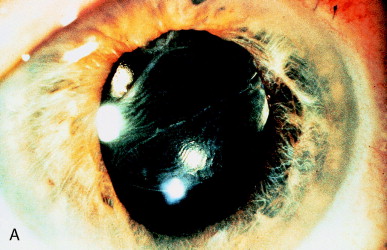

Figure 14. (A) Anterior capsule was inadvertently retained after PC IOL implantation. Opacification of the anterior capsule occurred rapidly. (B) A window has been cut in the anterior capsule with the Nd:YAG laser, with a small amount of the anterior capsule remaining attached in the lower right to avoid a free-floating capsular remnant in the anterior chamber. No damage has occurred to the anterior IOL surface.
Contracture of a continuous curvilinear capsulorhexis may cause progressive blockage of an initially clear pupillary zone. This may be due to eccentric contracture of an anterior capsule (Figure 15A), or a symmetric contracture (Figure 16A). This "capsule contraction syndrome" or "capsular phimosis syndrome" is seen when the diameter of the original cataract surgical capsulorhexis is 4 mm or smaller. It is attributed to contracture of the structurally strong round anterior capsule opening by the lens epithelial cells that have undergone myofibroblastic differentiation. The capsular contracture stretches the peripheral zonules, with the risk of frank rupture of the zonules and potential weakening of the IOL support. Capsular contracture is best avoided by keeping the diameter of continuous curvilinear capsulorhexis to 5 mm or greater.
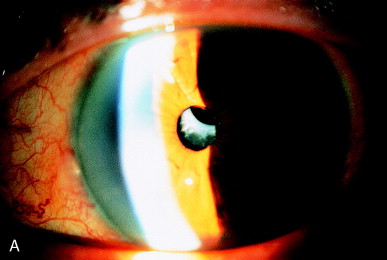
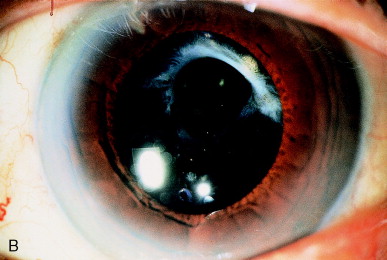
Figure 15. (A) Contracture of the anterior capsule inferiorly has nearly occluded the optical zone. (B) Nd:YAG laser cutting of the inferior capsule adhesion restores an adequate visual axis.
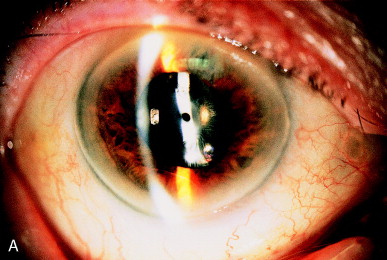
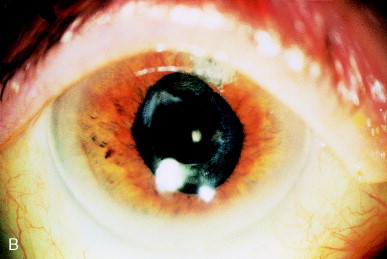
Figure 16. (A) Symmetric contracture of the anterior capsulorhexis leaves an inadequate visual axis. (B) Photodisruption of the anterior capsulotomy edge restores an adequate visual axis.
Nd:YAG laser photodisruption of the capsular margin should be undertaken when capsular contracture syndrome is recognized postoperatively. This will prevent further contraction of the capsule because the disrupted anterior capsule no longer has mechanical integrity. In addition, the pupillary zone is clear of the opaque capsule.
The technique typically consists of 2- to 3-mJ pulses at the edge of the capsulorhexis, transecting the round capsulorhexis edge into at least four quadrants. The capsule contracts and eventually resumes a relatively round appearance with a much larger opening (see Figure 16B).
Damage to the underlying PC IOL is avoided by deliberate anterior defocusing of the laser beam for removal of the IOL precipitates and opening of retained anterior capsule.
Retained Cortical Material
Occasionally cortex is retained postoperatively. It may be unrecognized or deliberately left due to a defect in the posterior capsule or a difficult-to-reach location, particularly under the incision. Cortex will become hydrated after surgery, swelling and loosening its position. Occasionally, on the day after surgery the visual axis is occluded by hydrated retained cortical material (Figure 17A).
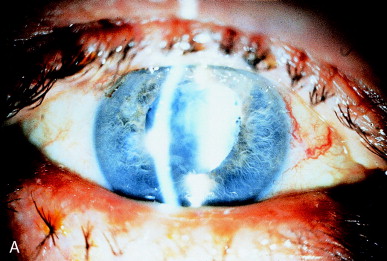
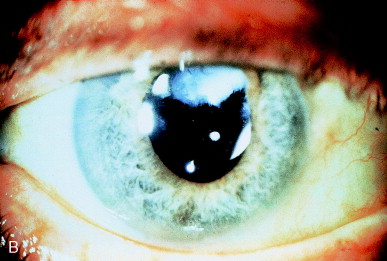
Figure 17. (A) Retained cortex is hydrated but trapped behind the posterior chamber IOL optic. (B) Nd:YAG laser pulses have disrupted the retained cortex in the pupillary zone.
Retained cortex may slowly resorb, but it is particularly slow when the cortex is trapped between the posterior capsule and the PC IOL anteriorly. Gradual clearing can take months. The retained material may slowly become a dense fibrotic sheet that is difficult to open with laser techniques and may require more invasive surgery.
The hydrated opaque cortical material can be liquefied with photodisruptive laser pulses. Typically 2 mJ pulse to cause optical breakdown within the cortical material. The pressure wave from Nd:YAG pulses within the cortex will emulsify the hydrated cortex, creating an appearance of a more uniform lens "milk." Within 24 hours, this more liquefied material will usually clear, restoring a good visual zone (Figure 17B).
The rapid liberation of lens protein through photodisruption may cause secondary inflammation and pressure elevation. Prophylactic antiglaucoma medical therapy is indicated, as is close monitoring of the patient. The surgeon should be prepared to surgically irrigate the retained cortical material if a clinically intolerable level of inflammation or pressure elevation occurs.
IOL Repositioning
In rare circumstances, Nd:YAG laser photodisruption can be used to manipulate the position of an IOL. The pressure wave from optical breakdown can shift an IOL optic if the optic is sufficiently mobile. A previously well-centered ciliary sulcus–fixated PC IOL became captured in the pupil after pupillary dilation (Figure 18). The pupil could not be redilated beyond the border of the IOL optic. Mechanical manipulation of the IOL optic by placing the patient in the supine position or with pressure of a cotton-tipped applicator over the ciliary sulcus failed to cause the IOL optic to shift posteriorly.
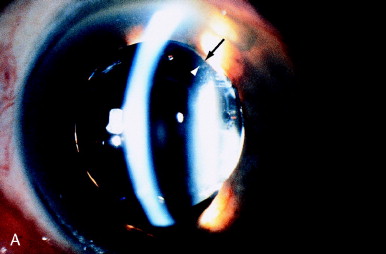
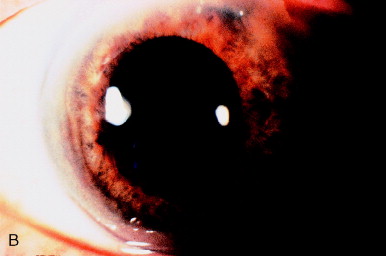
Figure 18. (A) Iris capture of the optic of a PC IOL (arrow). A single 6-mJ pulse focused at an area just anterior to the lens surface, approximately 1 mm centrally from the optic edge (arrowhead), retropulsed the optic behind the iris. (B) IOL is now in proper position behind the iris. From Steinert RF, Puliafito CA: The Nd:YAG Laser in Ophthalmology: Principles and Clinical Applications of Photodisruption, Philadelphia, 1985, WB Saunders, p 130.
A 6-mJ pulse from an Nd:YAG laser was applied to the peripheral edge of the IOL. The laser was focused just anterior to the anterior IOL surface. The anterior pressure wave caused the IOL optic to shift posteriorly, just behind the pupillary sphincter. The sphincter was then constricted with pilocarpine, successfully maintaining the PC IOL in the posterior chamber (Figure 18B).
Conclusion
Nd:YAG laser photodisruption allows a surgeon to effectively reach inside the eye with a pair of microscissors delivered on a beam of light. In rare circumstances, photodisruptive laser energy can be constructively used to push and cut. Photodisruption gives the surgeon additional options in the correction of a wide range of complications following cataract surgery.
Suggested Reading
- Shaffer RN. The role of vitreous detachment in aphakic and malignant glaucoma. Trans Am Ophthalmol Otolaryngol. 1954;28(2):217-231.
- Shaffer RN. A suggested anatomic classification to define the pupillary block glaucomas. Invest Ophthalmol. 1973;12(7):540-542.
- Epstein DL, Steinert RF, Puliafito CA. Neodymium-YAG laser therapy to the anterior hyaloid in aphakic malignant (ciliovitreal block) glaucoma. Am J Ophthalmol. 1984;98(2):137-143.
- Irvine SR. A newly defined vitreous syndrome following cataract surgery. Am J Ophthalmol. 1953;36(5):599-619.
- Gass JDM, Norton EWD. Cystoid macular edema and papilledema following cataract extraction. A fluorescein fundoscopic and angiographic study. Arch Ophthalmol. 1966;76(5):646-661.
- Iliff CE. Treatment of the vitreous tug syndrome. Am J Ophthalmol. 1966;62(5):856-859.
- Katzen LE, Fleischman JA, Trokel S. YAG laser treatment of cystoid macular edema. Am J Ophthalmol. 1983;95(5):589-592.
- Gass JDM, Norton EWD. Follow-up study of cystoid macular edema following cataract extraction. Trans Am Acad Ophthalmol Otolaryngol. 1969;73(4):665-682.
- Jacobson DR, Dellaporta A. Natural history of cystoid macular edema after cataract extraction. Am J Ophthalmol. 1974;77(4):445-447.
- Steinert RF, Wasson PJ. Neodymium:YAG laser anterior vitreolysis for Irvine-Gass cystoid macular edema. J Cataract Refract Surg. 1989;15(3):304-307.
- Davison JA. Capsule contraction syndrome. J Cataract Refract Surg. 1993;19(5):582-589.