It has become axiomatic that we still do not understand the mechanism of accommodation despite more than 200 years of scientific research and debate. However, the technological advances and research of the past decade have solved some of the mysteries surrounding accommodation. This article discusses some of this new knowledge and integrates it into a consolidated theory of accommodation that can help us discover ways to surgically correct presbyopia.
Technology advances understanding
Ongoing international scientific research has rapidly advanced our knowledge and tested our theories. 1 The use of ultrasound biomicroscopy (UBM) and optical coherence tomography (OCT) have enabled researchers to accurately measure the anatomic components involved in accommodation. Ray tracing and wavefront analysis now correlate direct measurements of the anatomic structures with UBM and OCT. The EVAS II (ex vivo accommodation simulator developed by Jean-Marie Parel, PhD, at Bascom Palmer Eye Institute and modified in Australia) integrated with 3-D image processing is a lens stretcher allowing for accurate measurements of accommodative structures under controlled experimental conditions.2 All of these technologies are used in developing finite element analyses and mathematical models of accommodation. Currently, there is an emphasis on correlating changes in wavefront with direct morphologic changes in the lens during accommodation. Improvements in UBM (e.g., Artemis 3) and OCT (Heidelberg) allow the thickness of the lens and curvature of the anterior and posterior capsule to be measured.
It is helpful to view the structures of accommodation in motion. By viewing this video of a UBM of a young adult on continuous loop and stopping to observe structures during the accommodative and disaccommodative stages, the following can be seen:
- Ciliary body contraction resulting in anterior and centripetal movement of the ciliary body.
- Changes in lens shape and position. As a result of ciliary body contraction, the lens equator diameter decreases by 3.5 percent; lens thickness increases by 300 microns, with the anterior lens surface moving forward by 250 microns and the posterior lens surface moving backward by 50 microns; and the lens has a net forward movement of 100 microns. More importantly, the anterior capsule steepens centrally, comprising the largest element of the refractive change during accommodation.
The main limitation of UBM is that the depth of examination is limited to 4 mm, with poor visualization of the posterior capsule and vitreous membrane. Other significant findings are:
- Posterior bowing of the iris in the midperiphery, with an increase in anterior chamber depth (ACD) in the midperiphery at the same time that ACD decreases centrally.
- A decrease in ciliary sulcus diameter with accommodation and a decrease in distance from the scleral spur to the origin of the posterior zonule and vitreous membrane.
Two hundred years of research
In 1801, Thomas Young introduced his theory of the mechanism of the eye. Subsequently, in the 1850s, Hermann von Helmholtz formulated his theory on accommodation based on changes in the lens and lens capsule. The lenticular theory is largely confirmed by current science, although Helmholtz did not recognize changes in the curvature of the posterior capsule. The theory of and changes predicted in the lens by Ronald A. Schachar, MD, PhD, have not been supported by other research.3
Another major theme in accommodative theory relates to the role of extralenticular components, including the vitreous, with origins in the theories of Cramer in 1851 and Danish ophthalmologist Marius Tscherning in 1900. In the 1970s and 1980s, D. Jackson Coleman, MD, used superb reasoning and experiments to formulate a strong case for his hydraulic suspension or caternary theory of accommodation.4,5 His research explored the anatomy of the posterior lens and its zonular attachments to the vitreous membrane. It set the stage for further advancing our knowledge of accommodation.
A recently-published study by Mary Ann Croft and colleagues represents a breakthrough in our knowledge of the anatomy of the vitreous or posterior zonule.6 It underscores the tenet that understanding anatomy is the key to understanding physiology. Using UBM correlated with improved scanning electron microscopy (EM) in human and rhesus monkey eyes, the authors were able to visualize three components of the posterior vitreous zonule, including anterior, intermediate and posterior elements. Prior understanding of this anatomy is based on Zinn's description of the anterior zonule and Weiger's description of the ligament attaching the vitreous membrane to the posterior lens capsule.
Elasticity of the lens capsule and zonules has been demonstrated according to Young's modulus, as described by Finchem in 1930 and Fisher in 1969. Rohen described the EM of zonules in 1979 but did not observe the complexity of the vitreous zonule due to limitations in fixation techniques. The intermediate vitreous zonule, also called pars plana zonule, was visualized with UBM by Strenck, Kaufman and Coleman.
The Croft study demonstrates the anatomy of the vitreous zonule without separation artifacts on EM and correlates the loss of forward movement of the ciliary body with aging.6 The experiments on monkeys demonstrate that lysis of the pars plana zonule (see Figure 1) improves the age-related loss of ciliary body movement. This illustrates an extralenticular mechanism of the pathophysiology of presbyopia, as well as a therapeutic target for presbyopia surgery.
Click on image to enlarge.
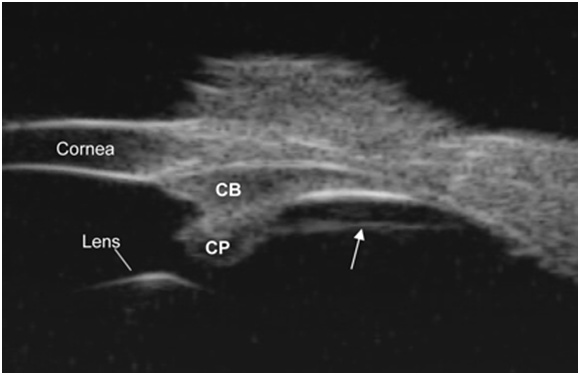
Figure 1. Pars plana zonule ultrasound biomicroscopy (UBM) overview image in a live rhesus monkey shows a prominent straight line (see the arrow) extending from the pars plicata region of the ciliary body to the ora serrata region and separated from the pars plana epithelium by a cleft. CP=ciliary processes. CB=ciliary body.
Image courtesy of Mary Ann Croft.
The posterior vitreous zonule with adjacent vitreous membrane, pars plana zonule and inner limiting membrane of the ciliary epithelium over the pars plana are joined in an attachment zone located circumferentially immediately anterior to the ora serrata. The posterior vitreous zonule attached peripherally to the vitreous membrane (which attaches to the posterior lens capsule in a ring behind the insertion of the posterior tines of the anterior zonule of Zinn) is pulled forward by the ciliary muscle during accommodation.
The intermediate pars plana zonule originates in the pars plana attachment zone and inserts at the base of the ciliary processes. It acts like a tether that pulls on the vitreous zonule when the ciliary body contracts.
Croft demonstrates a decreased distance between the scleral spur and posterior zonule attachment during accommodation. Therefore, during accommodation, the ciliary body moves forward and centripetally. At the same time, the posterior zonular attachments are interconnected with the vitreous membrane, and the peripheral posterior lens capsule is pulled forward. The effect of aging on the pars plana zonule with the reduction in the cleft may explain how Schachar scleral implants can improve accommodation and possibly how scleral relaxing incisions allow the sclera to mold during accommodation, resulting in less restriction of the pars plana zonule. In this instance, even though the Schachar theory has not been scientifically validated, the scleral implants or relaxing incisions may work in some eyes because of reduced forward restriction of ciliary body movement due to reduced restriction of the pars plana zonule.
Modifying accommodation theory
Based on these findings, as well as additional scientific evidence accumulated over the past 10 years, there is a compelling need to incorporate into accommodation theory the role of the posterior zonule and vitreous membrane. Below I present a consolidated theory of accommodation. It is my hope that it will be received with the recognition that these ideas are based on the monumental scientific accomplishments documented in the literature. I hope that the scientific community will carefully analyze and test these ideas so that further progress can be made on the surgical correction of presbyopia.
Goldberg's Postulate of Reciprocal Zonular Action and Consolidated Theory of Accommodation
The engine of accommodation is the contraction of the ciliary body mediated by the distribution of forces via all elements of the zonular apparatus. The resultant changes in refraction are due to the increased thickness of the lens and the increased curvature of the anterior and posterior capsule. The complex anatomy of the vitreous zonule results in traction on the vitreous membrane peripherally with anterior displacement of the vitreous membrane peripherally. At the same time, there is posterior displacement of the vitreous membrane centrally. This creates space for the thickening of the lens centrally and posterior movement of the posterior lens capsule during accommodation. The volume of fluid in the anterior aqueous compartment, including both the anterior and posterior chamber, and the volume of fluid in the posterior vitreous compartment does not change. However, the shape of the anterior compartment changes with decreased anterior chamber depth centrally and increased depth in the midperiphery (as shown by posterior bowing of the iris on UBM). A portion of the energy supplied by the ciliary body contraction results in the stretching of the posterior zonular apparatus.
During disaccommodation, the lens returns to its original nonaccommodative state as a result of traction of the anterior zonules accompanied by a return of the vitreous compartment and the shape of the vitreous membrane to their nonaccommodative states. There is a reciprocal action of the anterior zonules and posterior zonules. During ciliary body contraction, the anterior zonules lose tension and relax while the posterior zonules stretch and exert force on the posterior lens capsule. They play some role in shaping posterior lens thickness and curvature. During ciliary body relaxation, the posterior zonules lose tension as the lens flattens and is pulled back by the increasing tension of the anterior zonules.
Implications for surgical correction of presbyopia
This theory of accommodation has many implications for the surgical correction of presbyopia. As the Croft study demonstrates, lysis of the pars plana zonule in monkeys can improve the age-related loss of ciliary body movement. Perhaps laser lysis of the pars plana zonule in humans will enhance the performance of accommodating IOLs.
Single-optic accommodating IOLs based on anterior movement of the optic are limited in performance.7 Since the scientific findings with UBM demonstrate that the forward movement of the human lens during accommodation is only 100 microns, it is unlikely that this mechanism will be sufficient. It is also possible that single-optic IOLs (e.g., Crystalens) can, at least in some eyes, arch during ciliary body contraction, as evidenced by dynamic wavefront measurements.
It may be possible to enhance the accommodative arching by making design changes to the IOL or adding enhancements, such as capsular tension rings (CTRs), to optimize the shape and tension of the posterior capsule and zonular apparatus. Many new IOL designs are under development, some of them with moving parts built into the IOL (e.g., NuLens, FluidVision and Akkolens). Several of these new designs use ciliary sulcus fixation while leaving the posterior capsule for support. This represents a new direction in accommodative IOL design that is consistent with the UBM and EM findings discussed.
Related research demonstrates that the capsular bag flattens after extracapsular cataract extraction (ECCE). Initially, there is an increase in bag diameter followed by contraction and fibrosis. Studies demonstrate that zonular tension on the capsule still occurs following ECCE, but capsule diameter and elasticity are unpredictable. The anterior-posterior position of the IOL in relation to the ciliary ring may also cause results to vary.
Wasilewski showed that lens surgery that leaves the posterior capsule intact facilitates forward accommodative ciliary body movement. 8 Therefore, the posterior capsule should remain. It may be better to keep the posterior capsule as a support but to utilize ciliary sulcus fixation instead of capsular bag fixation. There may be improvement in accommodative function with a controlled size or shape of the contracted post-ECCE capsule.
Currently, studies in Europe are investigating the ideal diameter of the contracted capsule as IOL sizing may be a critical variable.9 While it is evident that a circular-shaped capsular bag would distribute the zonular tension over 360 degrees, it is possible that accommodating IOLs (e.g., Crystalens) would perform better if the capsular bag contracted to form an oval shape that could distribute the forces along the long axis of the IOL and improve arching.
I welcome serious debate and scientific validation of the 'reciprocal-zonule' postulate and the consolidated theory of accommodation.
References
- Glasser, A. Restoration of accommodation: surgical options for correction of presbyopia. Clin Exp Optom. 2008;91(3):279-295.
- Urs, R. Investigation of Accommodation and Presbyopia using Ultrasound Imaging during Ex Vivo Simulated Accommodation. 2010:University of Miami, Open Access Dissertations. Paper 360.
- Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106(5):863-872.
- Coleman DJ. Unified model for accommodative mechanism. American Journal of Ophthalmology. 1970;69:1063-1079.
- Coleman DJ. On the hydraulic suspension theory of accommodation. Trans Am Ophthalmol Soc. 1986;84:846-868.
- Luetjen-Drecoll E, Kaufman PL, Wasilewski R, Ting-Li L, Croft MA. Morphology and Accommodative Function of the Vitreous Zonule in Human and Monkey Eyes. Invest Ophth Vis Sci. 2010;51(3):1554-1564.
- Menapace R, Findl O, KriechbaumK, Leydolt-Koeppl Ch. Accommodating intraocular lenses: a critical review of present and future concepts. Graefes Arch Clin Exp Ophthalmol. 2007;245(4):473-489.
- Wasilewski R, McDonald JP, Heatley G, et al. Surgical Intervention and Accommodative Responses, II: forward Ciliary Body Accommodative Movement is Facilitated by Zonular Attachments to the Lens Capsule. Invest Ophth Vis Sci. 2008;49(12):5495-5502.
- Marchini G, Pedrotti E, Modesti M, Visentin S, Tosi R. Anterior segment changes during accommodation in eyes with a monofocal intraocular lens: high-frequency ultrasound study. J Cataract Refract Surg. 2008;34(6):949-956.
Author Disclosure
Dr. Goldberg has no financial interests to disclose.