SEP 14, 2017
By Karen G. Zaarour, MD; Elias I. Traboulsi, MD
A Compendium of Inherited Disorders and the Eye, Oxford University Press
Genetics
OMIM Numbers
Inheritance
- X-linked dominant
- Lethal in hemizygous males
- De novo mutation
Gene/Gene Map
Epidemiology
- The incidence rates of Aicardi syndrome are estimated at 1:105,000 in the US, 1:93,000 in the Netherlands, and 1:110,000 in Northern Ireland. There are more than 850 cases in the US and the worldwide estimate of prevalence is several thousand. A prevalence of 0.63 per 100,000 females was found in Norway.1
- Kroner et al. (2008) found that the risk of death peaked at age 16 years. The probability of survival to 27 years of age was 0.62 and the risk of death by age follows other congenital neurological disorders, with a wide range in severity of functional disability.2
Description
- Jean Aicardi et al. first described this condition, now named after him, in 1965.3 It is a rare neurodevelopmental disorder originally characterized by the triad of:
- Infantile spasms
- Partial or total agenesis of the corpus callosum
- Typical lacuna-shaped chorioretinal lesions
- Sutton et al. (2005) proposed modified diagnostic criteria for Aicardi syndrome.4 It was suggested that if all 3 classic features of the triad were present, the diagnosis would be clinically confirmed. If 2 classic features plus at least 2 other major or supporting features were present, the diagnosis would be highly suspected.
- Major features include:
- Cortical malformations
- Periventricular and subcortical heterotopia
- Cysts around the third cerebral ventricle and/or choroid plexus
- Optic disc/nerve coloboma or hypoplasia
- Supporting features:
- Vertebral or rib abnormalities
- Microphthalmia
Clinical Findings
- The clinical outcome is generally severe, with poor cognitive development, moderate to severe global developmental delay, and difficult-to-treat epilepsy, although Grosso et al. (2007) reported a case of Aicardi syndrome with normal cognitive functions. In most cases, seizures manifest before one year of age, and many girls present with severe episodes during the first 3 months of life.5
- Multiple structural central nervous system abnormalities have been documented, including: cortical migration anomalies (eg, pachygyria, cortical heterotopia and polymicrogyria), cysts around the third cerebral ventricle, pineal cyst, arachnoid cyst, cerebral hemispheric asymmetry, microcephaly, ventricular septal defect, colpocephaly, enlargement of the tectum, and a Dandy-Walker variant.
- Aicardi syndrome may also be associated with systemic anomalies such as vertebral malformations (eg, fused vertebrae, scoliosis, spina bifida), costal malformations (eg, absent ribs, fused or bifurcated ribs), hip dysplasia, muscular hypotonia, polydactyly, cleft lip and/or palate, auricular anomalies, hiatal hernia, gastrointestinal tract dysfunction, autism traits, and precocious or delayed puberty.
- A constellation of facial anomalies (prominent premaxilla, upturned nasal tip, decreased angle of nasal bridge, sparse lateral eyebrows, deep philtrum, upslanting palpebral fissures) has also been described in Aicardi syndrome.
- A few patients have developed tumors such as choroidal plexus papilloma, teratoma of the soft palate, embryonal carcinoma of the cheek, hepatoblastoma, nevoid hypertrichosis of the face, oral extragonadal yolk sac tumor, orbital ectopic brain tissue, orbital cyst, arachnoid cyst, metastatic angiosarcoma with scalp lipoma, and large cell medulloblastoma.6-12
Ocular Findings
- In his report of a large case series in 1969, Aicardi stated that the ocular findings of chorioretinal lacunae are an essential feature of this syndrome. Although virtually pathognomonic of Aicardi syndrome, they have been seen in other conditions, including orofaciodigital syndrome type IX. The chorioretinal lacunae consist of well-circumscribed, full-thickness defects limited to the retinal pigment epithelium (RPE) and choroid, with an intact overlying retina that may appear histologically abnormal. These lesions are most commonly found around the optic nerve head and the posterior pole (Figure 1) and typically decrease in size towards the peripheral fundus (Figure 2). Previous reports have noted increased pigmentation or fading of lacunae over time. Unilateral chorioretinal lacunae do not rule out the diagnosis of Aicardi syndrome in the presence of the clinical picture of the syndrome.13
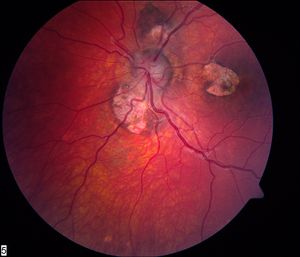
Figure 1. Left eye of a patient with Aicardi syndrome. The optic nerve head is dysplastic and has a grayish appearance. There are 3 lacunar lesions around the disk.
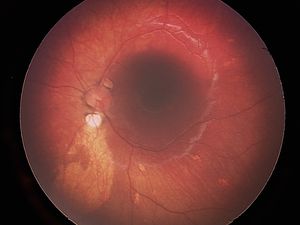
Figure 2. Left eye of another patient with Aicardi syndrome revealing an inferior para-papillary lacuna, as well as a large oval area of RPE depigmentation inferiorly. Note numerous midperipheral ovoid depigmented lesions.
- Description of RPE structures in postmortem histopathological studies have shown variable findings ranging from absence of RPE, attenuated RPE, or hyperplasia of RPE with lacunae. The choriocapillaris vessels were found to be decreased in caliber and number at the level of lacunae. Some histopathological specimens showed well-preserved neurosensory retina, whereas others showed glial tissue replacing the neurosensory retina overlying lacunae.
- A more recent chorioretinal architecture study by optical coherence tomography and fluorescein angiography in an 8-month-old girl with Aicardi syndrome demonstrated that lacunae are, in fact, subretinal fluid-filled cavities overlying typically absent RPE. Thin choroid and sclera form the base of these cavities. An abnormal neurosensory retina, characterized by disruption of the photoreceptors and intraretinal cysts, overlies the cavities.14
- Other ophthalmic features include optic nerve coloboma or hypoplasia and microphthalmia. Other features have been reported more sporadically, including optic nerve aplasia, increased rate of excavated disc anomalies, morning glory abnormality, nystagmus, sixth cranial nerve palsy, persistent pupillary membrane, iris cyst, iris coloboma, aniridia, peripheral retinal dysplasia, glial tissue extending from the optic disc, hyperplastic primary vitreous, detached retina, choroidal neovascularization (treated successfully with bevacizumab), retinopathy of prematurity, severe congenital ptosis, and late onset retinoblastoma.15–20
Neuroimaging findings
- The diagnosis of Aicardi syndrome can be suspected by prenatal ultrasound with color Doppler identifying the agenesis or dysgenesis of the corpus callosum. In the neonatal period, dysgenesis can be identified by transfontanellar ultrasound.21
- Hopkins et al. (2008) reported detailed brain magnetic resonance imaging (MRI) findings of 23 patients with Aicardi syndrome. Apart from corpus callosum partial or total agenesis, additional neuroimaging findings included ventriculomegaly with colpocephaly, polymicrogyria, periventricular and subcortical heterotopias, intracranial cysts, and cerebellar abnormalities.22
- Diffusion tensor imaging at 3 Tesla performed on 2 subjects with Aicardi syndrome by Wahl et al. revealed a widespread disruption in the corticocortical white matter organization, which appeared to be specific to Aicardi syndrome and not shared by other neurodevelopmental disorders with similar anatomic manifestations.23
Genetic studies
- Despite a well-recognized clinical picture for more than 50 years, the etiology of Aicardi syndrome is still unknown. Considering Aicardi syndrome as a monogenic X-linked dominant disease caused by a de novo mutation in a gene in the X chromosome has been a leading hypothesis.6,24
- Ropers et al. (1982) found a case of Aicardi syndrome in a girl in whom the breakpoint was in Xp22, between p22.2 and p22.3.25,26 Donnerfeld et al. (1989) found normal chromosomes in 17 out of 18 patients with Aicardi syndrome, except for an unbalanced X;3 translocation involving a breakpoint at Xp22.3 in a girl with chorioretinal lacunar lesions characteristic of the syndrome, in addition to developmental delay and infantile seizures. However, her brain MRI and CT failed to show any dysgenesis of the corpus callosum.27 Nielson et al. (1991) also failed to find any evidence of microdeletion in a patient with Aicardi syndrome using 5 DNA markers from the Xp22.3-p21.3 region.28
- Astrocytic inclusions containing filamin have been found, but the molecular defect is not yet known.29,30 Genetic studies attempting to identify the mutated gene using array comparative hybridization with genome-wide DNA-microarrays31 and X –chromosome DNA-microarrays32 have not been successful to date. The variability in the severity of the Aicardi syndrome phenotype could be attributed to the presumed mutated gene undergoing X-chromosome inactivation. Studies on this topic have shown contradictory results, showing either random or skewed X-chromosome inactivation patterns. A non X-linked cause of Aicardi syndrome has been suggested, partly due to a lack in several X-inactivation studies of nonrandom X chromosome inactivation, which would be expected in a disorder with an X-linked genetic defect.33 However, this is not always present in X-linked disorders such as Rett syndrome. Furthermore, a causal mutation in an autosomal gene with a sex-limited effect that causes Aicardi syndrome in females has also been considered.34
- Aicardi syndrome has been reported several times in 47, XXY males35–37, which is still consistent with an X-linked dominant inheritance with a possibility of mosaic pathogenic variants in the presumed Aicardi syndrome gene. However, the affected XY males reported by Curatolo et al. (1980), Aggarwal et al. (2000) and Chappelow at al. (2008), argue against this putative hypothesis.38–40
- Until the genetic etiology of Aicardi syndrome is confirmed, the possibility of a de novo pathogenic variant on an autosome with gender-limited expression in females remains. A recent publication identified de novo mutation in 2 affected girls: one carried a nonsense mutation in TEAD1 (OMIM 189967) and the second manifested a missense mutation in OCEL1.41 These data suggested that the clinical diagnosis of Aicardi syndrome may be genetically heterogeneous, and may be caused by mutation in autosomal genes. However, Bibiana Wong et al. (2017), sequenced TEAD1 and OCEL1 coding regions using DNA from 38 clinically well-characterized girls with the syndrome and failed to detect the previously reported variants, suggesting that these previously published variants represent either an extremely rare cause of Aicardi syndrome or an incidental finding.42
- Peripapillary chorioretinal lacunae in a girl with 3q21.3 to 3q22.1 microdeletion with features of Aicardi syndrome was described.43 A molecular characterization of a monosomy 1p36, presented as an Aicardi syndrome, raised the question of whether to consider the monosomy 1p36 in the differential diagnosis of the syndrome. However, the girl lacked typical chorioretinal lacunae.44
- Nemos et al. (2009) excluded mutations in the CDLK5 gene (300203) previously attributed to early infantile epileptic encephalopathy and the development of Rett syndrome-like features in 10 French patients with Aicardi syndrome.45
Differential diagnosis
- Agenesis of the corpus callosum in isolation or in association with other brain malformations or syndromes.
- Microcephaly with or without chorioretinopathy, lymphedema, or mental retardation (MCLMR) (OMIM 152950).
- Neuronal migration disorders, such as polymicrogyria, pachygyria and heterotopia.
- Oculocerebrocutaneous syndrome (OCCS) (OMIM 164180).
- Infantile spasms in isolation or as part of other syndromes.
- Orofaciodigital syndrome type IX (OFD 9) (OMIM 258865) also may manifest chorioretinal lacunae.
- Goltz syndrome (microphthalmia and other developmental eye defects) and microphthalmia with linear skin (MLS) defects syndrome.
Therapeutic considerations
- Treatment generally consists of multiple antiepileptic medications and general supportive measures. Chau et al. (2004) demonstrated a role of early treatment with vigabatrin in improving outcome in Aicardi syndrome.46 However, seizures are frequent, of different types (predominantly spastic flexion seizure) and typically refractory to medical therapy.
- Palliative epilepsy surgery such as corpus callostomy of a partial corpus callosum, vagus nerve stimulator implantation and hemispherectomy, showed variable outcomes ranging from near complete resolution of seizures to worsening of seizure profile.47
- Associated health issues, such as tumors or aspiration pneumonia, should be treated promptly to improve prognosis.
- Physical therapy, speech therapy, occupational therapy, and vision therapy should be started as soon as possible to ensure the maximum functionality and quality of life.
- Appropriate musculoskeletal therapy for management of scoliosis-related complications and spasticity is of high importance.
- Genetic counseling is advocated.
References
- Lund C, Bjørnvold M, Tuft M, Kostov H, Røsby O, Selmer KK. Aicardi syndrome: an epidemiologic and clinical study in Norway. Pediatr Neurol. 2015 Feb;52(2):182–186.e3.
- Kroner BL, Preiss LR, Ardini M-A, Gaillard WD. New incidence, prevalence, and survival of Aicardi syndrome from 408 cases. J Child Neurol. 2008 May;23(5):531–535.
- Aicardi Syndrome - Ophthalmology [Internet]. [cited 2017 May 26]. Available from: http://www.aaojournal.org/article/S0161-6420(91)32059-1/fulltext
- Sutton VR, Hopkins BJ, Eble TN, Gambhir N, Lewis RA, Van den Veyver IB. Facial and physical features of Aicardi syndrome: infants to teenagers. Am J Med Genet A. 2005 Oct 15;138A(3):254–258.
- Grosso S, Lasorella G, Russo A, Galluzzi P, Morgese G, Balestri P. Aicardi syndrome with favorable outcome: case report and review. Brain Dev. 2007 Aug;29(7):443–446.
- Sutton VR, Van den Veyver IB. Aicardi Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, et al., editors. GeneReviews(®) [Internet]. Seattle (WA): University of Washington, Seattle; 1993 [cited 2017 May 26]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1381/
- Happle R, Kroll P. Nevoid hypertrichosis of the face in a 3-month-old girl with Aicardi syndrome. Eur J Dermatol. 2013 Aug;23(4):547–548.
- Kamien BA, Gabbett MT. Aicardi syndrome associated with hepatoblastoma and pulmonary sequestration. Am J Med Genet A. 2009 Aug;149A(8):1850–1852.
- Yüksel D, Yilmaz D, Usak E, Senbil N, Gürer Y. Arachnoid cyst and costovertebral defects in Aicardi syndrome. J Paediatr Child Health. 2009 Jun;45(6):391–392.
- Taggard DA, Menezes AH. Three choroid plexus papillomas in a patient with Aicardi syndrome. A case report. Pediatr Neurosurg. 2000 Oct;33(4):219–223.
- Trifiletti RR, Incorpora G, Polizzi A, Cocuzza MD, Bolan EA, Parano E. Aicardi syndrome with multiple tumors: a case report with literature review. Brain Dev. 1995 Aug;17(4):283–285.
- Kiriştioğlu I, Kiliç N, Gürpinar AN, Doğruyol H. Aicardi syndrome associated with palatal hemangioma. Eur J Pediatr Surg. 1999 Oct;9(5):325–326.
- Guerriero S, Sciruicchio V, De Blasi R, Furino C, Smaldone G, Ciracì L, et al. Chorioretinal lacunae: pathognomonic findings for Aicardi syndrome. J Pediatr Ophthalmol Strabismus. 2010 May 21;47 Online:e1-3.
- Martel JN, Rutar T, Lujan BJ, de Alba Campomanes A. Chorioretinal architecture in Aicardi syndrome: an optical coherence tomography and fluorescein angiography study. J AAPOS. 2011 Jun;15(3):308–310.
- Shirley K, O’Keefe M, McKee S, McLoone E. A clinical study of Aicardi syndrome in Northern Ireland: the spectrum of ophthalmic findings. Eye Lond Engl. 2016 Jul;30(7):1011–1016.
- Fruhman G, Eble TN, Gambhir N, Sutton VR, Van den Veyver IB, Lewis RA. Ophthalmologic findings in Aicardi syndrome. J AAPOS. 2012 Jun;16(3):238–241.
- Aziz HA, Sisk RA, Berrocal AM, Murray TG. Optic nerve aplasia in Aicardi syndrome. J Pediatr Ophthalmol Strabismus. 2010 May 21;47 Online:e1-4.
- Wenick AS, Paskowitz DM, Tauqir MZ, Nguyen QD. Choroidal neovascularization and bevacizumab therapy in Aicardi syndrome. Graefes Arch Clin Exp Ophthalmol. 2013 Mar;251(3):1015–1017.
- Chappaz A, Barthelmes D, Buser L, Funk J, Gerth-Kahlert C. Iris cyst in a child with Aicardi syndrome: a novel association. J AAPOS. 2016 Oct;20(5):451–452.
- Leng T, Moshfeghi DM. Retinopathy of prematurity in an infant with Aicardi’s syndrome. Eye Lond Engl. 2011 Feb;25(2):257–258.
- Aicardi syndrome: Neonatal diagnosis by means of transfontanellar ultrasound [Internet]. [cited 2017 May 26]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4109104/
- Hopkins B, Sutton VR, Lewis RA, Van den Veyver I, Clark G. Neuroimaging aspects of Aicardi syndrome. Am J Med Genet A. 2008 Nov 15;146A(22):2871–2878.
- Wahl M, Strominger ZA, Wakahiro M, Jeremy RJ, Mukherjee P, Sherr EH. Diffusion tensor imaging of Aicardi syndrome. Pediatr Neurol. 2010 Aug;43(2):87–91.
- Aicardi J, Chevrie JJ, Rousselie F. [Spasma-in-flexion syndrome, callosal agenesis, chorioretinal abnormalities]. Arch Fr Pediatr. 1969;26(10):1103–1120.
- Ropers HH, Zuffardi O, Bianchi E, Tiepolo L. Agenesis of corpus callosum, ocular, and skeletal anomalies (X-linked dominant Aicardi’s syndrome) in a girl with balanced X/3 translocation. Hum Genet. 1982;61(4):364–368.
- Neidich JA, Nussbaum RL, Packer RJ, Emanuel BS, Puck JM. Heterogeneity of clinical severity and molecular lesions in Aicardi syndrome. J Pediatr. 1990 Jun;116(6):911–917.
- Donnenfeld AE, Packer RJ, Zackai EH, Chee CM, Sellinger B, Emanuel BS. Clinical, cytogenetic, and pedigree findings in 18 cases of Aicardi syndrome. Am J Med Genet. 1989 Apr;32(4):461–467.
- Nielsen KB, Anvret M, Flodmark O, Furuskog P, Bohman-Valis K. Aicardi syndrome: early neuroradiological manifestations and results of DNA studies in one patient. Am J Med Genet. 1991 Jan;38(1):65–68.
- Van den Veyver IB, Panichkul PP, Antalffy BA, Sun Y, Hunter JV, Armstrong DD. Presence of filamin in the astrocytic inclusions of Aicardi syndrome. Pediatr Neurol. 2004 Jan;30(1):7–15.
- Prayson RA. Hyaline Protoplasmic Astrocytopathy: A Clinicopathologic Study. Am J Clin Pathol. 2016 Oct;146(4):503–509.
- Wang X, Sutton VR, Eble TN, Lewis RA, Gunaratne P, Patel A, et al. A genome-wide screen for copy number alterations in Aicardi syndrome. Am J Med Genet A. 2009 Oct;149A(10):2113–2121.
- Yilmaz S, Fontaine H, Brochet K, Grégoire M-J, Devignes M-D, Schaff J-L, et al. Screening of subtle copy number changes in Aicardi syndrome patients with a high resolution X chromosome array-CGH. Eur J Med Genet. 2007 Oct;50(5):386–391.
- Eble TN, Sutton VR, Sangi-Haghpeykar H, Wang X, Jin W, Lewis RA, et al. Non-random X chromosome inactivation in Aicardi syndrome. Hum Genet. 2009 Mar;125(2):211–216.
- Prontera P, Bartocci A, Ottaviani V, Isidori I, Rogaia D, Ardisia C, et al. Aicardi syndrome associated with autosomal genomic imbalance: coincidence or eEvidence for autosomal inheritance with sex-limited expression? Mol Syndromol. 2013 Apr;4(4):197–202.
- Shetty J, Fraser J, Goudie D, Kirkpatrick M. Aicardi syndrome in a 47 XXY male - a variable developmental phenotype? Eur J Paediatr Neurol. 2014 Jul;18(4):529–531.
- Zubairi MS, Carter RF, Ronen GM. A male phenotype with Aicardi syndrome. J Child Neurol. 2009 Feb;24(2):204–207.
- Chen T-H, Chao M-C, Lin L-C, Jong Y-J, Yang SN, Lai Y-H, et al. Aicardi syndrome in a 47, XXY male neonate with lissencephaly and holoprosencephaly. J Neurol Sci. 2009 Mar 15;278(1–2):138–140.
- Chappelow AV, Reid J, Parikh S, Traboulsi EI. Aicardi syndrome in a genotypic male. Ophthalmic Genet. 2008 Dec;29(4):181–183.
- Curatolo P, Libutti G, Dallapiccola B. Aicardi syndrome in a male infant. J Pediatr. 1980 Feb;96(2):286–287.
- Aggarwal KC, Aggarwal A, Prasad MS, Salhan RN, Upadhaya A. Aicardi’s syndrome in a male child: an unusual presentation. Indian Pediatr. 2000 May;37(5):542–545.
- Schrauwen I, Szelinger S, Siniard AL, Corneveaux JJ, Kurdoglu A, Richholt R, et al. A De Novo Mutation in TEAD1 Causes Non-X-Linked Aicardi Syndrome. Invest Ophthalmol Vis Sci. 2015 Jun;56(6):3896–3904.
- Wong BK, Sutton VR, Lewis RA, Van den Veyver IB. Independent variant analysis of TEAD1 and OCEL1 in 38 Aicardi syndrome patients. Mol Genet Genomic Med. 2017 Jan 25;5(2):117–121.
- Broomall E, Renaud D, Ghadban R, Gavrilova R, Brodsky MC. Peripapillary chorioretinal lacunae in a girl with 3q21.3 to 3q22.1 microdeletion with features of aicardi syndrome. JAMA Ophthalmol. 2013 Nov;131(11):1485–1487.
- Molecular Characterization of a Monosomy 1p36 Presenting as an Aicardi Syndrome Phenocopy [Internet]. PubMed Journals. [cited 2017 May 26]. Available from: https://ncbi.nlm.nih.gov/labs/articles/19842196/
- Nemos C, Lambert L, Giuliano F, Doray B, Roubertie A, Goldenberg A, et al. Mutational spectrum of CDKL5 in early-onset encephalopathies: a study of a large collection of French patients and review of the literature. Clin Genet. 2009 Oct;76(4):357–371.
- Chau V, Karvelas G, Jacob P, Carmant L. Early treatment of Aicardi syndrome with vigabatrin can improve outcome. Neurology. 2004 Nov 9;63(9):1756–1757.
- Kasasbeh AS, Gurnett CA, Smyth MD. Palliative epilepsy surgery in Aicardi syndrome: a case series and review of literature. Childs Nerv Syst. 2014 Mar;30(3):497–503.
Resources
- Medline Plus
Aicardi syndrome