Superficial Foreign Body and Corneal Abrasions
Corneal abrasions (Figure 1) are a very common presentation to the outpatient primary care and emergency room physician. Though some differences exist, management is similar to the adult algorithm. Most childhood corneal abrasions occur during general play, and often children cannot give a good history of the exact traumatic event. With that said, suspect abrasion in any child who will not open the eye or who has tearing and pain.
It is very important to perform a thorough physical exam to rule out alternative or additional pathology such as corneal foreign body, a corneal ulcer, or infectious etiology such as herpes simplex keratitis. Pay special attention to the eyelids and fornices because a retained superficial foreign body can cause continued damage to the corneal epithelium during blinking. A clue to this can be the characteristic vertical linear abrasion lines seen on fluorescein staining.
Treatment typically involves a topical antibiotic as prophylaxis against infection while the protective corneal epithelium is absent. Either ointment or drops can be used, based on the physician's and the family's preference.1 Evidence is lacking for antibiotic treatment in an uncomplicated corneal abrasion, but the general consensus is that it helps to prevent superinfection, so it is commonplace to treat in most communities. There is evidence for antibiotic treatment in complicated abrasions, including in those who wear contacts, or abrasions with retained foreign bodies or trauma with organic matter.2
If the patient wears contacts, provide antipseudomonal coverage such as a topical fluoroquinolone.
Topical NSAIDs such as diclofenac 0.1% have been shown effective for pain control and do not delay wound healing.3 They carry a risk of corneal toxicity, so it is best for patients to use topical NSAIDs for only 2–3 days.4
Cycloplegics have not been shown to carry a benefit in pain relief5 unless there is also coexistent traumatic iritis. Historically, patching has been used, but recent studies have shown no improvement in healing time or reduction of pain, so most physicians no longer use a pressure patch.6
Most corneal abrasions heal well without any complications in 1–2 days. If the abrasion is uncomplicated and less than 4 mm in size and the patient has no decrease in vision, there is no need to follow up unless symptoms worsen or do not resolve in the expected time frame. If the patient is a contact-lens wearer or if the lesion is greater than 4 mm or otherwise complicated, follow-up is recommended in 1–2 days to ensure resolution.
Rarely, repeat corneal abrasions can lead to recurrent corneal erosion syndrome, which is characterized by the epithelial layer failing to adhere fully to its basement membrane (Bowman's layer). This leads to recurrent small abrasions, classically waking patients with pain after REM sleep. Attacks are treated as with a typical corneal abrasion. If the recurrences are frequent, consideration can be given to anterior stromal puncture or YAG laser treatments to Bowman's layer to stimulate better adherence to the epithelium.7
As mentioned previously, it is imperative to evert the upper eyelid to search the entire arc of the superior cul de sac, especially if there are foreign bodies or particulate matter suspected. This can be performed with a Demarres retractor, a cotton-tipped applicator, or with experience, even a finger tip. Utilizing a bent paper clip, although effective, is not considered appropriately professional by many families and should be discouraged. Irrigating the fornices with saline solution is effective, and a sweep with a moistened cotton swab can be used to eliminate further debris. For an embedded foreign body, apply topical anesthetic and remove the foreign body with a cotton swab, a platinum spatula, forceps, or even a sterile hypodermic 25‑gauge needle.
Be sure to rule out an intraorbital or intraocular foreign body, especially if there is a history of high-speed metallic injury such as those emanating from grinders or hammering. This is less common in the pediatric population. Corneal foreign bodies, including "rust rings" that are commonly seen from iron foreign bodies, can be removed in the same way as conjunctival foreign bodies. Rust rings might require a corneal burr to remove fully. If they are near the pupil, caution is advised because irregular diffraction can occur around the thinned area from a burr that is visually annoying, or in rare cases, even disabling.
Pay careful attention to the depth of the body in the cornea and whether it extends into the anterior chamber, which would result in the need for removal in a sterile OR setting (Figure 2).
Additionally, if there are multiple small foreign bodies that are visually insignificant and not a concern for infection or ongoing inflammation, consider forgoing removal because continued aggressive manipulation of the cornea with resultant scarring and inflammation might cause more harm than good.
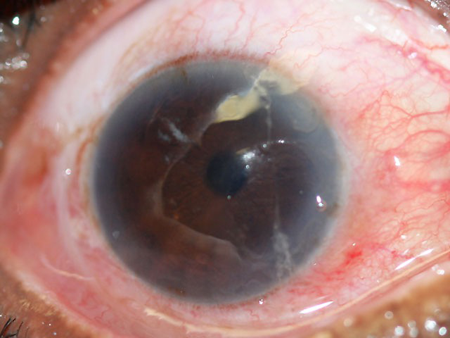
Figure 1. Traumatic corneal abrasion.
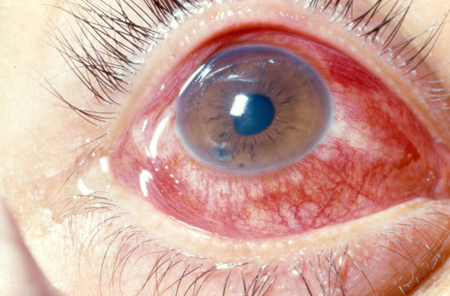
Figure 2. Foreign body in anterior chamber.
Chemical Injuries
Boys sustain two-thirds of the ocular chemical injuries found in children.8,9,10 These injuries tend to be caused primarily by household products, so prevention is the key to reducing the incidence of injury and its associated morbidity. The most common culprits are organic solvents and soaps from household cleaning agents — chemicals that are easily accessible to children in the home. These can occur as solids, liquids, gels, powders, and vapors.
Chemical injuries can be caused by either alkaline or acidic substances. Alkali injuries cause more significant epithelial cell disruption because these substances easily penetrate into the corneal stroma and cause collagen and proteoglycan ground substance destruction. They can also penetrate into the anterior chamber, causing inflammation, increased IOP, and cataracts. Acidic substances, on the other hand, denature proteins and cause precipitation, limiting their penetration into the cornea and anterior chamber. They can, however, still cause direct damage and inflammation.
The initial key to treatment of any chemical injury is irrigation. Immobilize the eyelid with a retractor and apply topical anesthetic. Then use copious irrigation, up to 20 liters of normal saline, lactated ringers, or BSS, with frequent pH testing to ensure the resolution to a physiologic level.11 Irrigation can be facilitated with a Morgan Medi-Flow lens (MorTan, Misoula, MT). It is important to sweep the fornices and ensure no additional chemical matter remains.
Table 1. Acute management.
- Use topical anesthesia if necessary.
- Separate the lids manually or with a lid retractor if possible.
- Gently rinse the cornea and fornices with saline or Ringer's Lactate for at least 15 minutes.
- If saline or Ringer's Lactate are not available, use tap water.
- Remove all particles with a cotton-tip swab or forceps.
- Rinse the palpebral conjunctiva and the fornices using double eversion.
- Perform slit lamp, visual acuity, and IOP exams.
- Measure pH and grade the injury.
- Continue irrigation until neutral pH is achieved (7.0).
- Injuries Grade I or II: Start topical steroids, ascorbic acid, and antibiotics.
- Injuries Grade III and IV: Continue irrigation with phosphate-free solutions and admit patient.
|
Prognosis can be determined by either the Roper-Hall (Table 2) or Dua12 (Table 3) classification systems. The Roper-Hall classification system grades prognosis based on the extent of corneal damage and degree of limbal ischemia. The Dua system relies on the degree of limbal and conjunctival involvement to predict prognosis after corneal burns. Both have been shown to be relatively accurate and similar in determining prognosis of different severities of ocular burns, although the Dua system might be superior in grading severe burns.
Table 2. Grading ocular burns: classification of severity of ocular surface burns by Roper-Hall.12
|
Grade
|
Prognosis
|
Cornea
|
Conjunctiva/Limbus
|
|
I
|
Good
|
Corneal epithelial damage
|
No limbal ischemia
|
|
II
|
Good
|
Corneal haze, iris details visible
|
< 1/3 limbal ischemia
|
|
III
|
Guarded
|
Total epithelial loss, stromal haze, iris details obscured
|
1/3–1/2 limbal ischemia
|
|
IV
|
Poor
|
Cornea opaque, iris and pupil obscured
|
> 1/2 limbal ischemia
|
Table 3. DUA classification of ocular surface burns.12
|
Grade
|
Prognosis
|
Limbal Involvement
|
Conjunctival Involvement
|
Analog Scale
|
|
I
|
Very good
|
0 clock hours
|
0%
|
0/0%
|
|
II
|
Good
|
3 clock hours
|
30%
|
0.1–3/1–29.9%
|
|
III
|
Good
|
> 3–6 hours
|
> 30–50%
|
3.1–6/31–50%
|
|
IV
|
Good to guarded
|
> 6–9 hours
|
> 50–75%
|
6.1–9/51–75%
|
|
V
|
Guarded to poor
|
> 9–< 12 hours
|
> 75–100%
|
9.1–11.9/
75.1–99.9%
|
|
VI
|
Very poor
|
Total limbus: 12 hours
|
Total conjunctiva: 100%
|
12/100%
|
The analog scale accurately records the limbal involvement in clock hours of affected limbus/percentage of conjunctiva involvement. When calculating percentage of conjunctival involvement, consider involvement of bulbar conjunctiva only up to and including the conjunctival fornices.
Grade I and Grade II
- Use short-acting cycloplegic drops.
- Avoid phenylephrine.
- Use topical antibiotic ointment q1‑2 h while awake.
- If IOP is elevated, use oral acetazolamide and preservative-free artificial tears or gel q1h and oral pain medication.
Grade III and Grade IV
In addition to the treatment for Grades I and II:
- Debridement of necrotic tissue
- If significant reaction in the AC or severe corneal inflammation: topical steroids (prednisolone acetate 1% or dexamethasone 0.1%) q 2–4 hours
Ten-percent sodium citrate and ascorbate4–7 can also be used to promote corneal healing. Though ascorbate has mostly been studied in rabbits, general consensus is that, unless the patient has a history of kidney disease, the benefit outweighs the small risk profile.
In the subacute phase, management includes frequent lubrication (without preservatives) and debridement of necrotic tissue. A bandaged contact lens or tarsorrhaphy can be used to protect epithelium that has begun to heal. When using a contact lens, monitor closely for infection. Additionally, monitor for other complications such as ulceration, glaucoma, conjunctival disease or symblepharon, or limbal stem cell loss.
Amniotic membrane transplant has been shown to reduce inflammation and promote re-epithelialization within the first 2 weeks after injury.13 This might also help reduce corneal neovascularization and set up for successful autologous stem-cell transplantation or PKP in the future.
If a normal ocular surface cannot be restored, a keratoprosthesis might be an option. These have a high failure rate in children and should be an option of last resort only. Remember that treatment and recovery after chemical injury can be long and the risk of amblyopia in children is high, so maintain a clear visual axis whenever possible to limit subsequent vision loss from amblyopia.
Hyphema and Glaucoma
When a child presents with hyphema and a history of trauma, the differential for etiology is broad. Always rule out nonaccidental trauma as well as other less-common medical causes of hyphema such as intraocular tumors or bleeding diathesis from systemic disease such as leukemia. If any of these causes are suspected, additional workup is appropriate including orbital MRI or ultrasound if the fundus cannot be seen and intraocular tumor is suspected.
Traumatic hyphema is more common in children than in adults14 and its incidence is estimated to be about 17 in 100,000 children.15 In a retrospective case review of 138 patients, Soohoo et al. showed that the majority of hyphemas in children result from general play (27%), though projectiles from toy guns and injuries from sports account for 26% and 23%, respectively.16
Hyphemas can be graded as either macroscopic or microscopic. Macroscopic hyphemas are characterized by the extent to which they fill the anterior chamber17 (Table 4). Microhyphemas can be graded on the number of cells (1+ to 4+) in the anterior chamber (Figures 2–4). As with any traumatic event to the eye, it is important to rule out any additional pathology on presentation such as an open globe.
Table 4. Hyphema grades.
|
0
|
No visible layering, but red blood cells within the anterior chamber (microhyphema)
|
|
I
|
Layered blood occupying less than 1/3 anterior chamber
|
|
II
|
Blood filling 1/3–1/2 the anterior chamber
|
|
III
|
Layered blood filling 1/2 to less than total of the anterior chamber
|
|
IV
|
Total clotted blood, often referred to as blackball or 8‑ball hyphema
|
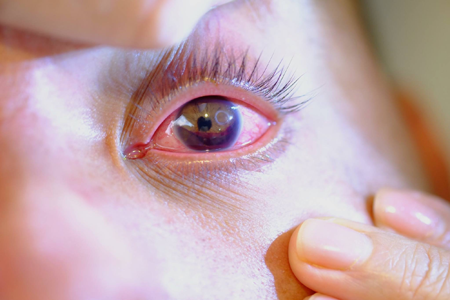
Figure 2. Traumatic hyphema (grade II).
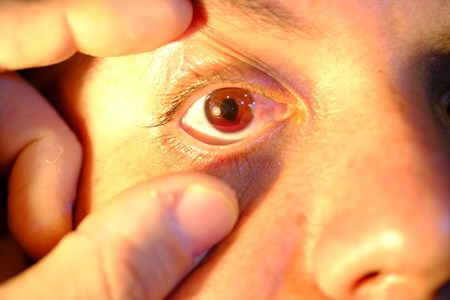
Figure 3. Traumatic hyphema (grade I).
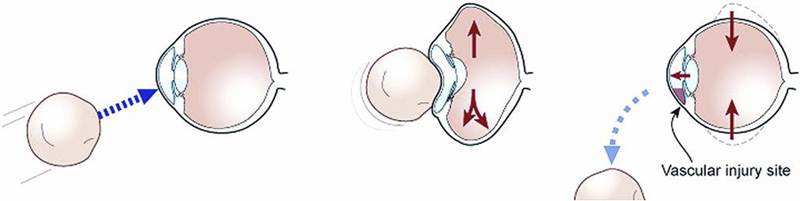
Figure 4. Mechanism of hyphema after blunt trauma.
Treatment of hyphema is aimed at preventing complications such as increased IOP, corneal blood staining, and rebleeding. As such, it was once commonplace to admit patients to the hospital to monitor them closely. However, outpatient management is now widely accepted and has been shown to be effective.18,19,20 It is important to take precautions such as placing a protective eye shield, limiting the child's activities, and elevating the head of the bed. Discuss the importance of this with both the child and the parents to ensure compliance and the best possible outcome.
Intraocular pressure can be problematic after a hyphema. Crouch et al. showed 32% of patients had an IOP elevation to 22 mm Hg within the first 24 hours. If the IOP is more than 25 mm Hg or if the child has sickle-cell trait or disease with any elevation of IOP, use IOP-lowering medications such as a topical beta blocker. Avoid prostaglandins because they can cause increased ocular inflammation.21 Also avoid alpha agonists in infants due to the risk of CNS and respiratory depression.22 Finally, avoid carbonic anhydrase inhibitors in patients with sickle-cell trait or disease because the relative acidosis created by this class of medication can induce sickling. As always, medication choice depends on the specific scenario (see Glaucoma below). Evidence for topical steroids is mixed, but they are often given to decrease inflammation. It is important to limit their long-term use due to risk of secondary glaucoma and cataract formation.
Corneal blood staining can occur with large hyphemas. Early signs include tiny yellow granules that appear in the deep stroma centrally and extend out peripherally. In adults, an anterior chamber washout is considered if corneal blood staining occurs or if IOP remains high after 5–7 days. In a child, blood staining might be difficult to see and IOP more difficult to measure. Given the risks of amblyopia, the threshold for washout is low and should be considered at 4–5 days if the IOP cannot be controlled medically.
Rebleeding is another feared complication of traumatic hyphemas because it has been shown to carry a worse long-term prognosis. The critical time for rebleeding to occur is 4–7 days after injury, so close follow-up is necessary. It is important to distinguish old blood and clot from new blood and to pay close attention to the size of hyphema in the anterior chamber. Some studies have shown a higher incidence of rebleeding in younger patients. This might be secondary to the difficulty of reducing a child's activity, so it is important to emphasize rest and to explain the risks to the family.
- Avoid aspirin and NSAIDs to minimize bleeding risk.
- Cycloplegics can also help with reducing secondary bleeding as well as formation of posterior synechiae from inflammation.
- Atropine is a good choice because it requires fewer applications and is theoretically easier in children.
- Aminocaproic acid has been successfully used to decrease the risk of rebleeding because it inhibits fibrin clot degradation. Its use has been proven safe in children, but is contraindicated in anyone with an intravascular thrombosis.23
Late complications from traumatic hyphemas include peripheral anterior synechiae, posterior synechiae, angle recession, and subsequent secondary glaucoma. Angle recession occurs in up to 85% of patients with hyphema, and the relative risk of glaucoma according to one study is about 2.21.24 It is important to counsel children and parents about the risk of these late complications and the need for yearly eye exams.
Due to the complications caused by sickle-cell anemia, take extreme caution when managing an African American child with hyphema. Order a hemoglobin S test for children at risk: family members of those with sickle-cell disease or trait, people whose ancestors are from Africa, India, the Middle East, the Mediterranean (Turkey, Italy, Greece), the Caribbean, and South and Central America. Even small hyphemas can predispose sickle-cell patients to CRAO and optic nerve damage, and the rebleeding rate is higher. Maintain tight control of IOP and avoid carbonic anhydrase inhibitors because they can cause sickling. Consider anterior chamber washout when pressures remain greater than 25 mm Hg for more than 24 hours.
Glaucoma
Ocular trauma can lead to secondary glaucoma, most often caused by multiple factors. There is a 3.39% risk of developing post-traumatic glaucoma after blunt injury. Bai et al. divided traumatic glaucoma into stages based on timing after injury:25
- The early stage, 0–1 month, is caused by inflammation, hyphema, and lens dislocation.
- From 1–6 months, pupillary block with poster synechiae or peripheral anterior synechiae can cause angle closure.
- After 6 months, angle recession or siderosis can be the etiology.
After trauma, pigment is released, plugging up the trabecular meshwork and subsequently being phagocytosed by its endothelial cells.26 The relative risk for chronic glaucoma is 20.8 in patients with heavy trabecular pigmentation.27 In patients with coexisting vitreous hemorrhage, ghost-cell glaucoma can be seen in 2 weeks to 3 months.28
In general, features that are significantly associated with long-term glaucoma risk are hyphema, angle recession of more than 180 degrees, displacement of the lens, and trabecular pigmentation.29
When treating elevated intraocular pressure in the setting of trauma, it is important to note a few differences compared to adults. Avoid beta blockers in small or premature infants and take caution in those with asthma or cardiac disease. If needed, use a selective beta blocker such as betaxolol. Do not give prostaglandins to those with intraocular inflammation or uveitis. Brimonidine is also contraindicated in children younger than 2 years old, as are carbonic anhydrase inhibitors in infants with renal insufficiency or sickle-cell disease.
Angle Recession
Angle recession is a common result of blunt trauma. As contact with the globe occurs, anterior-to-posterior compression forces the aqueous laterally, stressing the limbus. The longitudinal fibers of the ciliary muscle become separated from the circular fibers. This can also shear small vessels of the anterior ciliary arteries, leading to hyphema. As mentioned previously, angle recession is seen in 85% of traumatic hyphemas.24
The key finding on gonioscopy is a widened ciliary band. It is important to always check the contralateral eye first for comparison. Check visual fields and evaluate for any other signs of trauma in any patient presenting with angle recession, if possible. Follow these patients closely over time because the relative risk for developing glaucoma is 2:1. This risk is also directly related to the amount of degrees of angle recession on exam. In general, studies show risk is significantly increased when more than 180 degrees of recession exists.31
Prostaglandins avoid the traditional aqueous outflow pathway and increase uveoscleral outflow instead, so they can be useful in treatment of elevated IOP as long as inflammation has subsided. Avoid pilocarpine because it has been shown to worsen angle recession. If IOP cannot be controlled with medication, trabeculectomy with mitomycin C might be needed.32 Argon laser trabeculoplasty (ALT) has largely proven unsuccessful for the treatment of angle-recession glaucoma.33
Cyclodialysis and Iridodialysis
When the longitudinal portion of the ciliary body separates from the scleral spur, creating a direct communication between the anterior chamber and the suprachoroidal space, this is termed cyclodialysis. As a result, there is a risk of hypotony from increased aqueous outflow into the suprachoroidal space. Alternately, in any eye with hypotony and a history of trauma, consider cyclodialysis.
An abnormal region posterior to the scleral spur will be seen on gonioscopy. However, this can be difficult to appreciate in an eye that is hypotonous with a flat anterior chamber or cloudy cornea. Ultrasound biomicroscopy (UBM) does a good job of visualizing cyclodialysis when gonioscopy is difficult.34 Recently, anterior segment OCT has proven to be accurate in visualizing the anterior chamber angle and correlates well with ultrasound biomicroscopy.35 It has the added benefit of being noncontact, in comparison to UBM, which can be uncomfortable and difficult to perform in pediatric patients.
The management of cyclodialysis is aimed at reopposing the ciliary body back to the scleral wall and normalizing intraocular pressure. This can be attempted medically with 1% atropine sulfate BID for 6–8 weeks. If this fails, surgery might be indicated. A variety of surgical techniques have been described. These attempt either to manually oppose the ciliary body to the sclera or to noninvasively burn or produce local inflammation in the cleft to facilitate apposition. They include, but are not limited to, direct cyclopexy,36 argon laser photocoagulation,37 trans-scleral YAG,38 and cryoablation.39
Iridodialysis occurs after blunt trauma when the iris root becomes disinserted from the sclera (Figures 5 and 6). If it is small and the patient is asymptomatic, there is no need to intervene. Often a hyphema coexists, which warrants treatment as described above. If the pupil distortion is large or the patient has polycoria, monocular diplopia, or other symptoms such as photophobia or glare, this warrants surgical intervention. There have been many reports on the approach to repair of iridodialysis. Brown describes a technique specific to children using a scleral tunnel incision with minimal scleral manipulation and double-armed 10‑0 polypropylene suture, citing the concern for softer sclera in the pediatric population and the need for the repair to last for multiple decades.40
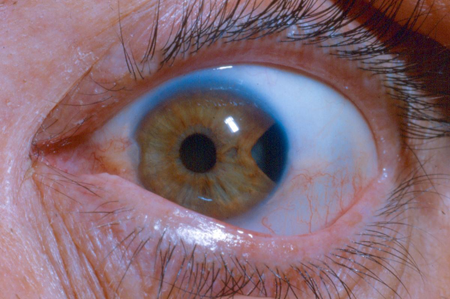
Figure 5. Traumatic iridodialysis secondary to blunt trauma.
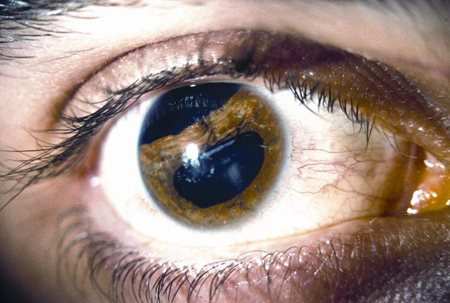
Figure 6. Traumatic iridodialysis.
Cataracts
Pediatric traumatic cataracts present some unique challenges. Often there are coexisting injuries to the eye, and management requires a multispecialty approach. Further complicating the situation is the problem of amblyopia in the pediatric population. In general, the younger the child the more urgent the removal of cataract after trauma. Amblyopia treatment should begin immediately after surgery. There are many questions and points of contention regarding management such as:
- contact lens versus IOL placement
- timing of IOL placement
- primary versus secondary IOL placement
- type and power of IOL
- timing and approach to posterior capsulotomy or capsulectomy
To minimize the risk of amblyopia, infants with visually significant cataracts should undergo extraction by about 6 weeks old or as soon as possible after a traumatic cataract. There has been contention as to whether to place an IOL or to leave the child aphakic and manage visual correction with a contact lens. There are complicating factors in placing IOLs in infants that can make aphakia with contact lens correction a better option for most infants. The eye rapidly grows within the first year of life, so it can be difficult to predict the implant power and size. Additionally, significant visual axis opacification can occur after cataract surgery in infants.41
The Infant Aphakia Treatment Study,42 a multicenter randomized control trial looking at 1‑year visual acuity outcomes of infants who underwent congenital cataract extraction with or without primary IOL implantation (those without were managed with contact lenses) showed that there was no difference in visual acuity at 1 year of age. However, the group that underwent primary IOL placement more frequently required additional operations. For this reason, aphakia with contact lens replacement is preferred for most infants undergoing cataract surgery. Good outcomes can be achieved to preserving central vision by placing a contact lens to maintain optical correction and by patching. These children can then safely undergo secondary IOL implantation when they are older and IOL power predictions and axial elongation are more predictable.
Another scenario to warrant secondary implantation is in older children with additional posterior-segment trauma requiring multiple operations. Secondary implantation allows time for the eye to stabilize and makes for more precise biometry using the traumatic eye rather than the fellow eye, as fellow-eye measurements can prove inaccurate. Additionally, larger changes in axial lengths have been noted in pseudophakic patients.43,44 As such, the decision for secondary IOL implantation should take into account the patient's age and extent of injury.
Posterior-capsule opacification has been noted in 40–100% of pediatric patients following cataract surgery and can occur early in children less than 6 years old. Because of this, a primary posterior capsulotomy with anterior vitrectomy to remove scaffolding for vitreous face opacification is indicated at the time of lensectomy. This will establish a clear visual axis for patching and aphakic contact correction. For older children who can cooperate, a Nd:YAG capsulotomy can be performed when the opacification occurs, and a primary posterior capsulotomy is not required.
In performing the posterior capsulotomy and anterior vitrectomy, there are a few common approaches. The first is after lensectomy but prior to IOL placement. After anterior capsulotomy, the vitrector can be set to low suction and a high cut rate to perform the posterior capsulotomy along with anterior vitrectomy through the clear corneal incision. The lens is inserted following this procedure, making sure there is no vitreous to the wound and that the haptics are in the capsular bag and did not go through posterior opening of the capsule.
Alternately, the IOL can be placed in the bag, then the vitrector slid around the edge of the IOL to access the posterior capsule and vitreous.45 This method requires more skill, but helps minimize vitreous to the wound. Finally, a posterior capsulotomy and anterior vitrectomy can be performed through the pars plana using standard or small-gauge vitrectomy equipment.
There are a host of considerations in choosing an intraocular lens for implantation following a traumatic cataract:
- Though commonly used in adults, anterior chamber IOLs should be avoided in children due to high rates of complications such as glaucoma, pupillary distortion and PAS, and corneal endothelial damage.
- The Artisan (Ophtec, Groningen, The Netherlands), an iris-fixated lens, appears to have some promise with good short-term outcomes, though study sizes have been small and longer-term follow-up is needed to ensure safety in children who might have the lens in place for many decades.46 Posterior chamber IOL placement into the capsular bag is preferable with a foldable single-piece acrylic lens or larger PMMA (polymethylmethacrylate) lens.
- Implantation of a ciliary sulcus lens is an alternative in pediatric cataracts, but it has been shown to increase postoperative complications such as uveitis and pupillary capture by the IOL in traumatic cataracts and should be avoided if possible.47 Three-piece lenses are required when placing a lens in the sulcus to help minimize the risk of these complications.
- If there is poor capsular support, a scleral-fixated posterior chamber IOL can be used, but is not without long-term risk for complications such as broken sutures, vitreous hemorrhage, retinal detachment, and endophthalmitis.48 It is generally best to use conservative options for visual rehabilitation, including contact lenses or safer IOLs, as primary treatment before using a scleral-fixated lens.
Choosing the power of IOL can be difficult and is often provider-specific. As mentioned, the eye grows very rapidly in infants with ‑6D of change in the first 24 months.49 Crouch et al. followed refractive error of patients undergoing cataract surgery between the ages of 1 and 18 years, noting that there was a myopic shift of ‑5.69D in children who underwent surgery in the 2nd year of life, ‑3.66D when performed in the 3rd, ‑2.03D when in the 7th and 8th year, and ‑0.97D when performed at 11–14 years.50 Because of this, any IOL aiming for emmetropia in a young child will result in myopia later in life.
Two approaches typically occur:
- The first is to choose a lens power aiming for emmetropia at the time of surgery. This will improve binocular function and reduce risk of amblyopia postoperatively, but the child will likely need additional surgery or correction in the future as the eye grows and undergoes considerable myopic shift.
- Alternatively, an IOL power based on what the child's vision would be expected to be in adulthood can be chosen. This would leave the child significantly hyperopic postoperatively, and this can make it more difficult to manage amblyopia. It will require corrective glasses or contacts postoperatively, but it has the potential to reduce the need for further intervention at a later date.
Lens Subluxation and Dislocation
Subluxation or dislocation occur when the lens is displaced or malpositioned and no longer in correct anatomical position. This can occur in a variety of genetic conditions including ectopia lentis, Marfan syndrome, homocystinuria, and Weill-Marchesani syndrome, as well as after trauma. In approaching a child with subluxation, first try optical correction. If the degree of subluxation is minimal, optical correction works well to help balance focus and eliminate amblyopia. If more severe optical distortion exists, an aphakic correction can provide the patient with the best vision because the visual axis can be bisected or so distorted that a clear retinal image cannot be achieved with glasses or contact lenses.
If optical correction fails to improve vision, consider surgical lensectomy through either an anterior incision or pars plana approach. If zonular incompetence exists, this can complicate lensectomy and IOL placement. If zonular weakness is less than 120 degrees, capsular hooks or a capsular tension ring can be used to support the capsular bag and allow safe lens removal. Traumatic cataracts in young children are soft and require only aspiration, which can reduce the need for capsular support. In some dislocations, fixation with a Cionni-modified capsular tension ring or ring segment in combination with lensectomy and IOL placement can be effective and these have been shown to be effective and safe in children.51
References
- Wipperman JL, Dorsch JN. Evaluation and management of corneal abrasions. American family physician 2013;87:114-120.
- Upadhyay MP, Karmacharya PC, Koirala S, et al. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. The British journal of ophthalmology 2001;85:388-392.
- Calder LA, Balasubramanian S, Fergusson D. Topical nonsteroidal anti-inflammatory drugs for corneal abrasions: meta-analysis of randomized trials. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine 2005;12:467-473.
- Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Survey of ophthalmology 2010;55:108-133.
- Meek R, Sullivan A, Favilla M, Larmour I, Guastalegname S. Is homatropine 5% effective in reducing pain associated with corneal abrasion when compared with placebo? A randomized controlled trial. Emergency medicine Australasia : EMA 2010;22:507-513.
- Michael JG, Hug D, Dowd MD. Management of corneal abrasion in children: a randomized clinical trial. Annals of emergency medicine 2002;40:67-72.
- Avni Zauberman N, Artornsombudh P, Elbaz U, Goldich Y, Rootman DS, Chan CC. Anterior stromal puncture for the treatment of recurrent corneal erosion syndrome: patient clinical features and outcomes. American journal of ophthalmology 2014;157:273-279 e271.
- El-Mekawey HE, Abu El Einen KG, Abdelmaboud M, Khafagy A, Eltahawy EM. Epidemiology of ocular emergencies in the Egyptian population: a five-year retrospective study. Clinical ophthalmology 2011;5:955-960.
- Pfister RR. Chemical injuries of the eye. Ophthalmology 1983;90:1246-1253.
- Vajpayee RB, Shekhar H, Sharma N, Jhanji V. Demographic and clinical profile of ocular chemical injuries in the pediatric age group. Ophthalmology 2014;121:377-380.
- Fish R, Davidson RS. Management of ocular thermal and chemical injuries, including amniotic membrane therapy. Current opinion in ophthalmology 2010;21:317-321.
- Gupta N, Kalaivani M, Tandon R. Comparison of prognostic value of Roper Hall and Dua classification systems in acute ocular burns. Br J Ophthalmol. 2011 Feb;95(2):194-8.
- Meller D, Pires RT, Mack RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology 2000;107:980-989; discussion 990.
- Kennedy RH, Brubaker RF. Traumatic hyphema in a defined population. American journal of ophthalmology 1988;106:123-130.
- Agapitos PJ, Noel LP, Clarke WN. Traumatic hyphema in children. Ophthalmology 1987;94:1238-1241.
- SooHoo JR, Davies BW, Braverman RS, Enzenauer RW, McCourt EA. Pediatric traumatic hyphema: a review of 138 consecutive cases. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 2013;17:565-567.
- Edwards WC, Layden WE. Traumatic hyphema. A report of 184 consecutive cases. American journal of ophthalmology 1973;75:110-116.
- Crouch ER, Jr., Crouch ER. Management of traumatic hyphema: therapeutic options. Journal of pediatric ophthalmology and strabismus 1999;36:238-250; quiz 279-280.
- Gharaibeh A, Savage HI, Scherer RW, Goldberg MF, Lindsley K. Medical interventions for traumatic hyphema. The Cochrane database of systematic reviews 2011;CD005431.
- Rocha KM, Martins EN, Melo LA, Jr., Moraes NS. Outpatient management of traumatic hyphema in children: prospective evaluation. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 2004;8:357-361.
- Walton W, Von Hagen S, Grigorian R, Zarbin M. Management of traumatic hyphema. Survey of ophthalmology 2002;47:297-334.
- Al-Shahwan S, Al-Torbak AA, Turkmani S, Al-Omran M, Al-Jadaan I, Edward DP. Side-effect profile of brimonidine tartrate in children. Ophthalmology 2005;112:2143.
- Teboul BK, Jacob JL, Barsoum-Homsy M, et al. Clinical evaluation of aminocaproic acid for managing traumatic hyphema in children. Ophthalmology 1995;102:1646-1653.
- Girkin CA, McGwin G, Jr., Long C, Morris R, Kuhn F. Glaucoma after ocular contusion: a cohort study of the United States Eye Injury Registry. Journal of glaucoma 2005;14:470-473.
- Bai HQ, Yao L, Wang DB, Jin R, Wang YX. Causes and treatments of traumatic secondary glaucoma. European journal of ophthalmology 2009;19:201-206.
- Richardson TM, Hutchinson BT, Grant WM. The outflow tract in pigmentary glaucoma: a light and electron microscopic study. Archives of ophthalmology 1977;95:1015-1025.
- Sihota R, Sood NN, Agarwal HC. Traumatic glaucoma. Acta ophthalmologica Scandinavica 1995;73:252-254.
- Campbell DG. Ghost cell glaucoma following trauma. Ophthalmology 1981;88:1151-1158.
- Sihota R, Kumar S, Gupta V, et al. Early predictors of traumatic glaucoma after closed globe injury: trabecular pigmentation, widened angle recess, and higher baseline intraocular pressure. Archives of ophthalmology 2008;126:921-926.
- Blanton FM. Anterior Chamber Angle Recession and Secondary Glaucoma. A Study of the Aftereffects of Traumatic Hyphemas. Archives of ophthalmology 1964;72:39-43.
- Alper MG. Contusion angle deformity and glaucoma. Gonioscopic observations and clinical course. Archives of ophthalmology 1963;69:455-467.
- Manners T, Salmon JF, Barron A, Willies C, Murray AD. Trabeculectomy with mitomycin C in the treatment of post-traumatic angle recession glaucoma. The British journal of ophthalmology 2001;85:159-163.
- Robin AL, Pollack IP. Argon laser trabeculoplasty in secondary forms of open-angle glaucoma. Archives of ophthalmology 1983;101:382-384.
- Gentile RC, Pavlin CJ, Liebmann JM, et al. Diagnosis of traumatic cyclodialysis by ultrasound biomicroscopy. Ophthalmic surgery and lasers 1996;27:97-105.
- Mateo-Montoya A, Dreifuss S. Anterior segment optical coherence tomography as a diagnostic tool for cyclodialysis clefts. Archives of ophthalmology 2009;127:109-110.
- Kuchle M, Naumann GO. Direct cyclopexy for traumatic cyclodialysis with persisting hypotony. Report in 29 consecutive patients. Ophthalmology 1995;102:322-333.
- Naumann G, Kuchle M. Noninvasive closure of persistent cyclodialysis cleft. Ophthalmology 1997;104:1207.
- Brooks AM, Troski M, Gillies WE. Noninvasive closure of a persistent cyclodialysis cleft. Ophthalmology 1996;103:1943-1945.
- Krohn J. Cryotherapy in the treatment of cyclodialysis cleft induced hypotony. Acta ophthalmologica Scandinavica 1997;75:96-98.
- Brown SM. A technique for repair of iridodialysis in children. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 1998;2:380-382.
- Trivedi RH, Wilson ME, Jr., Bartholomew LR, Lal G, Peterseim MM. Opacification of the visual axis after cataract surgery and single acrylic intraocular lens implantation in the first year of life. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 2004;8:156-164.
- Infant Aphakia Treatment Study G, Lambert SR, Buckley EG, et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Archives of ophthalmology 2010;128:810-818.
- Trivedi RH, Wilson ME, Jr., Facciani J. Secondary intraocular lens implantation for pediatric aphakia. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 2005;9:346-352.
- Leiba H, Springer A, Pollack A. Ocular axial length changes in pseudophakic children after traumatic and congenital cataract surgery. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 2006;10:460-463.
- Plager DA, Yang S, Neely D, Sprunger D, Sondhi N. Complications in the first year following cataract surgery with and without IOL in infants and older children. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 2002;6:9-14.
- Sminia ML, Odenthal MT, Wenniger-Prick LJ, Gortzak-Moorstein N, Volker-Dieben HJ. Traumatic pediatric cataract: a decade of follow-up after Artisan aphakia intraocular lens implantation. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 2007;11:555-558.
- Pandey SK, Ram J, Werner L, et al. Visual results and postoperative complications of capsular bag and ciliary sulcus fixation of posterior chamber intraocular lenses in children with traumatic cataracts. Journal of cataract and refractive surgery 1999;25:1576-1584.
- Asadi R, Kheirkhah A. Long-term results of scleral fixation of posterior chamber intraocular lenses in children. Ophthalmology 2008;115:67-72.
- O'Keefe M, Fenton S, Lanigan B. Visual outcomes and complications of posterior chamber intraocular lens implantation in the first year of life. Journal of cataract and refractive surgery 2001;27:2006-2011.
- Crouch ER, Crouch ER, Jr., Pressman SH. Prospective analysis of pediatric pseudophakia: myopic shift and postoperative outcomes. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus 2002;6:277-282.
- Kim EJ, Berg JP, Weikert MP, et al. Scleral-fixated capsular tension rings and segments for ectopia lentis in children. American journal of ophthalmology 2014;158:899-904.