AUG 26, 2016
By Arif O. Khan, MD, and Elias I. Traboulsi, MD
A Compendium of Inherited Disorders and the Eye, Oxford University Press
Genetics
OMIM Numbers
Inheritance
Gene/Gene Map
- BBS is caused by mutations in genes involved in the normal functioning of cilia. At least 21 genes have been associated with BBS to date: BBS1, BBS2, ARL6 (BBS3), BBS4, BBS5, MKKS (BBS6), BBS7, TTC8 (BBS8), BBS9, BBS10, TRIM32 (BBS11), BBS12, MKS1 (BBS13), CEP290 (BBS14), WDPCP (BBS15), SDCCAG8 (BBS16), LZTFL1 (BBS17), BBIP1 (BBS18), and IFT27 (BBS19), IFT172 (BBS20), C8orf37 (BBS21). Tri-allelic ciliary gene mutations do not seem to make a significant contribution to the phenotype.
Epidemiology
- Prevalence in Newfoundland is about 10 times than in Switzerland (1 in 160,000) and is similar to prevalence among the Bedouins of Kuwait (1 in 13,500).
Clinical Findings
- BBS is a ciliopathy with protean manifestations. Cilia gene mutations can cause a range of clinical conditions with retinal and renal dysfunction as common recurrent features. For BBS, the modified diagnostic criteria according to Beales et al. (1999) require the presence of four of six primary features or three of primary features plus two of the secondary features:
- Primary features
- Rod-cone dystrophy (Figure 1)
- Obesity
- Polydactyly (Figure 2)
- Learning disabilities
- Hypogonadism
- Renal anomalies
- Secondary features
- Speech disorder/delay
- Strabismus/cataracts/astigmatism
- Brachydactyly/syndactyly
- Developmental delay
- Polyuria/polydipsia (nephrogenic diabetes insipidus)
- Ataxia/poor coordination/imbalance
- Mild spasticity (lower limbs)
- Diabetes mellitus
- Dental crowding/hypodontia/high arched palate
- Left ventricular hypertrophy/congenital heart disease
- Hepatic fibrosis
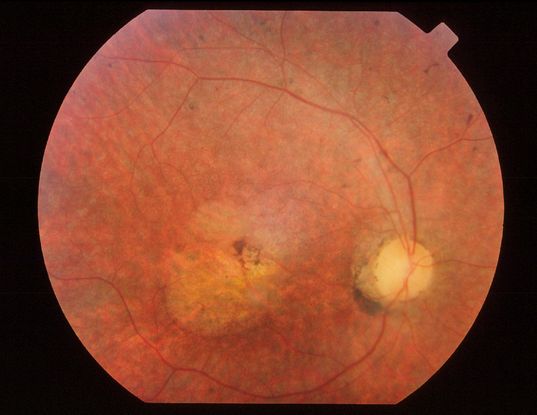
Figure 1. Fundus photograph of the right eye of a 30-year-old man with BBS. Note macular involvement, indicative of the cone-rod dystrophy type of retinal degeneration. This differentiates this retinal dystrophy from classic retinitis pigmentosa.
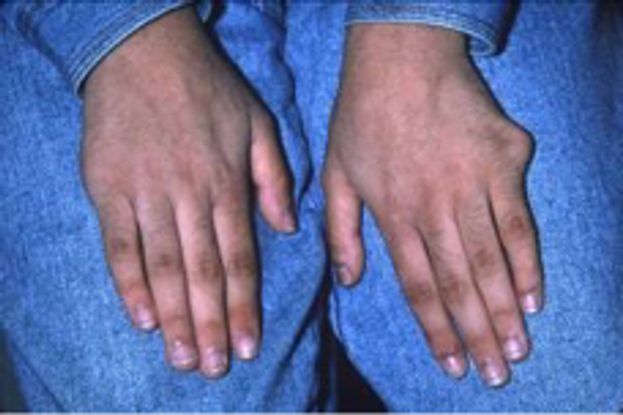
Figure 2. A 12-year-old girl who recently went into renal failure was examined because of poor vision. Ophthalmic examination confirmed rod-cone dystrophy. Targeted physical examination questioning revealed childhood amputations for polydactyly, which were not originally volunteered. A clinical diagnosis of BBS was made.
- Historically, Laurence-Moon syndrome (OMIM 245800) has been attached to the BBS. Laurence-Moon syndrome is characterized by juvenile chorioretinopathy resembling choroideremia, pituitary dysfunction, ataxia, peripheral neuropathy and spastic paraplegia. It is now recognized that Laurence-Moon syndrome is not a ciliopathy but rather is caused by bi-allelic mutations in the PNPLA6 gene, which encodes neuropathy target esterase, critical in phosphatidylcholine metabolism, membrane phospholipid trafficking and axonal integrity. Bi-allelic PNPLA6 mutations have also been implicated in Oliver–McFarlane syndrome (OMIM 275400), characterized by trichomegaly, juvenile chorioretinopathy, multiple pituitary hormone deficiencies, and spinocerebellar degeneration, as well as a spectrum of other neurodegenerative conditions.
- If iris coloboma is present instead of pigmentary retinopathy, patients have Biemond syndrome II (OMIM 210350), for which a genetic basis has not been identified to date.
- If diabetes mellitus, deafness, and cone-rod dystrophy are associated with a BBS-like picture, a diagnosis of Alström syndrome (OMIM 203800), another disorder of cilia function, should be considered, which is due to bi-allelic mutations in the gene ALMS1.
- If oculomotor apraxia and ataxia are associated with a BBS-like picture, a diagnosis of Joubert syndrome should be considered, which can be due to mutations in at least 20 different ciliary genes.
Ocular Findings
- Juvenile-onset pigmentary degeneration of the retina occurs in the vast majority of patients and is typically rod-cone, with atypical early macular involvement, although cone-rod dystrophy sometimes is seen.
- Visual acuity deteriorates rapidly with age so that by age 20 more than 75% of patients are legally blind (by age 30 more than 90%) from the retinal dystrophy.
- Visual fields become markedly constricted, severe abnormalities of color vision occur in the majority of cases.
- The electroretinogram typically becomes extinguished or substantially reduced, with elevated dark adaptation thresholds.
- Nystagmus is present in about 10% of patients.
- Biallelic mutations in BBS-related genes can cause retinal dystrophy without the full systemic BBS picture.
Therapeutic Considerations
- There is no treatment to prevent deterioration of vision.
- Surgical excision of extra digits can be performed. It is important to note that sometimes this has been performed before the child is seen by an ophthalmologist, and the parents may not offer this information unless specifically asked the question.
- Obesity is a major source of stress for patients and parents. Early referral to a dietician is important. A multidisciplinary approach to weight loss that includes a combination of dietary assessment, behavioral therapy and exercise is recommended.
- Educational needs for these developmentally challenged patients should be addressed early. This also applies for speech therapy when appropriate.
- The treatment of renal complications depends on the type of renal dysfunction. Monitoring for renal failure is essential. Patients may succumb to renal failure as juveniles or in middle age.
- Diabetes mellitus is usually of the non-insulin dependent type, but some patient will require insulin.
- Endocrinologic issues include hypogonadism and short stature and may need to be treated with hormonal replacement.
References
- Abu-Safieh L, Al-Anazi S, Al-Abdi L, et al. In search of triallelism in Bardet-Biedl syndrome. Eur J Hum Genet. 2012 Apr;20(4):420-42.
- Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet -Biedl syndrome: results of a population survey. J Med Genet. 1999 36:437-446.
- Farag TI, Teebi AS: High incidence of Bardet Biedl syndrome among the Bedouin. Clin Genet. 1989; 36:463-465.
- Green JS, Parfrey P, Harnett J, et al. The cardinal manifestations of Bardet-Biedl Syndrome, a form of Laurence-Moon-Biedl syndrome. New Eng J Med. 1989; 321:1002-1009.
- Hufnagel RB, Arno G, Hein ND, et al. Neuropathy target esterase impairments cause Oliver-McFarlane and Laurence-Moon syndromes. J Med Genet. 2015 Feb;52(2):85-94.
- Hurley RM, Dery P, Nogrady MB, Drummond KN. The renal lesion of the Laurence-Moon-Biedl syndrome. J Pediatr. 1975;87:206-209.
- Klein D, Ammann F: The syndrome of Laurence-Moon, Bardet-Biedl and allied diseases in Switzerland: clinical, genetic and epidemiological studies. J Neurol Sci. 1969;9:479-5l3.
- Mockel A, Perdomo Y, Stutzmann F, Letsch J, Marion V, Dollfus H. Retinal dystrophy in Bardet-Biedl syndrome and related syndromic ciliopathies. Prog Retin Eye Res. 2011 Jul;30(4):258-274.
Traboulsi EI. Compendium of Inherited Disorders and the Eye. New York: Oxford University Press; 2005. Adapted with permission.