By Elena Bitrian, MD; Sharon F. Freedman, MD
General Considerations
Cyclodestruction procedures aim to decrease intraocular pressure (IOP) by decreasing production of aqueous humor by the ciliary body (specifically the ciliary processes). There are 3 basic approaches to cyclodestruction (which some prefer to call cycloablation):
- Cyclocryotherapy
- Trans-scleral laser cycloablation
- Endoscopic laser cycloablation
All 3 techniques share the common goal of decreased aqueous production, and all share the possible side effects of inflammation (including possible sympathetic ophthalmia), ineffective treatment, and over-treatment with resultant hypotony or even phthisis. Although there is not uniform agreement, most surgeons reserve cycloablation procedures for those cases refractory to, or not amenable to, procedures that improve aqueous outflow such as angle surgery, glaucoma drainage implant surgery, and trabeculectomy (Table 1). Nonetheless, for especially refractory pediatric glaucoma (as with adult cases), cyclodestruction represents a valid method of attempting control of glaucoma that otherwise threatens residual vision or causes ongoing damage to the structure of the child's eye.
Table 1. Indications, advantages, and disadvantages of cyclodestructive procedures.
|
Method
|
Indications
|
Advantages
|
Disadvantages*
|
|
Cyclocryotherapy
|
Other treatment modalities exhausted:
- Cases when TDC or ECP is anatomically challenging to perform
- Repeated treatments in selected locations after cyclocryotherapy
|
- Short surgical time and rapid rehabilitation
- Technically easy
|
- Often needs to be repeated due to ciliary body recovery
- Most patients remain on medical therapy.
- Risk of phthisis bulbi
- Scleral thinning at cryo sites can affect future drainage device surgery.
- Proinflammatory and can accelerate cataract formation
|
|
Trans-scleral diode-laser cyclophotocoagulation (TDC)
|
- Failed angle surgery and minimal visual potential
- Failed trabeculectomy and/or aqueous drainage with poor central vision
- Inadequate IOP control after drainage device
- Anatomy precluding trabeculectomy or glaucoma drainage device, e.g., disorganized anterior segment, thin limbus
- Gravely ill children, poor follow-up, blind in fellow eye from complication of intraocular surgery
|
- Short surgical time and rapid rehabilitation
- Technically easy
|
- Often needs to be repeated due to ciliary body recovery
- Most patients remain on medical therapy.
- Risk of phthisis bulbi, but less than cryo
- Beware risk of perforation at sites of scleral thinning.
- Proinflammatory and can accelerate cataract formation
|
|
Endoscopic diode laser cyclophotocoagulation (ECP)
|
- Same indications as TDC (see above), but anatomy of eye allowing limbal or pars plana approach to ciliary processes; beware cataract in phakic eyes
- Consider after TDC has failed.
|
- Short surgical time and rapid rehabilitation
- Allows precise treatment and avoids risk of scleral perforation
- Can produce less inflammation than TDC and cryo; less power needed
|
- Often needs to be repeated due to ciliary body recovery
- Most patients remain on medical therapy.
- Risk of phthisis bulbi, but less than TDC and cryo
- Proinflammatory, but less than TDC and cryotherapy
- Intraocular approach with risk of infection and cataract formation
|
*Risk of sympathetic ophthalmia with all three procedures, but very low.
Cyclocryotherapy
This technique, the oldest cyclodestructive method, involves freezing the ciliary processes from an external approach (Figure 1). Success reported with cyclocryotherapy in pediatric glaucoma is rather poor, with Faran et al. reporting 30% success after 1 or more cryotherapy treatments in children with advanced congenital glaucoma,1 whereas Wagle et al. reported similarly modest 44% success with average 5‑year follow-up, allowing for multiple retreatments.2 The latter authors reported devastating complications — phthisis, blindness, retinal detachment — more likely in eyes with aniridic glaucoma than other types. In light of newer cyclodestructive procedures that can be gentler and more precise (see below), cyclocryotherapy should be reserved for those refractory pediatric glaucoma cases in which anatomy limits the likelihood of successful ciliary body treatment with either trans-scleral or endoscopic cyclophotocoagulation (below) or access to these technologies is truly unavailable and incisional surgery is impossible or has been exhausted.
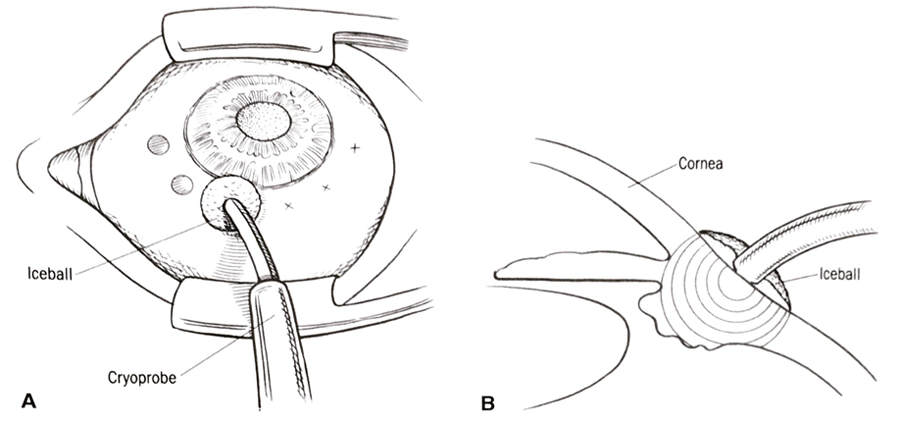
Figure 1. Cyclocryotherapy. A. The cryoprobe is placed with the tip approximately 2 mm posterior to the limbus. B. Cross-sectional view, showing the iceball that has encompassed the ciliary body.
When it is used, cyclocryotherapy in pediatric eyes should be limited to a maximum of 6 clock hours (180 degrees of the eye's circumference) at any 1 session, using approximately 1 freeze-spot per clock-hour (45–60 seconds each at ‑80ºC), with the anterior edge of a "large" 2.5‑mm diameter cryoprobe placed at 1–1.5 mm posterior to the limbus in a nonbuphthalmic globe and with care to avoid the 3 and 9 o'clock locations to minimize damage to the long posterior ciliary vessels. Any especially thin area of the limbus should also be avoided and care should be taken during a freeze to avoid contact with adjacent lid tissue and to shorten treatment if the "freeze zone" gets larger than approximately 1 cm, which can happen as the entire eye "cools down" with sequential freeze applications. Where limbal anatomy is distorted or difficult to evaluate, transillumination can help identify the proper treatment location.3 During cyclocryotherapy repeat sessions, care should be taken to leave at least 1 quadrant untreated because with overtreatment, chronic hypotony or phthisis can be permanent.2
Trans-Scleral Cyclophotocoagulation
Laser energy can be directed through the sclera to treat the ciliary processes using a probe in contact with the sclera over the intended treatment site. This technique, called trans-scleral cyclophotocoagulation, has been reported in pediatric glaucoma using both the Nd:YAG laser and the diode laser. In 1 small series, after a single 360‑degree treatment, the Nd:YAG used for trans-scleral cyclophotocoagulation was reported to reduce IOP in approximately 40% of eyes with advanced pediatric glaucoma.4 Although reported to produce less inflammation than cyclocryotherapy in adults, risks of serious complications remain with this technique.5 Recently, trans-scleral diode-laser has become the preferred cyclodestructive technique for pediatric cases refractory to other measures.6
Although trans-scleral diode-laser cyclophotocoagulation (TDC) has limited effectiveness and can produce all the side effects of other external cycloablation treatments, it has proven useful in refractory pediatric glaucoma cases. This technique, usually performed with the Iris Medical G‑probe (Iridex Corporation, Mountain View, CA, USA), has been reported successful in 30%–75% of eyes after 1 or more treatments (33%–70% retreatment rate), with variable follow-up times (Figure 2).7–10 Variable parameters for treatment are recommended, depending on the level of IOP elevation and the outflow capacity of the eye. For eyes without a filtration device, a reasonable initial treatment in eyes without drainage devices includes 270‑degree treatment (3 quadrants), placing treatment spots at intervals indicated by moving 1/2 probe width each spot, avoiding 3 and 9 o'clock positions, for a total of 13–16 spots; energy settings should be between 1,500 and 2,000 mW for 2,000 ms, with power titrated to a soft "pop." For eyes with a functioning glaucoma drainage device, but uncontrolled IOP, it is customary to decrease the extent of the initial treatment to 180–200 degrees.
Scleral perforation has been reported with TDC,11 so care should be taken to ensure that the probe surface is clean of debris and to avoid areas of obvious scleral thinning and the site of previous surgeries (do not laser where the tube passes and the previous trabeculectomy site). Among eyes with uncontrolled IOP after 1 glaucoma drainage device, TDC has been reported to have success similar to a second glaucoma drainage device implantation.12 Ultrasound biomicroscopy recently has been described as a guide to proper placement of the probe for TDC.13
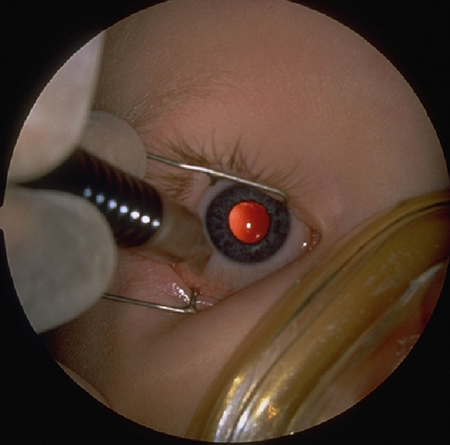

Figure 2. Trans-scleral diode-laser treatment, Iridex, Mountain View, CA, USA. A. Positioning of the laser probe on the surface of the patient's eye. B. Profile of the G probe trans-scleral laser probe.
Endoscopic Cyclophotocoagulation
The availability of a microendoscopic system for delivering energy to ablate the ciliary processes under direct view has expanded the options for cyclodestruction as a treatment for refractory pediatric glaucoma, allowing more precise localization of the target and treatment with much lower energy than required for trans-scleral laser (above). The most commonly used system uses a diode laser equipped with a 20‑gauge probe, within which is housed fiber optics for a video monitor, diode laser endophotocoagulation, and illumination (Figure 3, E2 Microprobe, Beaver-Visitec International, Waltham, MA, USA).14
Endoscopic cyclophotocoagulation (ECP) yields modest success when used for refractory pediatric glaucoma, with a suggestion that this technique might be gentler still than TDC, but with the same inherent risks of all cyclodestruction and the risk of infection given the need for an intraocular approach.15–17 When applied to eyes with glaucoma following removal of childhood cataract ("aphakic" and "pseudophakic" glaucoma), 1 series of 34 eyes reported cumulative success of 53% after relatively short mean follow-up of 44 months, and with an average of 1.5 treatment sessions per eye, and a generous definition of success.16 One small series reporting use of ECP in aphakic eyes with corneal opacification and refractory glaucoma found ECP success low as a primary treatment, but noted the utility of the endoscopic approach to facilitate subsequent glaucoma drainage implant surgery in these eyes.17
Many have remarked on subsequent ECP after failure of TDC to lower IOP; on direct visualization, the ciliary processes are often displaced from their "expected" location, resulting in many intact ciliary processes remaining despite repeated prior TDC.18 Although a variety of protocols exist for ECP, initial treatment conservatively should remain limited to a maximum of 3 quadrants; setting the power to 0.15 watts (recommended range 0.10 to 0.20 watts) and continuous treatment allows the individual ciliary processes to be "painted" so that they gradually shrink without the "explosion" that creates an audible "pop" (Video 1 and Figure 4).
Use of intraocular triamcinolone can be helpful in preventing exuberant post-laser inflammation. Although ECP can be attempted in a phakic eye, anatomic considerations usually limit this procedure to pediatric eyes that are either aphakic or pseudophakic; the probe can then be placed either anteriorly from a limbal approach or pars plana. It can be difficult to approach a child's pseudophakic eye from the limbal approach due to the high prevalence of synechiae and large Sommering ring presence in these eyes (personal unpublished data). Additional uses of the endoscopic system have included endoscopic goniotomy surgery.19
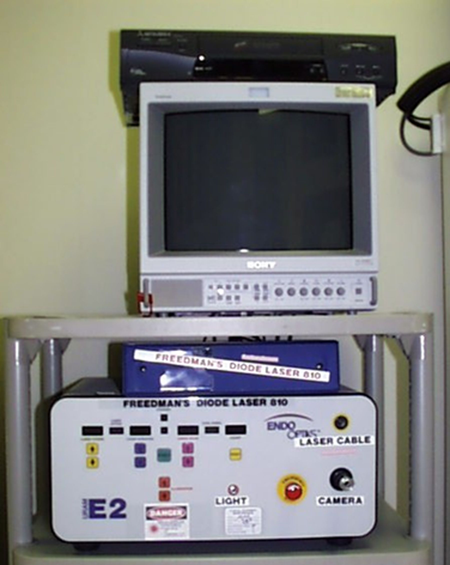
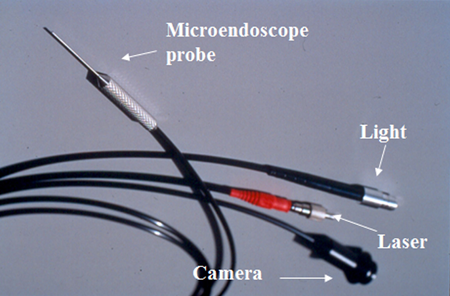
Figure 3. A. Endoscopic diode laser system. B. Diode laser equipped with a 20‑gauge probe, within which is housed fiber optics for a video monitor, diode laser endophotocoagulation, and illumination.
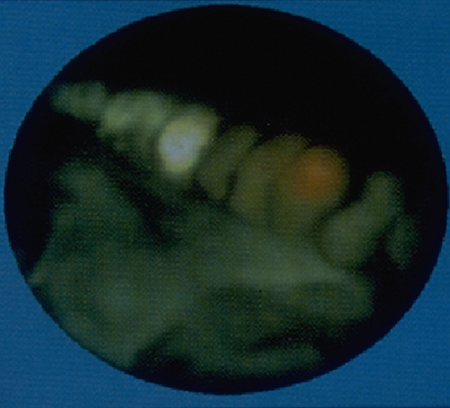
Figure 4. Endoscopic visualization of the ciliary processes and application of endoscopic diode laser (Endo Optiks, Beaver Visitec International, Waltham, MA, USA). The white ciliary processes to the left side have been treated to gently shrink and whiten them. The red spot on the ciliary process to the right of the center is the aiming beam, marking the area where the laser energy will be delivered when the foot pedal is depressed to fire the laser.
Video 1. This child with severe anterior segment dysgenesis, congenital glaucoma, and congenital aphakia in 1 eye has uncontrolled IOP and progressive central corneal ectasia despite multiple sessions of cyclocryotherapy. This video shows treatment of residual ciliary tissue using endoscopic diode laser. Extensive areas of atrophy form prior cryotherapy can be seen. Since the treatment, the eye is stable with IOP approximately 10 mm Hg and baseline vision of light perception.
Summary
Cyclodestruction has limited success and the risk of severe complications when used for treatment of refractory pediatric glaucoma. Nonetheless, this modality retains an important role in the management of selected eyes with refractory glaucoma. The position of cyclodestruction in the treatment algorithm depends on several factors, including the visual potential, anatomic features, prior surgical interventions, glaucoma severity, and child's overall health. Both TDC and ECP can represent viable adjunctive techniques after prior glaucoma drainage device surgery has incompletely controlled the IOP and can be reasonable primary surgery in selected eyes with challenging anatomy for other intraocular glaucoma procedures. Cyclodestruction should be used with extreme caution in eyes with uveitis because all 3 techniques produce significant inflammation; similarly all 3 techniques have the potential to produce retinal detachment, hypotony, phthisis, and even sympathetic ophthalmia.20,21
Cyclodestruction Pearls
- Choose the optimal type of ablation: trans-scleral versus endoscopic laser initially, cryotherapy rarely. Weigh considerations of anatomy, risk versus benefit of intraocular approach to treat ciliary processes more precisely, etc.
- Limit initial treatment with trans-scleral and endoscopic laser to 3 quadrants — or even 2 if prior glaucoma drainage implant is in place — to help avoid hypotony.
- Use adequate anti-inflammatory treatment to avoid complications of inflammation.
- Minimize risk of phthisis: Keep careful records of prior treatment, rarely allow 360 cumulative treatments.
- Discuss limitations of treatment, including sympathetic ophthalmia, fully with parents to achieve mutual understanding of risks and alternatives.
References
- Al Faran MF, Tomey KF, Al Mutlag FA. Cyclocryotherapy in selected cases of congenital glaucoma. Ophth Surg. 1990;21:794-798.
- Wagle NS, Freedman SF, Buckley EG, Davis JS, Biglan AW. Long-term outcome of cyclocryotherapy for refractory pediatric glaucoma. 1998;105(10):1921-1926; discussion 1926-1927.
- Bellows AR. Cyclocryotherapy: Its role in the treatment of glaucoma. Perspect Ophthalmol. 1980;4:139.
- Phelan MJ, Higginbotham EJ. Contact trans-scleral Nd:YAG laser cyclophotocoagulation for the treatment of refractory pediatric glaucoma. Ophthalmic Surg Lasers. 1995;26:401-403.
- Shields MB, Shields SE. Noncontact trans-scleral Nd:YAG cyclophotocoagulation: a long-term follow-up of 500 patients. Trans Am Ophthalmol Soc. 1994;92:271-283; discussion 283-277.
- Childhood Glaucoma. The 9th Consensus Report of the World Glaucoma Association Kugler Publications (Amsterdam, The Netherlands); 2013.
- Bock CJ, Freedman FF, Buckley EG, Shields MB. Trans-scleral diode laser cyclophotocoagulation for refractory pediatric glaucomas. J Pediatr Ophthalmol Strabismus. 1997;34:235-239.
- Autrata R, Rehurek J. Long-term results of trans-scleral cyclophotocoagulation in refractory pediatric glaucoma patients. 2003;217(6):393-400.
- Izgi B, Demirci H, Demirci FY, Turker G. Diode laser cyclophotocoagulation in refractory glaucoma: comparison between pediatric and adult glaucomas. Ophthalmic Surg Lasers. 2001;32(2):100-107.
- Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: role in the management of refractory pediatric glaucomas. 2002;109(2):316-323.
- Sabri K, Vernon SA. Scleral perforation following trans-scleral cyclodiode. Br J Ophthalmol. 1999;83(4):502-503.
- Sood S, Beck AD. Cyclophotocoagulation versus sequential tube shunt as a secondary intervention following primary tube shunt failure in pediatric glaucoma.J Aapos. 2009;13(4):379-383.
- Way AL, Nischal KK. High-frequency ultrasound-guided trans-scleral diode laser cyclophotocoagulation. Br J Ophthalmol. 2014;98(7):992-994.
- Uram M. Ophthalmic laser microendoscope endophotocoagulation. 1992;99:1829.
- Plager DA, Neely DE. Intermediate-term results of endoscopic diode laser cyclophotocoagulation for pediatric glaucoma. J Aapos. 1999;3(3):131-137.
- Carter BC, Plager DA, Neely DE, Sprunger DT, Sondhi N, Roberts GJ. Endoscopic diode laser cyclophotocoagulation in the management of aphakic and pseudophakic glaucoma in children. J Aapos. 2007;11(1):34-40.
- Al-Haddad CE, Freedman SF. Endoscopic laser cyclophotocoagulation in pediatric glaucoma with corneal opacities. J Aapos. 2007;11(1):23-28.
- Barkana Y, Morad Y, Ben-nun J. Endoscopic photocoagulation of the ciliary body after repeated failure of trans-scleral diode-laser cyclophotocoagulation. Am J Ophthalmol. 2002;133(3):405-407.
- Kulkarni SV, Damji KF, Fournier AV, Pan I, Hodge WG. Endoscopic goniotomy: early clinical experience in congenital glaucoma. J Glaucoma. 2010;19(4):264-269.
- Albahlal A, Al Dhibi H, Al Shahwan S, Khandekar R, Edward DP. Sympathetic ophthalmia following diode laser cyclophotocoagulation. Br J Ophthalmol. 2014;98(8):1101-1106.
- Aujla JS, Lee GA, Vincent SJ, Thomas R. Incidence of hypotony and sympathetic ophthalmia following trans-scleral cyclophotocoagulation for glaucoma and a report of risk factors. Clin Experiment Ophthalmol. 2013;41(8):761-772.