Introduction
Infants with disorders of the anterior segment often present to the ophthalmologist early in life. These ocular abnormalities are often readily apparent and identified by parents or primary care providers. It is as important to know how to manage and treat the obvious condition as it is to look for and detect associated ocular and systemic abnormalities.
Neural–crest-derived mesenchymal cells, the neuroectoderm of the optic cup, and surface ectoderm all contribute to tissues of the anterior segment. Iridocorneal anomalies in infants may be part of a spectrum of anterior segment developmental anomalies. Several genetic mutations associated with these anomalies have been identified, revealing the role of certain genes in ocular development. The majority of these genes encode transcription factors, which regulate the expression of downstream genetic targets by binding to specific DNA sequences and affecting transcription.
Three of the genes involved in developmental iridocorneal anomalies and mentioned in this chapter are discussed below.
PAX6
Paired box gene-6 (PAX6) is found on chromosome 11p13. It is considered the master control gene in eye morphogenesis. It encodes a transcription factor with two DNA-binding sites. It ultimately functions as a regulator of cellular proliferation, differentiation, migration, and adhesion.
The protein is expressed early in ocular tissue development. It is expressed in the lens, the inner and pigmented layers of the iris and ciliary body, the corneal epithelium, and the retina, and later in the trabecular meshwork.1 It continues to be expressed throughout life. PAX6 is also involved in the development of other tissues, including the brain, the central nervous system, and the pancreas.2
The most common reported malformation associated with pathogenic PAX6 mutations is aniridia.3 Non-aniridic PAX6 mutation phenotypes may include Peters anomaly, autosomal dominant keratitis, foveal hypoplasia, optic nerve hypoplasia, congenital cataracts, and neurodevelopmental abnormalities.
PITX2 and FOXC1
The PITX2 gene on chromosome 4q25 is a paired homeobox gene that encodes a transcription factor. The FOXC1 gene on chromosome 6p25 encodes a transcription factor with a characteristic DNA-binding fork head domain. Both factors play a role in the regulation of embryonic and ocular development. They are expressed within migrating periocular neural crest cells.
The spectrum of PITX2/FOXC1 mutations includes intragenic mutations, copy number changes, and deletions.4 Mutations in these two genes mainly result in complete or partial loss of function.5
Ocular findings in patients with mutations in PITX2 and FOXC1 include iridocorneal adhesions, iris hypoplasia, iridogoniodysgenesis, and Axenfeld-Rieger anomaly and syndrome.1,6 Mutations in these two genes have significant overlap in their ocular phenotypes.5
Systemic anomalies may provide a clue as to the affected gene. Mutations in the PITX2 gene seem more likely to result in ocular, dental, and umbilical abnormalities, while FOXC1 mutations are more likely to lead to isolated ocular anomalies or to a combination of ocular, heart, and hearing defects.5,7
PITX2 mutations may affect the pituitary axis and lead to growth disorders.5,8-10
Iridocorneal anomalies of neural crest origin
Posterior embryotoxon
Definition
Posterior embryotoxon describes a thickened and anteriorly displaced Schwalbe line. The Schwalbe line normally sits at the junction of the trabecular meshwork and the end of Descemet membrane. In individuals with posterior embryotoxon, the Schwalbe line is readily apparent by external examination and appears as an irregular opaque ridge concentric to the limbus. It is located about 0.5-2.0 mm central to the limbus, and it may contain pigmented spots on gonioscopy.11
It can be an isolated finding in approximately 8%-15% of the normal population. It is commonly bilateral and may be inherited in an autosomal dominant manner.
Isolated posterior embryotoxon is not associated with glaucoma.
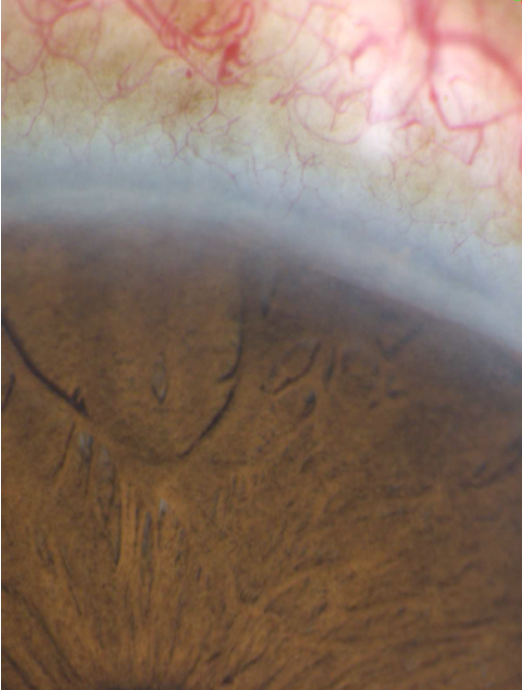
Figure 1. Posterior embryotoxon appears as an irregular opaque ridge concentric to the limbus. (Courtesy of Paul Harasymowycz, MD)
Associated conditions
1. Alagille syndrome and other systemic conditions
Alagille syndrome is the most common systemic association with posterior embryotoxon. It is an autosomal dominant disorder of neonatal jaundice due to intrahepatic cholestasis. Its features may include xanthomas, pulmonic stenosis, heart abnormalities, distinctive facial features, and "butterfly vertebrae.’’12-14
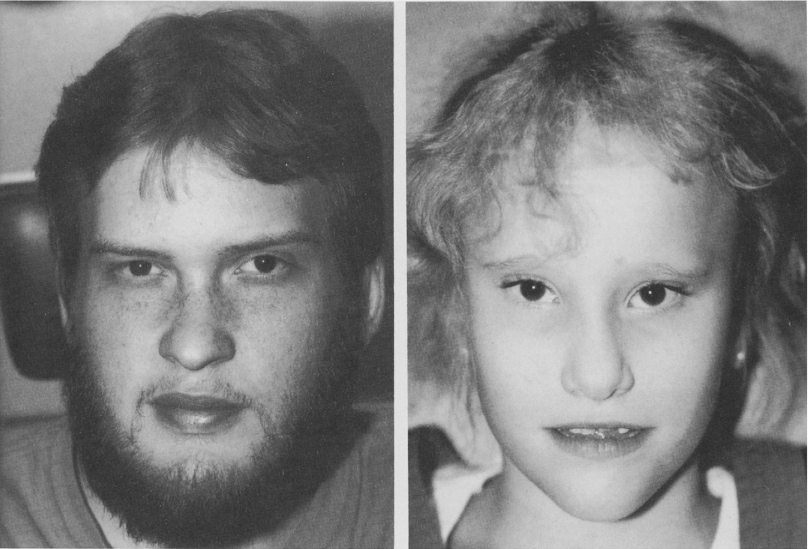
Figure 2. Facial photographs. (Brodsky, M.C.; Cunniff, C. Ocular Anomalies in the Alagille Syndrome [arteriohepatic dysplasia]. Ophthalmology, 1993. 100[12]: p. 1767–1774. Figure 3).
Velo-cardio-facial syndrome is another systemic association. It is a genetic syndrome of immune deficiency, cardiovascular abnormalities, cleft palate, learning difficulties, hypocalcemia, and abnormal facial appearance. Other than posterior embryotoxon, ocular findings include retinal vascular tortuosity, narrow palpebral fissures, iris nodules, and cataracts.15,16
Posterior embryotoxon has also been reported in X-linked ichthyosis.17
2. Axenfeld and Rieger anomalies
Historically, Axenfeld anomaly referred to posterior embryotoxon with adherent iris strands, which vary in appearance from thread-like to thick bands of tissue. Rieger anomaly described Axenfeld anomaly in the presence of congenital iris abnormalities, which include iris hypoplasia, corectopia, and pseudopolycoria. Rieger syndrome is known as the association of Rieger anomaly with systemic developmental defects, namely dental, facial, and umbilical abnormalities.
Today Axenfeld-Rieger anomaly (ARA) encompasses the ocular features of Axenfeld anomaly and Rieger anomaly.1 Axenfeld-Rieger syndrome (ARS) describes a heterogeneous group of conditions characterized by the ocular features of ARA and specific systemic anomalies.
From a developmental standpoint, ARA/ARS affects the iris-angle axis, and is classified as an iridotrabecular dysgenesis.18
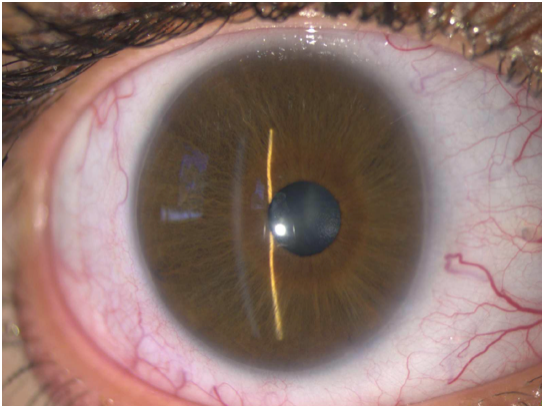
Figure 3. Posterior embryotoxon, iris stromal hypoplasia, and corectopia in a patient with ARA. (Courtesy of Paul Harasymowycz, MD)

Figure 4. Iris strands adherent to posterior embryotoxon in a patient with ARA. (Courtesy of Paul Harasymowycz, MD)
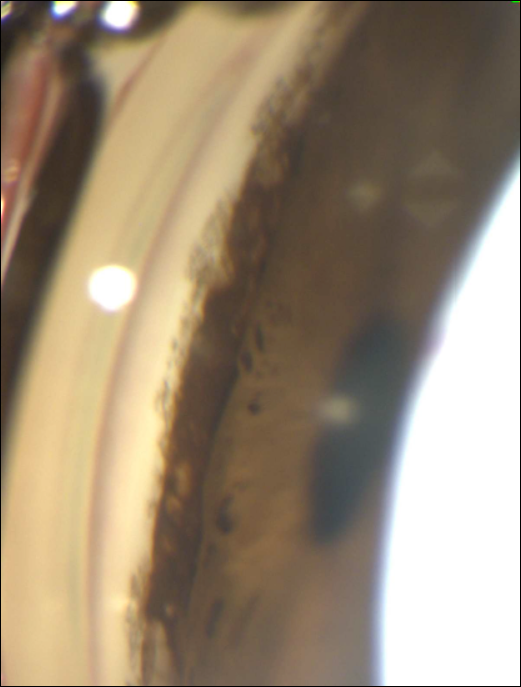
Figure 5. Thick bands of iris tissue adherent to posterior embryotoxon in a patient with ARA. (Courtesy of Paul Harasymowycz, MD)
3. Axenfeld-Rieger anomaly and syndrome
Ocular associations
Posterior embryotoxon with adherent iris strands is one of the most common manifestations of ARA/ARS. Its appearance is variable. It may be more pronounced, or it may be so subtle as to not be visible on slit-lamp examination.19
Other common iris manifestations include iris hypoplasia ranging from mild stromal thinning to severe atrophy, corectopia, pseudopolycoria, and ectropion of the iris. A high iris insertion into the posterior trabecular meshwork may be seen.19-21 Clinically, the iris stroma may be similar to iridocorneal endothelial syndrome.22 The distinction in an infant is readily made, as ARS is congenital, bilateral, and may include systemic disorders.
Increased intraocular pressure is a common association. Glaucoma appears during childhood or adulthood in approximately 50% of patients, though it may be found in infancy.7 The angle is usually open. Glaucoma is believed to be due to an incomplete development of the trabecular meshwork and of Schlemm canal during gestation.1,21 A high iris insertion seems to be more dramatic in eyes with glaucoma; however, iris abnormalities do not usually correlate with its severity.19
Other ocular features may include limbal dermoids, megalocornea, microcornea, Peters anomaly, cataracts, chorioretinal colobomas, retinal detachment, macular degeneration, and optic nerve hypoplasia.1,23,24
An infant with ARS typically presents with posterior embryotoxon, iris corectopia, and some degree of iris atrophy. Photophobia may be a presenting symptom.
Complete absence of posterior embryotoxon, however, in the presence of congenital corneal opacification and classic ARS systemic signs should alert the clinician that it may be a variation in phenotype.6 Testing for PITX2 and FOXC1 may still be warranted.
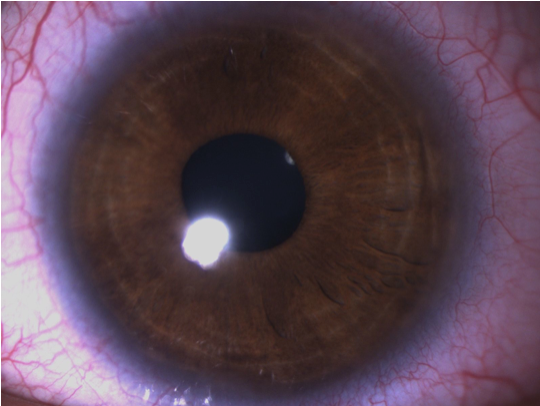
Figure 6. Iris stromal hypoplasia in a patient with ARS. (Courtesy of Paul Harasymowycz, MD)
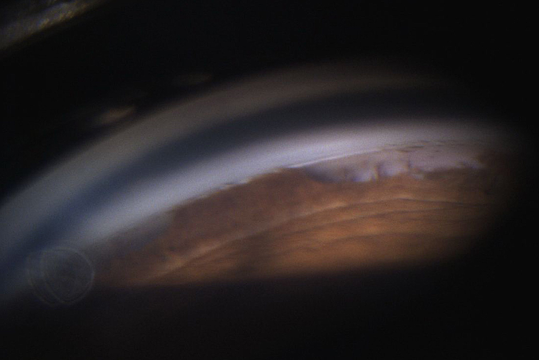
Figure 7. Thick bands of iris tissue adherent to posterior embryotoxon in a patient with ARS. (Courtesy of Paul Harasymowycz, MD)
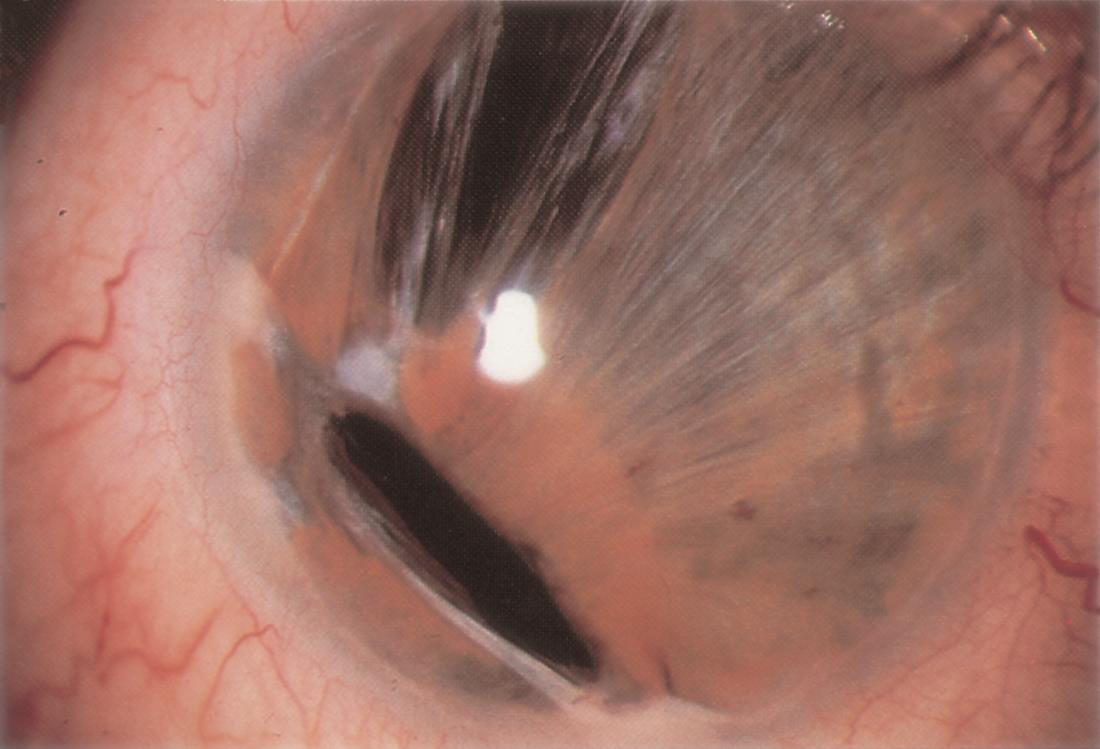
Figure 8. A patient with ARS exhibiting iris atrophy, corectopia, and pseudopolycoria. https://www.aao.org/image/axenfeldrieger-syndrome-3
Systemic associations
Typical nonocular findings in ARS are craniofacial dysmorphism, dental anomalies, and excess umbilical skin. Facial anomalies include maxillary hypoplasia, prognathism, hypertelorism, telecanthus, a prominent forehead, and a broad, flat nose.1,23,25,26 Dental anomalies may include microdontia, oligodontia, hypodontia, and cone-shaped teeth. Other systemic anomalies associated with ARS are disorders of the pituitary gland, growth hormone deficiency, and short stature, hypospadias, anal stenosis, cardiac abnormalities, abnormal ears, deafness, and mental deficiency.1, 20
De Hauwere syndrome is a systemic disorder associated with ARS.5 Findings in this condition include anterior segment anomalies, hypertelorism, hypotonia, psychomotor retardation, hearing loss, femoral head abnormalities and ventricular anomalies.27
Epidemiology
ARS is found in 1/200,000 live births. There is no sex predilection.
Pathogenesis
ARS is believed to be due to defects in neural crest cell differentiation, migration, or development. These defects lead to the finding of neural crest remnants and a monolayer of endothelial-like cells on the iris and the anterior chamber angle.
Genetics
ARS most commonly has an autosomal dominant pattern of inheritance, but it can also occur sporadically.21 ARS has high penetrance with variable phenotypic expression. ARA or ARS is caused by mutations in PITX2 or FOXC1 in approximately 40% of cases.4,5,26 Mutations in other gene loci have also been reported.
Systemic anomalies may point to the affected gene. ARS in a patient with significant ocular, dental, umbilical, and growth abnormalities may be more likely to result from a mutation in the PITX2 gene.5,7 A patient with ARS and heart defects is more likely to have a FOXC1 mutation.
4. Iridogoniodysgenesis
Iridogoniodysgenesis refers to bilateral iris stromal hypoplasia and trabecular meshwork maldevelopment. The iris is striking in color, a dark slate grey or bitter chocolate, due to the pigmented iris epithelium visible through the hypoplastic stroma. The pupillary sphincter appears as a tan-colored ring. These findings are congenital and nonprogressive. Patients usually do not have posterior embryotoxon, corectopia, or iris holes. Juvenile-onset glaucoma is associated in the majority of cases, though it has been reported to appear anytime from birth to late adulthood.28-30 Glaucoma management can be difficult, as medical therapy is often unsuccessful.31
Iridogoniodysgenesis anomaly (IGDA) refers to iridogoniodysgenesis caused by a mutation in the FOXC1 gene on 6p25. The disorder has an autosomal dominant inheritance pattern. Brain imaging in some affected patients has revealed cerebellar vermis abnormalities.32
Iridogoniodysgenesis syndrome (IGDS) refers to iridogoniodysgenesis caused by a mutation in the PITX2 gene on 4q25.33 The disorder has an autosomal dominant inheritance pattern. Affected individuals may present with maxillary hypoplasia, dental and periumbilical anomalies, and hypospadias in males.31,34
Management
Treatment is primarily directed at the presenting complication. In cases where glaucoma is absent, the prognosis is usually favorable.
When ARS is associated with infantile glaucoma, surgery is the first line of treatment, and medical therapy is usually an adjunct to surgery.1,35 Surgical options include goniotomy, trabeculotomy, trabeculectomy, or aqueous shunt procedures with or without antimetabolite augmentation. Cycloablation procedures may also be used. (See Secondary Glaucoma: Glaucoma Associated With Non-Acquired Ocular Anomalies) Life-long monitoring of patients is essential, as glaucoma may appear beyond adulthood.
Patients with ARS may experience significant photophobia or glare due to their iris defects. Tinted lenses may help alleviate their symptoms. When pupil corectopia is severe and pupillary stenosis becomes an issue, a large pupilloplasty is indicated. Even after surgery, the pupil may continue to move eccentrically or contract. Pupil corectopia with no significant stenosis is otherwise treated with occlusion therapy of the better eye.
Patients with ARA must be examined for possible associated facial, dental, periumbilical, and other anomalies. Any associated systemic conditions are managed using a multidisciplinary approach. Examination of the parents, along with genetic analysis, may be precious tools in providing clues about the etiology of an infant's condition, and may help provide accurate counseling.36
Congenital iris ectropion
Diagnostic features
This condition is characterized by hyperplasia and outward turning of the posterior pigment iris epithelium onto the anterior surface of the iris. It is usually a unilateral, isolated, nonprogressive finding. It may occupy the circumference of the pupil or only be seen sectorally.
- Patients may initially be referred for anisocoria, as the posterior pigment epithelium is dark.
- Bilateral iris ectropion should alert the clinician to look more thoroughly for another possible cause.
- In some cases, iris ectropion is acquired and may be progressive. Careful examination for underlying disorders may reveal iris ectropion is the result of a tractional process, as in rubeosis iridis.
Pathogenesis
In the literature, iris ectropion is often called ectropion uveae. Iris ectropion is the correct name for this condition, as the posterior epithelium of the iris is derived from neural ectoderm. This condition is believed to arise from an arrest in development in late gestation that leads to the retention of primordial epithelium. A primary vascular insult has been suggested as the initial trigger.37
Ocular associations
Congenital iris ectropion may be encountered in the presence of iris stromal hypoplasia, a high iris insertion, angle dysgenesis, glaucoma, and in a number of cases, unilateral blepharoptosis.38-40
Systemic associations
Systemic associations may include neurofibromatosis I, Prader-Willi syndrome, and facial hemihypertrophy.38
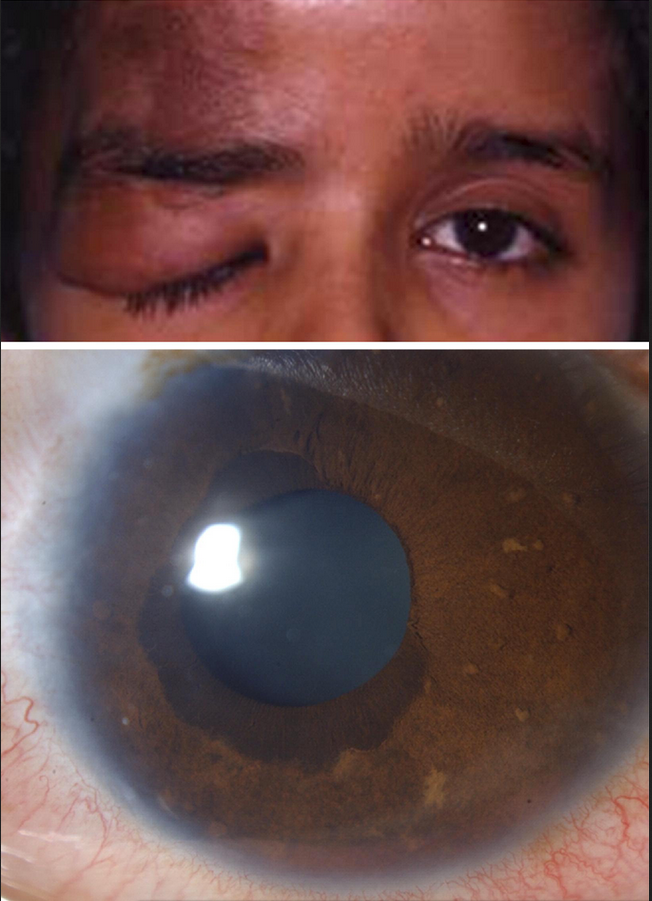
Figure 9. Iris ectropion with Lisch nodules (bottom) and plexiform neurofibroma (top) in a patient with neurofibromatosis-1. (Edward, D.P. et al. Congenital ectropion uvea and mechanisms of glaucoma in neurofibromatosis type 1: new insights. Ophthalmology. 2012 Jul;119(7):1485-94. Figure 1.)
Management
Glaucoma may first appear in infancy, though the majority of patients usually develop glaucoma in early childhood or later.38,40,41 It is important to mention that the extent of the iris ectropion does not correlate with the degree of angle involvement in the affected eye.40,41 Patients will often require surgical intervention for adequate intraocular pressure (IOP) control. Augmented trabeculectomy may be the procedure of choice.1 (See Secondary Glaucoma: Glaucoma Associated With Non-Acquired Ocular Anomalies.)
Due to the associated systemic conditions, a thorough systemic evaluation is needed.
Iridocorneal endothelial syndromes
Diagnostic features
Iridocorneal endothelial syndrome (ICE) is a term encompassing disorders characterized by abnormal corneal endothelium, iris anomalies, and peripheral anterior synechiae.42-44
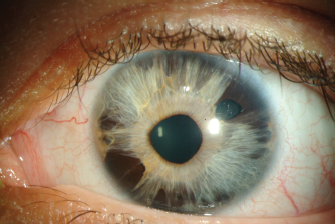
Figure 10. Iridocorneal endothelial syndrome with corectopia. (External Disease and Cornea, in Basic and Clinical Science Course (BCSC). 2013-2014, American Academy of Ophthalmology. Figure 12-15.)
Epidemiology
ICE typically presents between the ages of 20 and 50, though it has been reported in children as early as eleven years of age.42,44-46 It is more common in females. It appears to be a non-inherited disorder with no clear association to other ocular or systemic disorders.47,48
Pathogenesis
An abnormal clone of endothelial cells migrates posterior to the Schwalbe line, and onto the trabecular meshwork.44,49 Also known as "ICE cells,’’ these cells are large and pleomorphic. They exhibit the ultrastructural characteristics of epithelial cells, leading to their ability to migrate.47,49 The endothelial membrane may contain single to multiple layers of cells, and includes collagenous and fibrillar tissue. It progressively covers the anterior chamber angle and the iris surface. When the enlarging membrane and its surrounding tissues contract, tractional iris changes can be seen, and they include peripheral anterior synechiae (PAS), corectopia, iris ectropion, iris stromal atrophy, and iris pigment epithelial holes.50
The true etiology of ICE syndrome has not yet been elucidated. A microscopic study of the abnormal basement membrane secreted by the ICE cells showed that its secretion begins postnatally, suggesting ICE is a genetic disease with late onset, or that it may be an acquired disorder.51 It is thought that the abnormal clone of endothelial cells represents a primary neural crest cell disorder, and that these cells may in fact be congenitally and bilaterally abnormal.52,53 Others have suggested a viral etiology of ICE syndrome.54-56
Clinical features
Clinically, the abnormal corneal endothelium has a "beaten-bronze’’ or "hammered-silver’’ appearance, resembling corneal guttae.48 It is best seen when viewed with the slit lamp in specularly reflected light. Microcystic corneal edema may develop. It is due to defective endothelial cell pump function and/or to elevated IOP from secondary angle-closure glaucoma.57
Iris changes in ICE syndrome vary and include heterochromia, iris ectropion, corectopia, hole formation, and iris atrophy. Iris nodules may appear due to ICE-cell growth on the iris stroma, and a dark iris mass may be seen due to iris stromal atrophy uncovering darker pigment epithelium.50 These changes are seen on slit-lamp examination. Gonioscopy may reveal high and progressively broad-based PAS extending anterior to the Schwalbe line.
Specular microscopy can confirm the diagnosis when the iris and corneal signs are subtle. It shows normal endothelial cell loss and abnormal ICE cells in the affected eye. In cases where corneal edema prevents imaging by specular microscopy, confocal microscopy may be of great value for demonstrating epithelioid endothelial cells as dark areas with a bright central spot.57-60
Young patients typically present with a unilateral change in iris appearance. Older ones usually experience visual impairment and/or monocular pain from corneal edema or high IOP.
ICE syndrome almost always presents as a unilateral disease. The unaffected eye may have subtle irregularities of the corneal endothelium or changes of iris atrophy without other signs of the disease.42, 52
Clinical variants
1. Chandler Syndrome
Corneal and angle findings predominate in this variant. It has a higher prevalence of corneal edema compared to the other variants.48 It may present with little iris atrophy and corectopia, or with no iris changes at all.
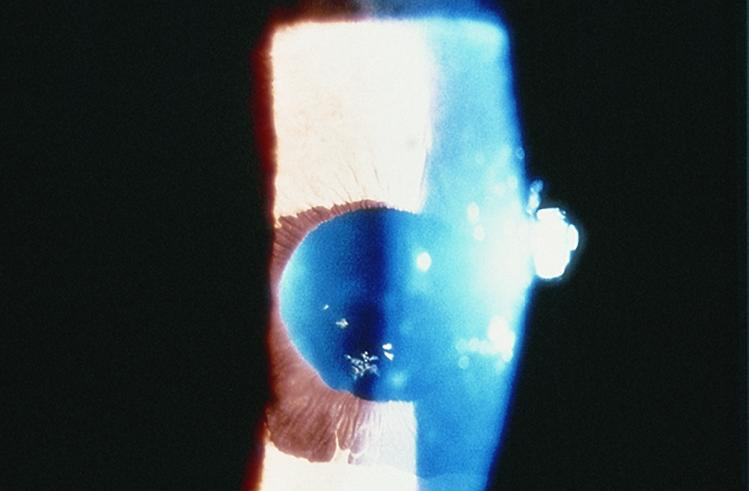
Figure 11. Chandler Syndrome. (Courtesy of Steven T. Simmons, MD. American Academy of Ophthalmology.)
2. Essential/Progressive Iris Atrophy
Severe progressive iris atrophy is the key feature of essential/progressive iris atrophy. The iris stroma undergoes extensive atrophy, leading to iris "melting holes.’’48 Corectopia and iris ectropion are formed in the quadrant with PAS, while in the opposite quadrant, tensile forces on the iris lead to "stretch holes.’’45,48 These iris degenerative changes may cause significant light-sensitivity and visual disturbance. Any corneal edema is typically mild.
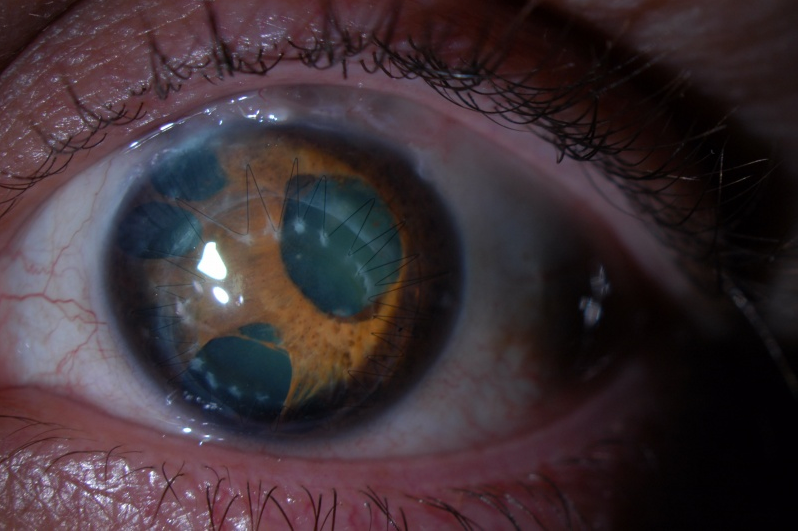
Figure 12. Essential/progressive iris atrophy in a patient with polycoria, iris hole formation, and corectopia. (Photo courtesy of Krieger Eye Institute [KEI] at Sinai Hospital of Baltimore, along with Dr. Donald Abrams and Dr. Gregory Oldham)
3. Cogan-Reese syndrome/Iris-nevus syndrome
This condition is unique due to its iris findings. It is characterized by multiple tan pedunculated nodules or diffuse pigmented lesions on the anterior surface of the iris.44 Iris atrophy may be present but is usually subtle.
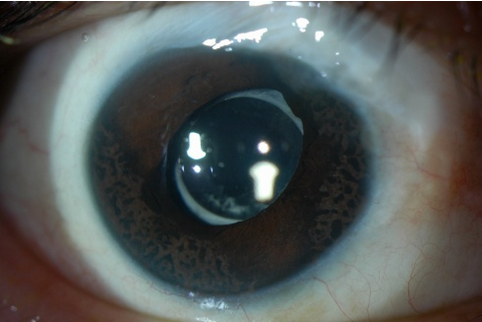
Figure 13. Cogan-Reese syndrome/iris-nevus syndrome. (Photo courtesy of Krieger Eye Institute [KEI] at Sinai Hospital of Baltimore, along with Dr. Donald Abrams and Dr. Gregory Oldham.)
Ocular associations
Secondary angle-closure glaucoma typically results from the PAS. It can sometimes be encountered without overt synechiae because the membrane overgrowth can functionally close the angle without contracting. These patients may be initially diagnosed with open-angle glaucoma because the membrane may be difficult to identify with gonioscopy.44 Glaucoma is associated in 43%-82% of patients with ICE syndrome.48,59,61,62 It may be more severe in patients with progressive iris atrophy and Cogan-Reese syndrome, compared to Chandler syndrome.44,48 One study emanating from an oncology department reported far less glaucoma in ICE cases (15%), suggesting the difference in numbers may vary based on referral patterns.50 (See Secondary Glaucoma: Glaucoma Associated With Non-Acquired Ocular Anomalies.)
Management
It is primarily directed toward preventing vision loss from glaucoma. Medical therapy with aqueous suppressants is the first step. In the long term, it often proves ineffective at controlling the IOP.59 Filtering surgery is needed at that juncture, as laser trabeculoplasty is not an option in patients with ICE syndrome. Augmented trabeculectomy is often the procedure of choice.59 Some advocate early consideration of drainage tubes in patients with ICE syndrome.63 (See Secondary Glaucoma: Glaucoma Associated With Non-Acquired Ocular Anomalies)
Achieving IOP control can clear the corneal edema resulting from high intraocular pressures. Mild corneal edema due to corneal endothelial cell dysfunction can be addressed with topical hypertonic saline drops and gels. In severe cases, surgical options include PKP and endothelial keratoplasty (DSAEK or DSEK).
Iridocorneal anomalies of a global origin
Congenital megalocornea
Diagnostic features
A newborn's cornea is normally 9.5-10.5 mm in horizontal diameter (white-to-white). It increases to the 12 mm average adult size by age 2. Megalocornea refers to a cornea with a horizontal diameter greater than 13 mm. The enlargement is nonprogressive.
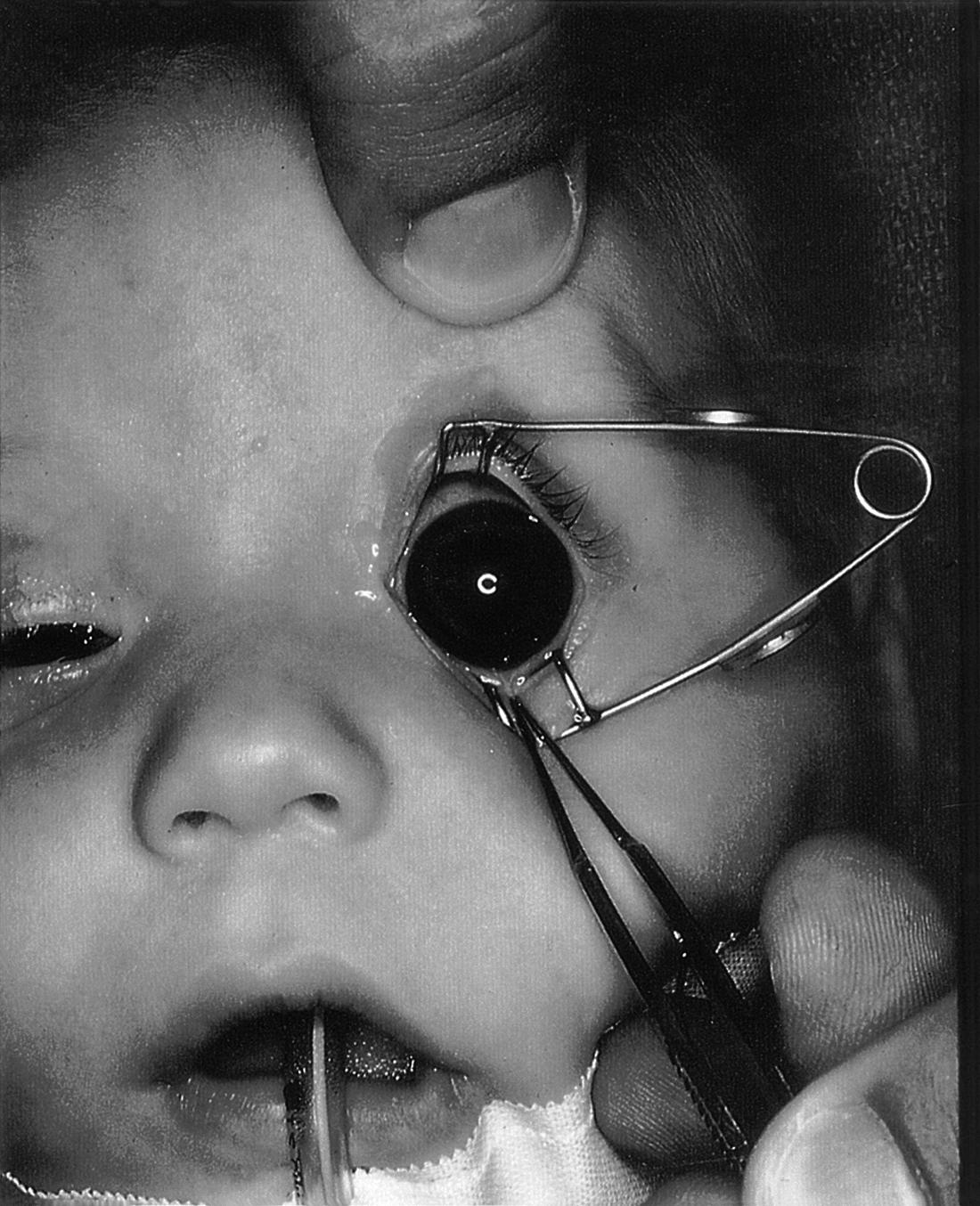
Figure 14. Corneal horizontal diameter in megalocornea is greater than 13 mm. (Courtesy of the American Academy of Ophthalmology.)
Initial clinical examination must distinguish megalocornea from infantile glaucoma where enlargement of the globe (buphthalmos) may occur. (See Glaucoma: Definitions and Classification.) Features of infantile glaucoma, such as photophobia, epiphora, corneal opacification, Haab striae, high IOP, and large optic nerve excavations, must be sought and the diagnosis must be excluded.
Keratoglobus may present at birth in association with Ehlers-Danlos type VI or with other conditions. It may be distinguished from megalocornea by its globular protrusion of the cornea and severe corneal thinning, especially in the periphery. Keratoglobus does not exhibit the ocular signs associated with megalocornea, such as iris stromal hypoplasia with iris transillumination.
Epidemiology
This rare congenital condition affects less than 1/50,000 persons.64 It is typically bilateral and symmetric.
Pathogenesis
The prevalent theory explaining this condition is the failure of the optic cup to grow, resulting in a larger space for the cornea to fill.
Genetics
Megalocornea is usually inherited in an X-linked recessive manner, where 90% of affected patients are male. Heterozygous women may present with a slight increase in corneal diameter. Associated ocular and systemic anomalies may be more often encountered with this inheritance pattern.64 Only one causative gene, CHRDL1 on Xq23, has been identified in this condition and encodes for the ventropin protein.65, 66 Ventropin is also expressed in the retina and the brain.
Autosomal dominant and autosomal recessive cases have been described. Examination of family members may be helpful in establishing an inheritance pattern.
Ocular associations
In cases of megalocornea, corneas are usually thin, the anterior chamber deep, and the iridocorneal angle may be readily seen at the slit-lamp without a gonioscope.65 Nocturnal lagophthalmos may occur and result in exposure keratitis. Myopia and with-the-rule astigmatism are commonly encountered refractive errors.1
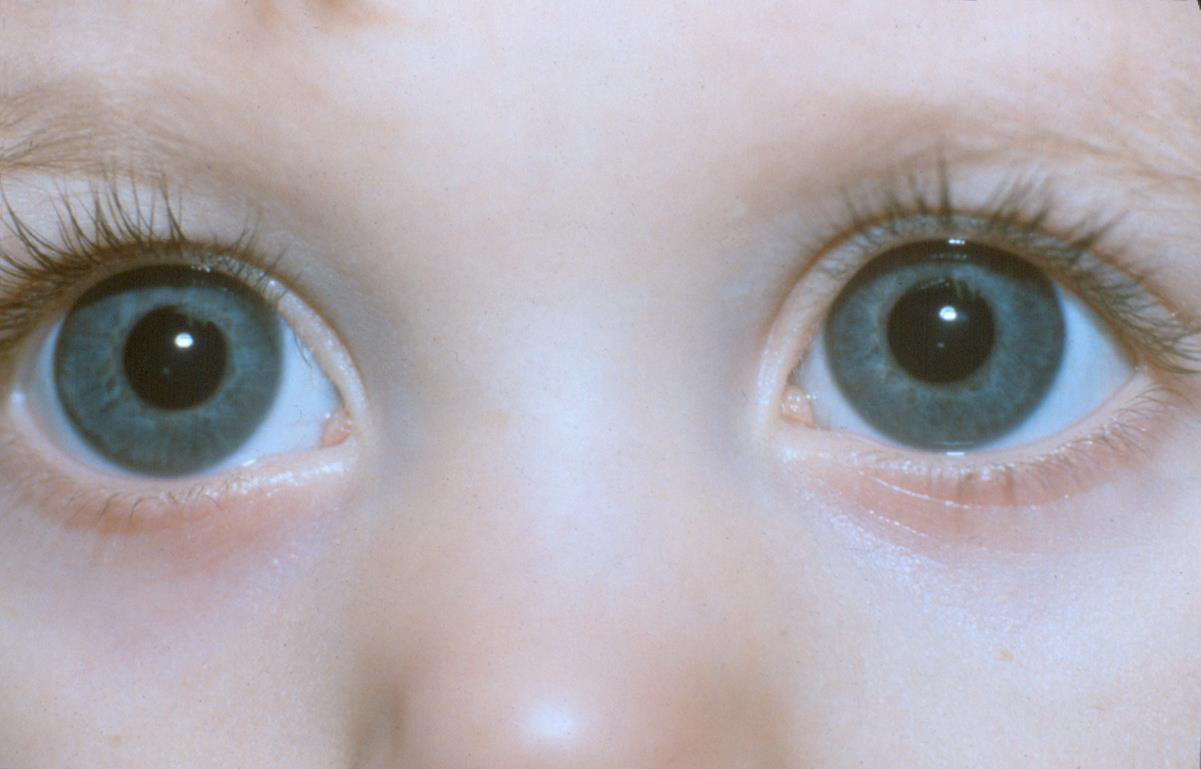
Figure 15. Megalocornea. (Courtesy of the American Academy of Ophthalmology)
Other ocular abnormalities may be encountered and be age related.67 They include zonular stretching, phacodonesis, ectopia lentis, cataracts, spherophakia, iris stromal hypoplasia with iris transillumination, iridodonesis, Krukenberg spindle, increased angle pigmentation, corneal arcus juvenilis, mosaic corneal dystrophy, and later-onset glaucoma.1,64,65,67-71
Systemic associations
Included among systemic associations of megalocornea are facial anomalies, craniosynostosis, frontal bossing, hypertelorism, facial hemiatrophy, dwarfism, intellectual disability, hypotonia, Alport syndrome, Down syndrome, Marfan syndrome, osteogenesis imperfecta, mucolipidosis type II, the megalocornea-mental retardation syndrome, or other genetic syndromes.64
Management
Given possible systemic associations, management includes a systematic general pediatric examination.
Optical correction of the refractive error and treatment of amblyopia are often needed. Contact lenses may be a good option in patients with high astigmatism. Tinted glasses can help patients with significant photophobia due to iris transillumination. A nightly lubricating ointment is a good prophylactic measure in patients to prevent exposure keratitis.
Regular screening throughout life for the development of glaucoma is indicated. When lens extraction is warranted, either due to a cataract or to a dislocated lens, the associated anterior segment abnormalities may complicate the procedure and require extra care.64,68 Traditionally, treatment of aphakia in patients with megalocornea includes aphakic spectacles, contact lenses, and intraocular lens (IOL) implantation. A formula that takes white-to-white and anterior chamber depth into account is recommended for IOL calculations.72,73 Pseudophacodonesis and dislocation of the lens within the capsular bag may occur following implantation.74 Scleral-fixated posterior chamber IOLs, peripheral-iris fixated IOLs, and iris-claw IOLs are other reported options for lens implantation in megalocornea.72,74-76
Microcornea
Diagnostic features
Microcornea is a nonprogressive condition in which the corneal horizontal diameter is less than 9 mm in a newborn, and less than 10 mm by age 2. Significant asymmetry in corneal diameter in the same patient is considered abnormal, even when both corneal diameters fall within the normal range.
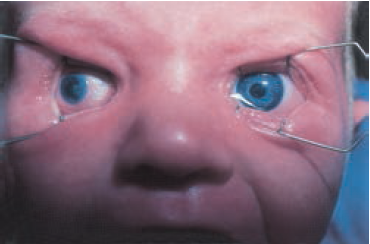
Figure 16. Microcornea, right eye. (Pediatric Ophthalmology and Strabismus, in Basic and Clinical Science Course [BCSC]). 2013-2014, American Academy of Ophthalmology. Figure 18-1.)
In microcornea, the globe is otherwise of normal dimensions, as opposed to microphthalmos, where all structures are proportionally small and malformed inside a small globe. In anterior microphthalmos, a proportionally small anterior segment is found in a globe of normal dimensions. In nanophthalmos, normal or thickened structures are contained in a small globe.
Epidemiology/Pathogenesis
The disorder may be unilateral or bilateral. It may follow an autosomal dominant or an autosomal recessive pattern.
Postulated mechanisms for microcornea include an overgrowth of the anterior tips of the optic cup, which limit growth of the cornea.
Ocular associations
The cornea is clear and of normal thickness. The corneal curvature is flatter than normal and patients tend to be hyperopic.
Infantile glaucoma and angle-closure glaucoma are associated with microcornea. (See Secondary Glaucoma: Glaucoma Associated With Non-Acquired Ocular Anomalies.) Open-angle glaucoma may develop later in life, suggesting that drainage-angle development may be anomalous. Cataracts may be encountered at birth or may develop later in life. Other associated ocular conditions may include corectopia, iris colobomas, ARA, persistent fetal vasculature, retinopathy of prematurity, rod-cone dystrophy, myopic chorioretinal atrophy, rod-cone dystrophy, posterior staphyloma, and optic nerve hypoplasia.77,78
Systemic associations
In microcornea, systemic associations include Rieger syndrome, Ehlers-Danlos syndrome, Marfan syndrome, trisomy 21, Turner syndrome, Weill-Marchesani syndrome, Warburg syndrome, Norrie syndrome, cataract-microcornea syndrome, and renal glucosuria.77
Management
As an isolated condition, microcornea has good visual prognosis. Management may only require optical correction of the refractive error and amblyopia treatment if necessary. Associated ocular abnormalities may affect visual outcome.
From a systemic standpoint, a thorough examination is required and an evaluation by a geneticist is recommended.
Peters anomaly
Definition
Peters anomaly is an anterior segment dysgenesis characterized by a congenital corneal opacity and iridocorneal adhesions, corneolenticular adhesions, or both. The opacity occurs at the stromal level and overlies a posterior corneal defect.
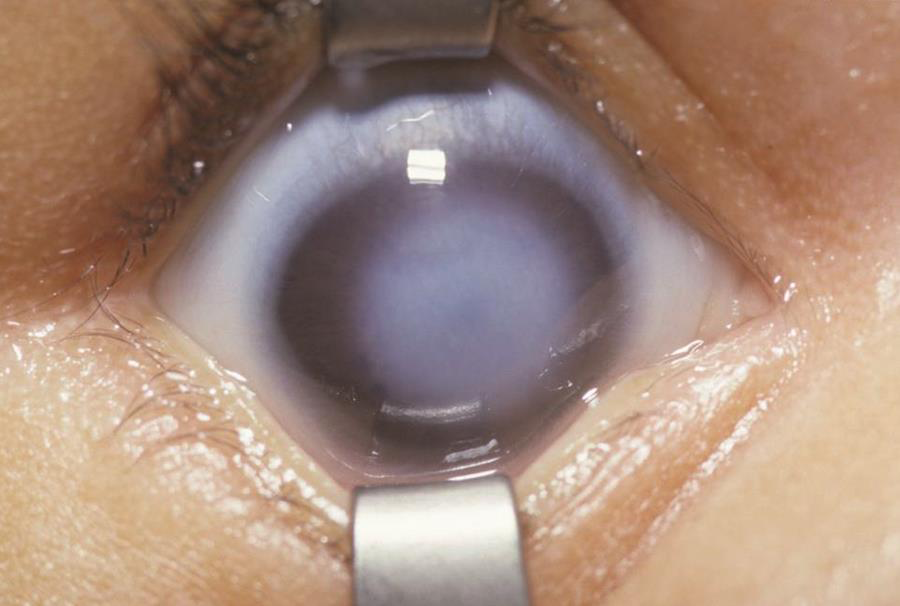
Figure 17. Central corneal opacity in a patient with Peters anomaly. (Courtesy of Irene H. Maumenee, MD. American Academy of Ophthalmology)
Epidemiology
This anomaly is rare. About 60%-80% cases of Peters anomaly are bilateral and possibly asymmetric.1,68,79,80 Risk factors may include intrauterine infection, maternal retinoic acid ingestion, and fetal alcohol syndrome.81,82
Pathogenesis
The presumed mechanism in a number of cases is failure during development of the separation of the lens from the overlying surface ectoderm.83 Late reattachment of the lens or the iris or both to the cornea during development has also been suggested. Several mechanisms may lead to Peters anomaly phenotypes.
Histopathology
Posterior stroma, Descemet membrane, and corneal endothelium underlying the characteristic corneal opacity are typically absent on histopathology. Bowman layer thickening and its absence have both been described in a number of reports.1
Genetics
Peters anomaly is most often sporadic.80 It can also be inherited in an autosomal recessive and an autosomal dominant manner.84
It is a genetically heterogeneous condition with a number of associated mutations. They may affect PAX6, PITX2, FOXC1, CYP1B1 (2p22-21), PITX3, or other genes.6,24,80,85,86 Heterozygous mutations in FOXE3 may cause Peters anomaly and are often associated with lens abnormalities and severe glaucoma.87 RIEG1 gene mutation has also been associated with Peters anomaly.88 A mutation of another gene, B3GALTL on chromosome 13q12.3, is found to result in the classic Peters Plus syndrome phenotype.89
Clinical features
The appearance of the characteristic corneal opacity can differ widely in size and density. It can range from a small, faint stromal haze, to a large, dense, protruding leukoma.1,68 It may be central or eccentric, and it may decrease in the first few years of life.1
Iridocorneal adhesions may vary significantly in number and thickness, and there may be adhesions between strands. Corectopia is a common finding. When the peripheral cornea is clear, corneolenticular adhesions may be seen on gonioscopy.
An infant with Peters anomaly will typically be referred for an abnormal red reflex. The corneal opacification is present from the infant's birth. It is typically a clinical diagnosis made by anterior segment examination.
High-frequency ultrasound biomicroscopy (UBM) may be very helpful in determining the presence and the degree of involvement of adhesions between lens, iris, and cornea.24 In cases of total corneal opacification, it may help with the diagnosis.
Ocular associations
Associated ocular anomalies include lens opacities and lens adherence to the posterior cornea. Lens opacities may be found without any contact between adjacent structures and the lens. In some cases along the spectrum of dysgenesis of the cornea-iris-lens axis, the lens may even fail to form.6
Glaucoma occurs in 50%-70% of patients.1,90 Anomalous angle-structure development may be encountered.80
Other associated ocular conditions include sclerocornea, microcornea, microphthalmia, iris defects, aniridia, ARA, persistent fetal vasculature (PFV), chorioretinal colobomas, and optic nerve hypoplasia.6,24 Spontaneous corneal perforation is a very rare occurrence.80
Classifications
Peters types
Peters anomaly has been traditionally classified as follows:
Peters Type I
Involves the iris, Descemet membrane, and the corneal endothelium. It is characterized by iris adhesions to the periphery of the corneal opacity. The corneal opacity is typically avascular.
Peters Type II
Involves the iris, Descemet membrane, the corneal endothelium, and the lens. It is characterized by adhesions, or adherence of the lens to the corneal defect.6 Vascularization of the leukoma may occur.
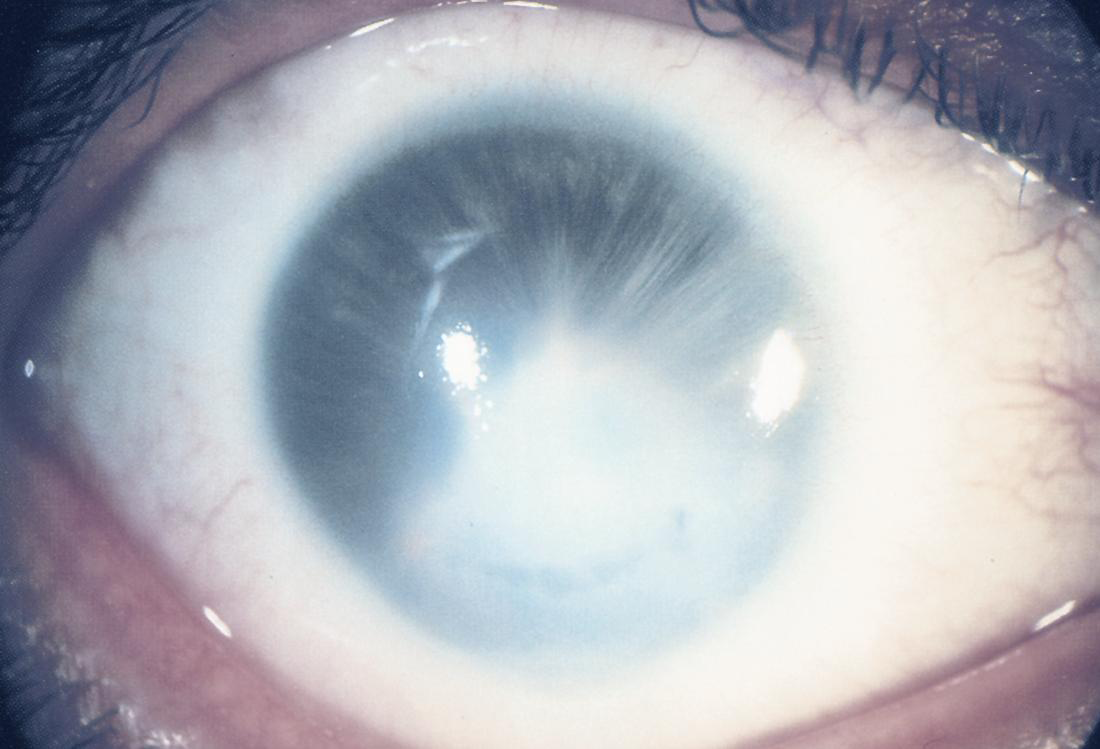
Figure 18. Corneolenticular adhesions in Peters type II. (Courtesy of the American Academy of Ophthalmology)
Peters anomaly types 1 and 2 may be found in each eye of the same patient, which suggests that this classification is a phenotypical one.24
Developmental causes of congenital corneal opacities18
Peters is a kerato-irido-lenticular dysgenesis (KILD) because it affects the cornea-iris-lens axis.6 KILD is further divided into:
I. Iridocorneal adhesions
II. Lens fails to separate
III. Lens separates but then becomes reapposed
IV. Lens separates but then fails to continue to form
V. Lens fails to form at all
Systemic associations
Peters anomaly is associated with systemic anomalies in up to 60% of patients.82,90,91 Associated systemic abnormalities are more common in bilateral Peters anomaly.80
Systemic malformations include cardiac, gastrointestinal, and genitourinary defects; central nervous system malformation; craniofacial anomalies; hearing deficits; and spinal and skeletal anomalies.1,81,82
Peters anomaly may be associated with genetically transmitted syndromes, such as Pfeiffer syndrome, fetal alcohol syndrome, and Peters plus syndrome.82,91
Peters plus syndrome is a rare autosomal recessive condition associated with mutations in the B3GALTL gene.89 It includes Peters anomaly, brachydactyly, short disproportionate stature, cleft lip and palate, abnormal ears, developmental delay, and other variable features.89,90,92
Two reported rare and life-threatening systemic associations are Wilms tumor and microphthalmia with linear skin defects syndrome.80,82,93 The latter is encountered in female patients. Typical findings include microphthalmia, hyperpigmented linear skin lesions, and possibly lethal cardiac arrhythmias.
Management
As soon as the Peters diagnosis is made, and it is usually at birth, the full support of a neonatologist and of a geneticist are warranted.80 Since many malformations are encountered in association with Peters anomaly, a thorough systemic examination and a genetic evaluation are needed. If a genetic mutation or an inheritance pattern is found, genetic counseling for future pregnancies should be considered. All patients with Peters anomaly should also be co-managed by a pediatric ophthalmologist, a corneal specialist and a glaucoma specialist.
Deprivation amblyopia due to Peters anomaly can result from a number of structural abnormalities. Corneal opacification can be a significant limiting factor for visual development in infancy, and in these cases penetrating keratoplasty should be considered within the first 3 months of life to clear the visual axis. UBM may be a very helpful preoperative tool in determining anterior and posterior chamber structural anomalies in cases of Peters anomaly.24 Anterior segment optical coherence tomography (OCT) may also be helpful.
Following penetrating keratoplasty, suture removal, refraction, and contact lens fitting are performed. Treatment of any amblyopia must be initiated promptly.
It has been suggested that an optical iridectomy could be an option as a temporary measure or as a definitive measure in selected cases of corneal opacification.94 There must be enough peripheral clear cornea, and the pupillary opening must be large enough for this option to be considered. In cases of corneal opacities that are not large, dilation may be another temporary option to reduce the chance of amblyopia before a definitive procedure.80
Visual development can be hindered by lens opacity, requiring surgical removal of the lens. When corneolenticular adhesion is present, combining penetrating keratoplasty, lensectomy, and vitrectomy is usually warranted. In cases of localized corneal opacity and lens apposition to the cornea, surgical removal of the lens alone without peeling the keratolenticular adhesion may be sufficient to clear the visual axis.6,95 If IOL implantation is also performed, refractive outcome may be unpredictable, due to the difficulty of accurate preoperative keratometry values and a possible postoperative myopic shift.95
Glaucoma associated with Peters anomaly is another major concern for visual prognosis. Frequent examinations are warranted for the monitoring of glaucoma. (See Secondary Glaucoma: Glaucoma Associated With Non-Acquired Ocular Anomalies)
Aniridia
Diagnostic features
Aniridia is characterized by varying degrees of congenital bilateral iris hypoplasia, ranging from mild iris hypoplasia to a rudimentary iris stump. It is a panocular disorder and findings include foveal hypoplasia, early onset nystagmus, glaucoma, cataracts, optic nerve hypoplasia, and limbal stem cell deficiency.
From a developmental standpoint, aniridia affects the iris-angle axis, and is classified as an iridotrabecular dysgenesis.18
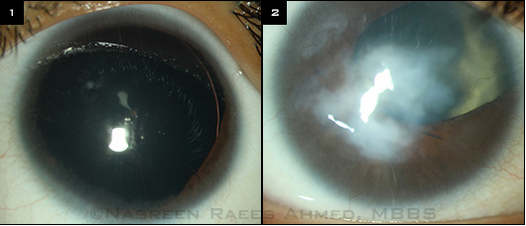
Figure 19. Classic form of aniridia, characterized by lack of iris tissue. (EyeNet Magazine, American Academy of Ophthalmology)
Epidemiology
It is a condition that affects 1/40,000 to 1/100,000 individuals.96,97 No racial or gender predilection has been found.
Pathogenesis
- The ectodermal theory postulates that aniridia results from a defect in development of the optic vesicle rim between the 12th and 14th weeks of gestation.96, 98
- The mesodermal theory proposes failure of adequate mesenchyme migration or proliferation during the second month of gestation.96,98,99
- A third theory is a fusion of the mesodermal theory and excessive iris remodeling or cell death.99
Histopathology
Iris hypoplasia is the key characteristic of the disorder. Iris tissue appears to always be present, albeit to a variable degree. The iris surface is hypoplastic, with few crypts. The ciliary body also appears to be hypoplastic.
Congenital abnormalities of the anterior chamber angle are seen on histopathology. In some cases, it is anomalous development of the angle and in others the angle recess is reportedly filled with loose mesenchymal tissue.100 In a study using UBM, the trabecular meshwork and Schlemm canal were not found in aniridic eyes.98 The trabecular-iris angle, however, was not significantly different from that of normal age-matched individuals.
Anterior subcapsular, cortical, nuclear, and posterior subcapsular cataracts may be found. The anterior lens capsule is reportedly remarkably thin, which may affect cataract surgery.101,102
Inheritance
Familial aniridia is the most common form, found in two-thirds of aniridic patients. It is inherited in an autosomal dominant manner with complete penetrance and variable expressivity.103 Wilms tumor in children of affected parents is unlikely but possible, due to a familial deletion extending to WT1.103-106 Surprisingly good central vision and relatively mild associated ocular anomalies have been reported in a large autosomal dominant pedigree.107
Sporadic aniridia is encountered in approximately one-third of cases.108 Approximately thirty percent of patients with sporadic aniridia will develop Wilms tumor due to a contiguous deletion of WT1.
Autosomal recessive aniridia is encountered in 2% of affected patients. It is associated with a group of conditions known as Gillespie syndrome.
In patients without a family history of aniridia, examination of the parents may be helpful in uncovering a mild iris hypoplasia or other anomalies on the PAX6 spectrum.
Genetics
The genetic defect in these patients involves the paired box gene-6 (PAX6), found on chromosome 11p13. The aniridic phenotype appears to be caused by loss of function of one allele, either by intragenic mutation, or by deletion.109 Null mutations tend to cause classical aniridia with iris hypoplasia and other associated sight-threatening ocular anomalies.2,103
In a number of cases of aniridia, no pathogenic PAX6 mutation has been uncovered.108,110,111 Some of these patients present with an aniridia phenotype and normal vision.
Wilms tumor (WT1) is the pediatric nephroblastoma predisposition gene also on chromosome 11p13. Neighboring genes WT1 and PAX6 may be deleted together in large chromosomal deletion. WT1 is a tumor suppressor gene and its deletion along with PAX6 increases the risk of developing the tumor to up to 50%.97,112
Wilms tumor and aniridia may be part of the Wilms tumor, genitourinary abnormalities, and mental retardation (WAGR) syndrome, a group of conditions caused by hemizygous deletions in the 11p13 region.113,114 Kidney and genitourinary anomalies found in WAGR syndrome are related to hemizygosity for WT1.115 Other affected genes in or close to the WAGR locus in even larger deletions may play a role in the WAGR phenotype. The brain-derived neurotrophic factor (BDNF) gene and the LIN7C gene may play a role in behavioral and neurodevelopmental phenotypes.114,116,117 The BDNF gene may also contribute to childhood-onset obesity.
Ocular associations
The aniridic phenotype may vary largely between and within families. Aniridic eyes, however, are usually affected equally in the same patient.
As with the aniridic phenotype as a whole, the iris appearance may vary greatly. In severe cases, iris tissue may appear to be completely absent. Gonioscopy or ultrasound biomicroscopy may help detect a residual iris stump.98 In less severe cases, iris hypoplasia may be subtle and the pupil may be round and normal-sized. Iris transillumination or loss of iris surface architecture can be telling signs.97,103,118 Other iris changes include partial iris defects, colobomas, corectopia, and iris ectropion.96
Foveal hypoplasia is a characteristic finding and leads to impaired visual acuity (typically 20/100 - 20/200). Nystagmus usually appears by six weeks of age. Posterior segment findings include a dampened foveal reflex, macular hypopigmentation, and retinal vessels in the foveal avascular zone. Phototoxicity as a result of iris hypoplasia may also contribute to maculopathy in aniridic patients.119
Electroretinography (ERG) testing in aniridic patients shows highly variable retinal dysfunction, ranging from nearly normal to severely abnormal.120 Typically the amplitude of the signal is depressed and ERG components are affected in an inner-to-outer gradient. OCT may also be used to better evaluate and document maculopathy in aniridic patients.119,121 Findings in foveal hypoplasia include an absent or reduced foveal pit and outer retinal anomalies. Handheld ultra-high-resolution spectral-domain OCT appears to reliably identify typical foveal hypoplasia in the presence of nystagmus.122 Other uses for OCT may include monitoring iridocorneal angle changes.119
Small polar opacities are commonly present at birth.123-125 Attached persistent pupillary membranes may be present.103 Visually significant cataracts most often develop in the first two decades of life and affect 50%-85% of patients.96,103,118 Subcapsular opacification may appear over time in the midperiphery and increase in density and size.123 Cortical and nuclear cataracts are also associated. Lens subluxation or dislocation may occur.
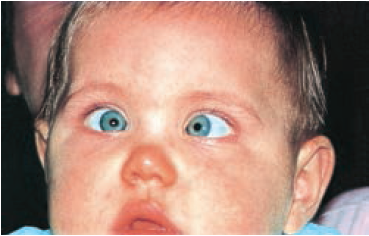
Figure 20. Ciliary processes and the edge of the lens are visible in an infant with aniridia. Persistent pupillary membrane fibers and a central anterior polar cataract are also found. (Pediatric Ophthalmology and Strabismus, in Basic and Clinical Science Course [BCSC]. 2013-2014, American Academy of Ophthalmology. Figure 19-1)
Secondary glaucoma develops in up to 75% of patients with congenital aniridia.96,97,110,118,126 It appears gradually in later childhood or adulthood, and develops due to angle abnormalities, which lead to obstruction of aqueous outflow. On gonioscopy, synechiae-like strands may extend from the iris stroma onto the trabecular meshwork, progressively becoming a more homogeneous sheet, leading to angle closure.100,127 In a minority of case , the angle may remain open until the second decade of life, and eventually close by the anterior rotation of the iris stump.126,127 Primary anomalous development of the drainage angle may also occur, in which case intraocular pressure is elevated earlier in life, leading to corneal edema and buphthalmos.128 (See Secondary Glaucoma: Glaucoma Associated With Non-Acquired Ocular Anomalies.)
Optic nerve hypoplasia is associated in approximately 10% of patients.124 Optic nerve colobomas are another possible finding.103
Aniridia-associated keratopathy (AAK) is a consequence of limbal stem-cell deficiency.110,129 It is a progressive condition that develops in the first decade of life. It has been reported to affect 60% of patients under 10 years of age, and 100% of those 41 years and older.130 Symptoms include red eye, foreign body sensation, photophobia, and epiphora. Early signs include a peripheral corneal pannus and corneal vascularization. Persistent epithelial defects, corneal ulcers, and scarring follow. Later signs are corneal conjunctivalization, opacification, and keratinization. Dry eye is common in aniridic patients; one study reported a prevalence of 56%.131 While deficient aqueous production has been suggested as a cause of dry eye, meibomian gland dysfunction also appears to play an important role.131-133
Central corneal thickness (CCT) is reportedly increased by up to 100 μm in aniridic patients, and the etiology may not only be due to thickened corneal stroma or epithelium, but may also be developmental.134,135
Retinal anomalies have been reported in aniridic eyes. They may present as multiple small circumferential lipoidal spots in the peripheral retina.136,137 Two cases of unilateral exudative retinopathy resembling Coats disease have been reported.118 Retinal tears and detachments are rare and have been associated with buphthalmic aniridic eyes.137
Microcornea, microphthalmia, and ptosis are other ocular associations.125,138
Refractive errors are common in aniridic patients. Strabismus, most frequently esotropia, may also be encountered.96
Most cases of aniridia are diagnosed in early infancy due to an apparent iris abnormality. In some cases, iris hypoplasia in an infant may be so mild as to be missed or, in other cases, confused with ARS. Aniridia can be distinguished by the presence of nystagmus and foveal hypoplasia. Posterior embryotoxon may be readily visible in ARS and also help make the distinction.
In the complete absence of iris due to a PAX6 mutation, the lens may become apposed to the cornea, resulting in a central corneal opacity. This phenotype may resemble Peters anomaly and from a developmental standpoint, it is on the KILD spectrum.6 Signs such as nystagmus, foveal hypoplasia, optic nerve hypoplasia, and corneal pannus point to the diagnosis of aniridia.
Systemic associations
1. WAGR
WAGR syndrome is a form of sporadic aniridia encountered in approximately 13% of aniridic patients. WAGR complex describes the associated systemic findings, which include Wilms tumor, Aniridia, Genitourinary malformations, and intellectual disability (Retardation). The phenotype is highly variable. While aniridia is often severe in WAGR, it may also be absent, and some patients do not get Wilms tumor.
Wilms tumor in patients with WAGR syndrome usually presents earlier in life, occurs bilaterally, and has a more favorable histology than non-WAGR Wilms tumors.106,139 It is diagnosed before age 5 in 80% of cases. One patient with WAGR syndrome was diagnosed with Wilms tumor as late as age 25, and two patients presented de novo disease within their contralateral kidney up to 12 years after the initial tumor was found.106,140
Kidney anomalies, focal segmental glomerulosclerosis, and end-stage renal disease occur more frequently than Wilms tumor. About 53% of patients with WAGR syndrome develop chronic renal failure at 20 years from diagnosis.139
Genitourinary abnormalities include cryptorchidism, hypospadias, uterine abnormalities, streak ovaries, ambiguous genitalia, ureteric and urethral anomalies, and gonadoblastoma.
Intellectual disability is present in 70% of individuals.
Behavioral difficulties include attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders. Neurologic conditions are encountered in up to a third of cases, including epilepsy, abnormal muscle tone, enlarged ventricles, and corpus callosum agenesis.
Polyphagia and hyperphagia, early-onset obesity, and high blood cholesterol levels are part of a specific WAGR phenotype including obesity, known as WAGRO.115,117,141
Dysmorphic features may be present and include craniofacial dysmorphism, hemihypertrophy, scoliosis, and kyphosis.
Depending on the size of the contiguous genetic deletion, WAGR may be associated with other gene deletion conditions or syndromes.115,142
2. Gillespie syndrome
It is a rare form of aniridia associated with systemic abnormalities including cerebellar ataxia and intellectual disability.97
A typical presentation may be fixed dilated pupils in a hypotonic child. The circumpupillary iris aplasia may be a unique sign for Gillespie syndrome, and may help establish the diagnosis before neurological signs appear.96
Gillespie syndrome may be inherited in an autosomal recessive or an autosomal dominant manner, though the genetic basis of it remains unclear.143,144
3. Central nervous system disorders
Reduced olfaction has been reported as the most common central nervous system deficit in isolated aniridia. Central auditory processing deficits may cause hearing disabilities. Cognitive and behavioral problems as well as developmental delay have been described.
Anomalies in a number of structures have been found in aniridic patients on MRI, including in the anterior commissure, anterior cingulated cortex, cerebellum, temporal and occipital lobes, corpus callosum, pineal gland, and olfactory bulb.
4, Other syndromes
Nonocular syndromes and chromosomal abnormalities may be associated with aniridia, including Marfan syndrome, Smith–Lemli–Opitz syndrome, Biemond syndrome, and the XXXXY chromosomal pattern.96
Management
Children with sporadic aniridia need to undergo genetic testing to look for deletions of the WT1 gene. Deletion analysis is also indicated in patients with familial aniridia, as some have been reported to develop Wilms tumor.6,103 If a deletion is found, a collaborative approach must be adopted with nephrology. Serial clinical examinations and renal ultrasounds to screen for Wilms tumor are indicated every 3 months until 8 years of age. A thorough physical examination should continue to be performed every 6 to 12 months to detect abdominal masses.145 A high index of suspicion must be kept for Wilms tumor in patients of any age.
Other associated systemic conditions are best managed using a multidisciplinary approach to treatment. Cryptorchidism and other genital or urinary disorders require monitoring and care. Detailed audiological examination is recommended, as children with aniridia may have abnormal hearing. Control of hyperphagia and tailored nutritional management may be needed to prevent major morbidities in patients suffering from obesity.103,117
Proper eye care includes regular eye examinations, correction of refractive errors, and treatment of amblyopia. Optical low vision aids may be of great help. Filter lenses are recommended to reduce glare. Artificial iris implants may also be of use in selected cases.146
Aniridic patients should undergo annual glaucoma screening to allow for timely intervention.134 Treatment of glaucoma associated with aniridia is difficult. Prophylactic goniotomy has been suggested.126 After failure of medical therapy, options are augmented trabeculectomy, drainage tubes, and cyclodiode laser treatment.147 (See Secondary Glaucoma: Glaucoma Associated With Non-Acquired Ocular Anomalies.)
Management of mild aniridic keratopathy (AAK) includes preservative-free lubricants.110 In moderate AAK, serum drops and amniotic membranes may be helpful for the survival and expansion of surviving limbal stem cells. In severe cases, limbal cell transplantation, PKP, or keratoprosthesis implantation are considered.
Cataract extraction is indicated when visually significant opacities develop. Cataract surgery in aniridic patients has a higher risk of intraoperative complications, owing to associated poor zonular stability and anterior capsule fragility.101,102,148 Black diaphragm aniridic IOLs are good options for reducing glare or light sensitivity.
References
- Nishal KK, Sowden JC, Anterior segmental developmental anomalies. In Pediatric Ophthalmology and Strabismus. Hoyt, C.S.; Taylor, D. eds. 2013, United Kingdom: Elsevier Saunders.
- Simpson TI. Price DJ. Pax6; a pleiotropic player in development. Bioessays. 2002. 24(11):1041-1051.
- The Human PAX6 Allelic Variant Database. http://pax6.hgu.mrc.ac.uk/
- D'haene B, Meire F, Claerhout I, et al. Expanding the spectrum of FOXC1 and PITX2 mutations and copy number changes in patients with anterior segment malformations. Invest Ophthalmol Vis Sci, 2011; 52(1):324-333.
- Reis LM, Tyler RC, Volkmann Kloss BA, et al. PITX2 and FOXC1 spectrum of mutations in ocular syndromes. Eur J Hum Genet, 2012; 20(12):1224-1233.
- Nischal, K.K., Genetics of Congenital Corneal Opacification-Impact on Diagnosis and Treatment. Cornea, 2015. 34 Suppl 10: p. S24-34.
- Strungaru MH, Dinu I, Walter MA. Genotype-phenotype correlations in Axenfeld-Rieger malformation and glaucoma patients with FOXC1 and PITX2 mutations. Invest Ophthalmol Vis Sci, 2007. 48(1): 228-237.
- Chang TC, Summers CG, Schimmenti LA, Grajewski A. Axenfeld-Rieger syndrome: new perspectives. Br J Ophthalmol. 2012; 96(3):318-322.
- Lines MA, Kozlowski K, Kulak SC, et al., Characterization and prevalence of PITX2 microdeletions and mutations in Axenfeld-Rieger malformations. Invest Ophthalmol Vis Sci. 2004; 45(3):828-833.
- Karadeniz NN, Kocak-Midillioglu I, Erdogan D, Bokesoy I. Is SHORT syndrome another phenotypic variation of PITX2? Am J Med Genet A. 2004; 130a(4):406-409.
- Basic and Clinical Science Course (BCSC), Section 6. Pediatric Ophthalmology and Strabismus. 2013-2014, San Francisco: American Academy of Ophthalmology.
- Raymond WR, Kearney JJ, Parmley VC, Ocular findings in arteriohepatic dysplasia (Alagille's syndrome). Arch Ophthalmol. 1989; 107(7):1077..
- Hingorani M, Nischal KK, Davies A, et al. Ocular abnormalities in Alagille syndrome. Ophthalmology. 1999; 106(2):330-337.
- Alagille syndrome, in Genetics Home Reference, U.S.N.L.o. Medicine, Editor. 2014.
- Mansour AM, Goldberg RB, Wang FM, Shprintzen RJ. Ocular findings in the velo-cardio-facial syndrome. J Pediatr Ophthalmol Strabismus. 1987; 24(5):263-266.
- Forbes BJ, Binenbaum G, Edmond JC, DeLarato N, McDonald-McGinn DM, Zackai EH, Ocular findings in the chromosome 22q11.2 deletion syndrome. J AAPOS. 2007; 11(2):179-182.
- Rodriguez Caballero ML, Unanumo Perez P, [Posterior embryotoxon in ichthyosis]. J Fr Ophtalmol. 1983; 6(10):793-795.
- Nischal KK. Congenital corneal opacities - a surgical approach to nomenclature and classification. Eye (Lond). 2007; 21(10):1326-1337.
- Shields MB. Axenfeld-Rieger syndrome: a theory of mechanism and distinctions from the iridocorneal endothelial syndrome. Trans Am Ophthalmol Soc. 1983; 81:736-784.
- Shields MB, Buckley E, Klintworth GK, Thresher R. Axenfeld-Rieger syndrome. A spectrum of developmental disorders. Surv Ophthalmol. 1985; 29(6):387-409.
- Alward WL. Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol. 2000; 130(1):107-115.
- Shields MB. Axenfeld-Rieger and iridocorneal endothelial syndromes: two spectra of disease with striking similarities and differences. J Glaucoma. 2001; 10(5 Suppl 1):S36-S38.
- Schultz SK, Feldman BH, Adamopoulou C, Salim S. Axenfeld Rieger Syndrome, in EyeWiki. http://eyewiki.org/Axenfeld_Rieger_Syndrome.
- Nischal KK, Naor J, Jay V, MacKeen LD, Rootman DS, Clinicopathological correlation of congenital corneal opacification using ultrasound biomicroscopy. Br J Ophthalmol. 2002; 86(1):62-69.
- Fryns JP, Van Den Berghe H, Rieger syndrome and interstitial 4q26 deletion. Genet Couns. 1992; 3(3):153-154.
- Tumer Z, Bach-Holm D, Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet. 2009; 17(12):1527-1539.
- Online Mendelian Inheritance in Man: An Online Catalog of Human Genes and Genetic Disorders. http://www.omim.org/
- Weatherill JR, Hart CT. Familial hypoplasia of the iris stroma associated with glaucoma. Br J Ophthalmol. 1969; 53(7):433-438.
- Jerndal T. Goniodysgenesis and hereditary juvenile glaucoma. A clinical study of a Swedish pedigree. Acta Ophthalmol Suppl. 1970; 107:3-100.
- Pearce WG, Wyatt HT, Boyd TA, Ombres RS, Salter AB. Autosomal dominant iridogoniodysgenesis: genetic features. Can J Ophthalmol. 1983; 18(1):7-10.
- Héon E, Sheth BP, Kalenak JW. Linkage of autosomal dominant iris hypoplasia to the region of the Rieger syndrome locus (4q25). Hum Mol Genet. 1995; 4(8):1435-1439.
- Aldinger KA, Lehmann OJ, Hudgins L, et al. FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat Genet. 2009; 41(9):1037-1042.
- Alward WL, Semina EV, Kalenak JW. Autosomal dominant iris hypoplasia is caused by a mutation in the Rieger syndrome (RIEG/PITX2) gene. Am J Ophthalmol. 1998; 125(1):98-100.
- Walter MA, Mirzayans F, Mears AJ, Hickey K, Pearce WG. Autosomal-dominant iridogoniodysgenesis and Axenfeld-Rieger syndrome are genetically distinct. Ophthalmology. 1996; 103(11):1907-1915.
- Beck AD. Diagnosis and management of pediatric glaucoma. Ophthalmol Clin North Am. 2001; 14(3):501-512.
- Kelberman D, Islam L, Holder SE, et al. Digenic inheritance of mutations in FOXC1 and PITX2 : correlating transcription factor function and Axenfeld-Rieger disease severity. Hum Mutat. 2011; 32(10): 1144-1152.
- Harasymowycz PJ, Papamatheakis DG, Eagle RC Jr, Wilson RP. Congenital ectropion uveae and glaucoma. Arch Ophthalmol. 2006; 124(2):271-273.
- Ritch R, Forbes M, Hetherington J Jr, Harrison R, Podos SM. Congenital ectropion uveae with glaucoma. Ophthalmology. 1984; 91(4):326-331.
- Wilson ME. Congenital iris ectropion and a new classification for anterior segment dysgenesis. J Pediatr Ophthalmol Strabismus. 1990; 27(1):48-55.
- Dowling JL Jr, Albert DM, Nelson LB, Walton DS. Primary glaucoma associated with iridotrabecular dysgenesis and ectropion uveae. Ophthalmology. 1985; 92(7):912-921.
- Laaks D, Freeman N. Congenital iris ectropion uveae presenting with glaucoma in infancy. J AAPOS. 2013; 17(2):214-216.
- Shields MB. Progressive essential iris atrophy, Chandler's syndrome, and the iris nevus (Cogan-Reese) syndrome: a spectrum of disease. Surv Ophthalmol. 1979; 24(1):3-20.
- Yanoff M. Iridocorneal endothelial syndrome: unification of a disease spectrum. Surv Ophthalmol. 1979; 24(1):1-2.
- Basic and Clinical Science Course, Section 10. Glaucoma. San Francisco: American Academy of Ophthalmology. 2013-2014.
- Salim S, Shields MB, Walton D. Iridocorneal endothelial syndrome in a child. J Pediatr Ophthalmol Strabismus. 2006; 43(5):308-310.
- Olawoye O, Teng CC, Liebmann JM, Wang FM, Ritch R. Iridocorneal endothelial syndrome in a 16-year-old. J Glaucoma. 2011; 20(5):294-297.
- Eagle RC Jr, Font RL, Yanoff M, Fine BS. Proliferative endotheliopathy with iris abnormalities. The iridocorneal endothelial syndrome. Arch Ophthalmol. 1979; 97(11):2104-2111.
- Wilson MC. Shields MB, A comparison of the clinical variations of the iridocorneal endothelial syndrome. Arch Ophthalmol. 1989; 107(10):1465-1468.
- Levy SG, Kirkness CM, Moss J, Ficker L, McCartney AC. The histopathology of the iridocorneal-endothelial syndrome. Cornea. 1996; 15(1):46-54.
- Shields CL, Shields MV, Viloria V, Pearlstein H, Say EA, Shields JA. Iridocorneal endothelial syndrome masquerading as iris melanoma in 71 cases. Arch Ophthalmol. 2011; 129(8):1023-1029.
- Alvarado JA, Murphy CG, Juster RP, Hetherington J, Pathogenesis of Chandler's syndrome, essential iris atrophy and the Cogan-Reese syndrome. II. Estimated age at disease onset. Invest Ophthalmol Vis Sci. 1986; 27(6):873-882.
- Kupfer C, Kaiser-Kupfer MI, Datiles M, McCain L. The contralateral eye in the iridocorneal endothelial (ICE) syndrome. Ophthalmology. 1983; 90(11):1343-1350.
- Hirst LW, Quigley HA, Stark WJ, Shields MB. Specular microscopy of iridocorneal endothelia syndrome. Am J Ophthalmol. 1980; 89(1):11-21.
- Alvarado JA, Underwood JL, Green WR, Wu S, Murphy CG, Hwang DG, Moore TE, O’Day D. Detection of herpes simplex viral DNA in the iridocorneal endothelial syndrome. Arch Ophthalmol, 1994; 112(12):1601-1609.
- Groh MJ, Seitz B, Schumacher S, Naumann GO. Detection of herpes simplex virus in aqueous humor in iridocorneal endothelial (ICE) syndrome. Cornea. 1999; 18(3):359-360.
- Tsai CS, Ritch R, Straus SE, Perry HD, Hsieh FY. Antibodies to Epstein-Barr virus in iridocorneal endothelial syndrome. Arch Ophthalmol. 1990; 108(11):1572-1576.
- Basic and Clinical Science Course, Section 8. External Disease and Cornea. San Francisco: American Academy of Ophthalmology; 2013-2014.
- Chiou AG, Kaurman SC, Beuerman RW, Ohta T, Yaylali V, Kaufman HE. Confocal microscopy in the iridocorneal endothelial syndrome. Br J Ophthalmol. 1999; 83(6):697-702.
- Laganowski HC, Kerr Muir MG, Hitchings RA. Glaucoma and the iridocorneal endothelial syndrome. Arch Ophthalmol. 1992; 110(3):346-350.
- Laganowski HC, Sherrard ES, Muir MG, Buckley RJ. Distinguishing features of the iridocorneal endothelial syndrome and posterior polymorphous dystrophy: value of endothelial specular microscopy. Br J Ophthalmol. 1991; 75(4):212-216.
- Shields MB, Campbell DG, Simmons RJ. The essential iris atrophies. Am J Ophthalmol. 1978; 85(6):749-759.
- Teekhasaenee C, Ritch R. Iridocorneal endothelial syndrome in Thai patients: clinical variations. Arch Ophthalmol. 2000; 118(2):187-192.
- Doe EA, Budenz DL, Gedde SJ, Imami NR. Long-term surgical outcomes of patients with glaucoma secondary to the iridocorneal endothelial syndrome. Ophthalmology. 2001; 108(10):1789-1795.
- Roche O, Dureau P, Uteza Y, Dufier JL. [Congenital megalocornea]. J Fr Ophtalmol, 2002; 25(3):312-318.
- Webb TR, Matarin M, Gardner JC, et al. X-linked megalocornea caused by mutations in CHRDL1 identifies an essential role for ventroptin in anterior segment development. Am J Hum Genet. 2012; 90(2): 247-259.
- Han J, Young JW, Frausto RF, Isenberg SJ, Aldave AJ. X-linked Megalocornea Associated with the Novel CHRDL1 Gene Mutation p.(Pro56Leu*8). Ophthalmic Genet. 2015; 36(2):145-148.
- Meire FM, Delleman JW. Biometry in X linked megalocornea: pathognomonic findings. Br J Ophthalmol. 1994; 78(10):781-785.
- Vasaiwala RA, Djalilian AR. Congenital Anterior Segment Abnormalities. Focal Points: Clinical Modules for Ophthalmologists. American Academy of Ophthalmology, Editor. 2015; 33:2.
- Mackey DA, Buttery RG, Wise GM, Denton MJ. Description of X-linked megalocornea with identification of the gene locus. Arch Ophthalmol. 1991; 109(6):829-833.
- Meire FM, Bleeker-Wagemakers EM, Oehler M, Gal A, Delleman JW. X-linked megalocornea. Ocular findings and linkage analysis. Ophthalmic Paediatr Genet. 1991; 12(3):153-157.
- Khan AO, Aldahmesh MA, Alkuraya FS. Congenital megalocornea with zonular weakness and childhood lens-related secondary glaucoma - a distinct phenotype caused by recessive LTBP2 mutations. Mol Vis. 2011; 17:2570-2579.
- de Sanctis U, Grignolo FM. Cataract extraction in X-linked megalocornea: a case report. Cornea. 2004; 23(5):533-535.
- Assia EI, Segev F, Michaeli A. Cataract surgery in megalocornea Comparison of 2 surgical approaches in a single patient. J Cataract Refract Surg. 2009; 35(12):2042-2046.
- Saffra N, Rakhamimov A, Masini R, Rosenthal KJ. Anterior Chamber Iris Claw Lens for the Treatment of Aphakia in a Patient with Megalocornea. Case Rep Ophthalmol. 2015; 6(2):164-169.
- Oetting TA, Newsom TH. Bilateral Artisan lens for aphakia and megalocornea: Long-term follow-up. J Cataract Refract Surg. 2006;. 32(3):526-528.
- Basti S, Koch DD. Secondary peripheral iris suture fixation of an acrylic IOL in megalocornea. J Cataract Refract Surg. 2005; 31(1):7; author reply 8.
- Online Mendelian Inheritance in Man (OMIM). http://www.omim.org/
- Mataftsi A, Islam L, Kelberman D, Sowden JC, Nischal KK. Chromosome abnormalities and the genetics of congenital corneal opacification. Mol Vis. 2011; 17:1624-1640.
- Peters' Anomaly, Eyewiki, Editor. http://eyewiki.org/Peters%27_Anomaly.
- Bhandari R, Ferri S, Whittaker B, Liu M, Lazzaro DR. Peters anomaly: review of the literature. Cornea. 2011; 30(8):939-944.
- Neilan E, Pikman Y, Kimonis VE. Peters anomaly in association with multiple midline anomalies and a familial chromosome 4 inversion. Ophthalmic Genet. 2006; 27(2):63-65.
- Traboulsi EI, Maumenee IH. Peters' anomaly and associated congenital malformations. Arch Ophthalmol. 1992; 110(12):1739-1742.
- Matsubara A, Ozeki H, Matsunaga N, et al. Histopathological examination of two cases of anterior staphyloma associated with Peters' anomaly and persistent hyperplastic primary vitreous. Br J Ophthalmol. 2001; 85(12):1421-1425.
- Berker N, Alanay Y, Elgin U, et al. A new autosomal dominant Peters' anomaly phenotype expanding the anterior segment dysgenesis spectrum. Acta Ophthalmol. 2009; 87(1):52-57.
- Weh E, Reis LM, Happ HC, et al. Whole exome sequence analysis of Peters anomaly. Hum Genet. 2014; 133(12): 1497-1511.
- Vincent A, Billingsley G, Priston M, et al. Further support of the role of CYP1B1 in patients with Peters anomaly. Mol Vis. 2006; 12:506-510.
- Ali M, Buentello-Volante B, McKibbin M, et al. Homozygous FOXE3 mutations cause non-syndromic, bilateral, total sclerocornea, aphakia, microphthalmia and optic disc coloboma. Mol Vis. 2010; 16:1162-1168.
- Doward W, Perveen R, Lloyd IC, Ridgway AE, Wilson L, Black GC. A mutation in the RIEG1 gene associated with Peters' anomaly. J Med Genet. 1999; 36(2):152-155.
- Weh E, Reis LM, Tyler RC, et al. Novel B3GALTL mutations in classic Peters plus syndrome and lack of mutations in a large cohort of patients with similar phenotypes. Clin Genet. 2014; 86(2):142-148.
- Ito YA, Walter MA. Genomics and anterior segment dysgenesis: a review. Clin Experiment Ophthalmol. 2014; 42(1):13-24.
- Heon E, Barsoum-Homsy M, Cevrette L, et al. Peters' anomaly. The spectrum of associated ocular and systemic malformations. Ophthalmic Paediatr Genet. 1992; 13(2):137-143.
- Maillette de Buy Wenniger-Prick LJ, Hennekam RC, The Peters' plus syndrome: a review. Ann Genet. 2002; 45(2):97-103.
- LINEAR SKIN DEFECTS WITH MULTIPLE CONGENITAL ANOMALIES 1; LSDMCA1, in OMIM. http://www.omim.org/entry/309801?search=LINEAR%20SKIN%20DEFECTS%20WITH%20MULTIPLE%20CONGENITAL%20ANOMALIES%201%3B%20LSDMCA1&highlight=congenital%20multiple%20linear%20with%20multiplicity%20defect%201%20skin%20lsdmca1%20anomaly.
- Zaidman GW, Rabinowitz Y, Forstot SL. Optical iridectomy for corneal opacities in Peter's anomaly. J Cataract Refract Surg. 1998; 24(5):719-722.
- Medsinge A, Nischal KK. Cataract surgery in children with congenital keratolenticular adhesion (Peters anomaly type 2). J AAPOS. 2015; 19(1):24-28.
- Nelson LB, Spaeth GL, Nowinski TS, Margo CE, Jackson L. Aniridia. A review. Surv Ophthalmol. 1984; 28(6):621-642.
- Grønskov K, Olsen JH, Sand A, et al. Population-based risk estimates of Wilms tumor in sporadic aniridia. A comprehensive mutation screening procedure of PAX6 identifies 80% of mutations in aniridia. Hum Genet. 2001; 109(1):11-18.
- Okamoto F, Nakano S, Okamoto C, Hommura S, Oshika T. Ultrasound biomicroscopic findings in aniridia. Am J Ophthalmol. 2004; 137(5):858-862.
- Beauchamp GR, Meisler DM. An alternative hypothesis for iris maldevelopment (aniridia). J Pediatr Ophthalmol Strabismus. 1986; 23(6):281-283.
- Margo CE. Congenital aniridia: a histopathologic study of the anterior segment in children. J Pediatr Ophthalmol Strabismus. 1983; 20(5):192-198.
- Schneider S, Osher RH, Burk SE, Lutz TB, Montione R. Thinning of the anterior capsule associated with congenital aniridia. J Cataract Refract Surg. 2003; 29(3):523-525.
- Hou ZQ, Hao YS, Wang W, Ma ZZ, Zhong YF, Song SJ. [Clinical pathological study of the anterior lens capsule abnormalities in familial congenital aniridia with cataract]. Beijing Da Xue Xue Bao. 2005. 37(5):494-497.
- Hingorani M, Hanson I, van Heyningen V. Aniridia. Eur J Hum Genet. 2012; 20(10):1011-1017.
- Fantes JA, Bickmore WA, Fletcher JM, Ballesta F, Hanson IM, van Heyningen V. Submicroscopic deletions at the WAGR locus, revealed by nonradioactive in situ hybridization. Am J Hum Genet. 1992; 51(6):1286-1294.
- Robinson DO, Howarth RJ, Williamson KA, van Heyningen V, Beal SJ, Crolla JA. Genetic analysis of chromosome 11p13 and the PAX6 gene in a series of 125 cases referred with aniridia. Am J Med Genet A. 2008; 146A(5):558-569.
- Breslow NE, Beckwith JB. Epidemiological features of Wilms' tumor: results of the National Wilms' Tumor Study. J Natl Cancer Inst. 1982; 68(3):429-436.
- Elsas FJ, Maumenee IH, Kenyon KR, Yoder F. Familial aniridia with preserved ocular function. Am J Ophthalmol. 1977; 83(5):718-724.
- Lim HT, Seo EJ, Kim GH, et al. Comparison between aniridia with and without PAX6 mutations: clinical and molecular analysis in 14 Korean patients with aniridia. Ophthalmology. 2012; 119(6):1258-1264.
- Tzoulaki I, White IM, Hanson IM. PAX6 mutations: genotype-phenotype correlations. BMC Genet. 2005; 6:27.
- Lee H, Khan R, O'Keefe M. Aniridia: current pathology and management. Acta Ophthalmol. 2008; 86(7):708-715.
- Traboulsi EI, Ellison J, Sears J, Maumenee IH, Avallone J, Mohney BG. Aniridia with preserved visual function: a report of four cases with no mutations in PAX6. Am J Ophthalmol. 2008; 145(4):760-764.
- Muto R, Yamamori S, Ohashi H, Osawa M. Prediction by FISH analysis of the occurrence of Wilms tumor in aniridia patients. Am J Med Genet. 2002; 108(4):285-289.
- Ton CC, Hirvonen H, Miwa H, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991; 67(6):1059-1074.
- Shinawi M, Sahoo T, Maranda B, et al. 11p14.1 microdeletions associated with ADHD, autism, developmental delay, and obesity. Am J Med Genet A. 2011; 155a(6):1272-1280.
- Brémond-Gignac D, Crolla JA, Copin H, et al. Combination of WAGR and Potocki-Shaffer contiguous deletion syndromes in a patient with an 11p11.2-p14 deletion. Eur J Hum Genet. 2005; 13(4):409-413.
- Froguel P, Blakemore AI. The power of the extreme in elucidating obesity. N Engl J Med. 2008; 359(9):891-893.
- Rodríguez-López, R, Pérez JM, Balsera, et al. The modifier effect of the BDNF gene in the phenotype of the WAGRO syndrome. Gene. 2013; 516(2):285-290.
- Hingorani, M, Williamson KA, Moore AT, van Heyningen V. Detailed ophthalmologic evaluation of 43 individuals with PAX6 mutations. Invest Ophthalmol Vis Sci. 2009; 50(6):2581-2590.
- Gregory-Evans K, Cheong-Leen R, George SM, et al. Non-invasive anterior segment and posterior segment optical coherence tomography and phenotypic characterization of aniridia. Can J Ophthalmol. 2011; 46(4):337-344.
- Tremblay F, Gupta SK, De Becker I, Guernsey DL, Neumann PE. Effects of PAX6 mutations on retinal function: an electroretinographic study. Am J Ophthalmol. 1998; 126(2):211-218.
- Holmström G, Eriksson U, Hellgren K, Larsson E. Optical coherence tomography is helpful in the diagnosis of foveal hypoplasia. Acta Ophthalmol. 2010; 88(4):439-442.
- Lee H, Sheth V, Bibi M, et al. Potential of handheld optical coherence tomography to determine cause of infantile nystagmus in children by using foveal morphology. Ophthalmology. 2013; 120(12):2714-2724.
- Edén U, Lagali N, Dellby A, et al., Cataract development in Norwegian patients with congenital aniridia. Acta Ophthalmol. 2014; 92(2):e165-167.
- McCulley TJ, Mayer K, DAhr SS, Simpson J, Holland EJ. Aniridia and optic nerve hypoplasia. Eye (Lond). 2005; 19(7):762-764.
- Xiao X, Li S, Zhang Q. Microphthalmia, late onset keratitis, and iris coloboma/aniridia in a family with a novel PAX6 mutation. Ophthalmic Genet. 2012; 33(2):119-121.
- Chen TC, Walton DS. Goniosurgery for prevention of aniridic glaucoma. Trans Am Ophthalmol Soc. 1998; 96:155-165; discussion 165-169.
- Grant WM, Walton DS. Progressive changes in the angle in congenital aniridia, with development of glaucoma. Am J Ophthalmol. 1974; 78(5):842-847.
- Wiggins RE Jr, Tomey KF. The results of glaucoma surgery in aniridia. Arch Ophthalmol. 1992; 110(4):503-505.
- Tseng SC, Li DQ. Comparison of protein kinase C subtype expression between normal and aniridic human ocular surfaces: implications for limbal stem cell dysfunction in aniridia. Cornea. 1996; 15(2):168-178.
- Mayer KL, Nordlund ML, Schwartz GS, Holland EJ. Keratopathy in congenital aniridia. Ocul Surf. 2003; 1(2):74-79.
- Shiple D, Finklea B, Lauderdale JD, Netland PA. Keratopathy, cataract, and dry eye in a survey of aniridia subjects. Clin Ophthalmol. 2015; 9:291-295.
- Mackman G, Brightbill FS, Optiz JM. Corneal changes in aniridia. Am J Ophthalmol. 1979; 87:497–502.
- Jastaneiah S, Al-Rajhi AA. Association of aniridia and dry eyes. Ophthalmology. 2005; 112(9):1535-1540.
- Brandt JD, Casuso LA, Budenz DL. Markedly increased central corneal thickness: an unrecognized finding in congenital aniridia. Am J Ophthalmol. 2004; 137(2):348-350.
- Whitson JT, Liang C, Godfrey DG, et al. Central corneal thickness in patients with congenital aniridia. Eye Contact Lens. 2005; 31(5):221-224.
- Jesberg DO. Aniridia with retinal lipid deposits. Arch Ophthalmol. 1962; 68:331-336.
- Dowler JG, Lyons CJ, Cooling RJ. Retinal detachment and giant retinal tears in aniridia. Eye (Lond). 1995; 9 ( Pt 3):268-270.
- Henderson RA, Williamson K, Cumming S, et al. Inherited PAX6, NF1 and OTX2 mutations in a child with microphthalmia and aniridia. Eur J Hum Genet. 2007; 15(8):898-901.
- Breslow NE, Norris R, Norkool PA, et al. Characteristics and outcomes of children with the Wilms tumor-Aniridia syndrome: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2003; 21(24):4579-4585.
- Fischbach BV, Trout KL, Lewis J, Luis CA, Sika M. WAGR syndrome: a clinical review of 54 cases. Pediatrics. 2005; 116(4):984-988.
- Marlin S, Couet D, Lacombe D, Cessans C, Bonneau D. Obesity: a new feature of WAGR (del 11p) syndrome. Clin Dysmorphol. 1994; 3(3): 255-257.
- Almind GJ, Brøndum-Nielsen K, Bangsgaard R, Baekgaard P, Grønskov K. 11p Microdeletion including WT1 but not PAX6, presenting with cataract, mental retardation, genital abnormalities and seizures: a case report. Mol Cytogenet. 2009; 2:6.
- Defreyn A, Maugery J, Chabrier S, Coullet J. [Gillespie syndrome: an uncommon presentation of congenital aniridia]. J Fr Ophtalmol. 2007; 30(1):e1.
- Ticho BH, Hilchie-Schmidt C, Egel RT, Traboulsi EI, Howarth RJ, Robinson D. Ocular findings in Gillespie-like syndrome: association with a new PAX6 mutation. Ophthalmic Genet. 2006; 27(4):145-149.
- Clericuzio CL, Johnson C. Screening for Wilms tumor in high-risk individuals. Hematol Oncol Clin North Am. 1995; 9(6):1253-1265.
- Brandlhuber U, Nentwich MM, Rudolph G, Haritoglou C. Artificial iris implantation in a 9-year-old boy. Eur J Ophthalmol. 2015:25; e109-111.
- Khan AO. A Surgical Approach to Pediatric Glaucoma. Open Ophthalmol J. 2015; 9:104-112.
- Khaw PT. Aniridia. J Glaucoma. 2002; 11(2):164-168.