A Compendium of Inherited Disorders and the Eye, Oxford University Press
OMIM Numbers
- 204000 (LCA1), 204100 (LCA2), 604232 (LCA3), 604393 (LCA4), 604537 (LCA5), 613826 (LCA6), 613829 (LCA7), 613835 (LCA8), 608553 (LCA9), 611755 (LCA10), 613837 (LCA11), 610612 (LCA12), 612712 (LCA13), 613341 (LCA14), 613843 (LCA15), 614186 (LCA16), 614620 (IFT40), 609237 (IQCB1), 616787 (CLUAP1), 179605 (PRPH2).
Inheritance
Autosomal recessive
Genes
There are currently 20 retinal genes whose mutations cause the phenotype of LCA, accounting for about 70% of the cases, while the genes underlying the remaining 30% of patients await discovery.1 The causal LCA genes include GUCY2D (LCA1), RPE65 (LCA2), SPATA7 (LCA3), AIPL1 (LCA4), LCA5 (LCA5), RPGRIP1 (LCA6), CRX (LCA7), CRB1 (LCA8), NMNAT1 (LCA9), CEP290 (LCA10), IMPDH1 (LCA11), RD3 (LCA12), RDH12 (LCA13), LRAT (LCA14), TULP1 (LCA15), IQCB1, CLUAP1, PRPH2, KCNJ13, and IFT140. These genes encode retinal proteins that affect one of seven pathways, the disruption of each of which causes retinal degeneration:
- The phototransduction cascade (GYCY2D, AIPL1, and RD3);
- The retinoid cycle (RPE65, LRAT, and RDH12);
- Retinal development (CRX);
- The intraflagellar transport (SPATA7, LCA5, RPGRIP1, CEP290, TULP1, NPHP5, CLUAP1, and IFT140);
- Photoreceptor structure (CRB1 and PRPH2);
- Photoreceptor ion channels (KCNJ13);
- Metabolic enzymes for cellular survival (IMPDH1 and NMNAT1).1
Epidemiology
Leber congenital amaurosis (LCA) is the second most common group of inherited retinal dystrophies after retinitis pigmentosa, accounting for about 5% of all retinal dystrophies. With an estimated prevalence ranging from 1 per 33,0002 to 1 per 81,000,3 LCA accounts for about 20% of legal blindness in children.4
Clinical Findings
LCA manifests itself in the first 6 months of life with significant visual loss and sensory, pendular nystagmus. Visual acuity (VA) in children with LCA varies significantly among patients with differing gene mutations and can be as low as no light perception (NLP). This variability has led in recent years to some confusion about terminology and the clinical diagnosis of this group of disorders. For example, patients with mutations in CRB1, LRAT, CEP290, or RPE65 may have VA better than 20/50 and may actually be diagnosed later in early childhood, and sometimes designated as having early-onset childhood retinal dystrophy or early-onset severe childhood retinal dystrophy, rather than LCA. There is a progressive decline in the visual function of all patients.5 The retinal phenotype in LCA is extremely variable and includes a relatively normal appearance (Figures 1 and 2); typical vascular attenuation and bone-spicule pigmentation (Figure 3); nummular pigmentation, maculopathy with atrophic changes (Figure 4), or colobomatous-like macular changes; thickening of the macula with sparing of the para-arteriolar retinal pigment epithelium (RPE) (Figure 5); and Coats-like exudative vasculopathy. Interestingly, there are phenotypic-genotypic correlations characteristic of some fundus changes. We have summarized the genetic and clinical characteristics of each genetic type of LCA in Table 1. Diagnostic findings such as non-recordable electroretinograms (ERGs) and specific optical coherence tomography (OCT) or fundus autofluorescence (FAF) findings add to the phenotypic-genotypic correlations (Table 1). Other clinical findings, such as refractive error, photophobia, photodysphoria, sluggish and poorly reactive pupils, oculodigital sign, keratoconus, and cataracts can be part of the phenotypic manifestations of LCA (Table 1).
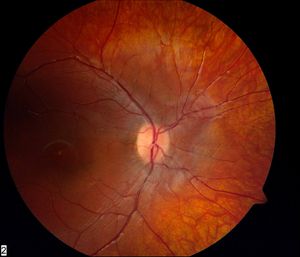
Figure 1. Fundus of a patient with LCA and GUCY2D mutations. Note normal appearance despite very poor vision.

Figure 2. Myopic-looking fundus with slight attenuation of the vasculature in this young child with RPE65 mutations. Pigmentary changes develop with age in this type of LCA.
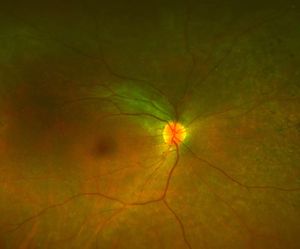
Figure 3A.
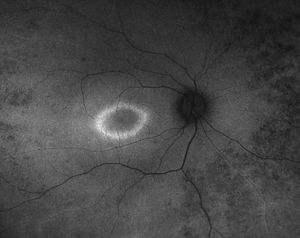
Figure 3B.
Figure 3: A. Fundus photo of a patient with CEP290. Note attenuated vessels and peripheral pigmentary changes. B. Corresponding fundus autofluorescence picture demonstrating peripheral hypopigmented spots and a ring of hyperfluorescence surrounding the fovea.

Figure 4. Older individual (30s) with RDH12 mutations and a macular atrophic lesion, as well as attenuated blood vessels and peripheral pigmentary changes.
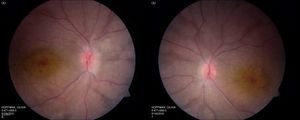
Figure 5. Right and left fundus photos of a young child with CRB1 mutations. Note prominence of the macular xanthophyll, thickening of the retina (see Figure 6), and preservation (darker areas) of the para-arteriolar RPE.
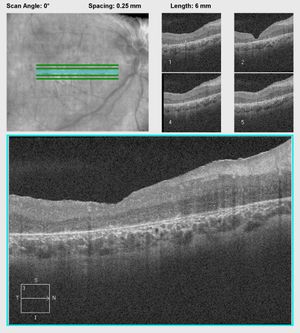
Figure 6A.
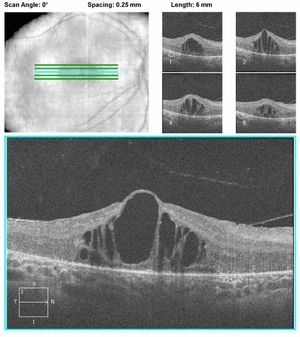
Figure 6B.
Figure 6: A. OCT of a patient with CRB1 mutations showing thickening and disorganization of the retina in the macular area. B. OCT of another patient with CRB1 mutations revealing edematous changes commonly found in patients with CRB1 mutations. These may respond to treatment with topical carbonic anhydrase inhibitors.
|
Table 1: List of LCA causal genes, their encoded proteins, and their most important phenotypic manifestations
|
|
Gene (LCA#)
OMIM#
Inheritance
Location
% of LCA Cases
# Mutations
|
Protein and Function (Pathway)
|
Visual Function and Clinical Features
|
Key Fundus Characteristics
|
Isolated/Syndromic
|
|
GUCY2D (LCA1)
OMIM# 204000
. AR
. 17p13.1
. 10-20%6
. 1327
|
Retinal guanylyl cyclase 1 (GC1)
(Phototransduction)
GC1 is a calcium-regulated module linked to retinal synaptic activity and thus phototransduction.8 Normally GC1 replenishes cGMP, but not in LCA. In GUCY2D-related LCA, the cells are low on cGMP, which results in a constant state of hyperpolarization.
|
Severe but stable visual function loss (likely due to poor cone function).9 VA typically varies between 20/200 and LP.10 Color vision is also significantly reduced.11
Significant photophobia, cataract, keratoconus13
OCT: relatively preserved outer retinal structures14,15
ERG: generally retains substantial rod function with poor cone function.14
|
Initially, patients have normal fundus appearance. The fundus appearance changes with age and results in widespread chorioretinal atrophic changes by age 50.12
|
Possible neurodevelopmental delay.16
No other syndromic associations.
|
|
RPE65 (LCA2)
OMIM# 204100
. AR
. 1p31.3
. 5-10%17
. 13218
|
Retinoid isomerohydrolase enzyme
(Retinoid visual cycle)
This enzyme normally isomerizes and hydrolyzes all-trans retinyl ester to 11-cis retinol each time a photon hits 11-cis retinal.
|
Relatively good vision early in life (due to good residual cone function and alternative supply of 11-cis-retinal18) with a possible mild improvement in visual function temporarily. Specifically, patients have significant nyctalopia (poor rod function).19
OCT: relatively preserved central macula surrounded by retina thinning/atrophy20,21
FAF: reduced/absent autofluorescence
signal, likely due to decreased levels of lipofuscin in the RPE layer.22
Mild nystagmus (when present)
|
Fundus exam is characteristic for a blond translucent appearance and whitish drusen-like deposits in about 90% of cases. Later in life, patients develop chorioretinal atrophy peripheral patches in 64% of cases10
|
None
|
|
SPATA7 (LCA3)
OMIM# 604232
. AR
. 14q31.3
. 3%23
. 1424
|
Spermatogenesis-associated protein 7
(Photoreceptor ciliary transport)
SPATA7 localizes to the connecting cilium of the photoreceptors. It is responsible for protein trafficking across the photoreceptor connecting cilium, and for recruiting and localizing retinitis pigmentosa GTPase regulator interacting protein 1 (RPGRIP1) to the photoreceptor connecting cilium.
|
OCT: preservation of the foveal inner/outer segment junction.25 FAF: parafoveal hyperautofluorescent ring.25
ERG: non-recordable scotopic and photopic.27
Infantile nystagmus. Moderate hypermetropic refractive error. Also cause juvenile autosomal recessive retinitis (arRP)
|
Variable foveal involvement with diffuse peripheral retinal atrophic changes25 and attenuated vessels.26
|
Midfacial hypoplasia with enophthalmos. Rare hearing loss and low sperm count.
No syndromic associations.
|
|
AIPL1 (LCA4)
OMIM# 604393
. AR
. 17p13.2
. <5%17
. 507
|
Aryl-hydrocarbon-interacting protein-like 1
(Phototransduction and protein biosynthesis)
AIPL1 is specific to rod photoreceptors in adult life only, showing a specific staining pattern extending from the inner segment to the synaptic junction in the outer plexiform layer.28 AIPL1 specifically targets processing of farnesylated proteins causing de-stabilization of phosphodiesterase 6 (PDE6), which is believed to be compromised in LCA, leading to early-onset retinal degeneration.29
|
Significantly decreased but stable visual acuity, which ranges from LP to NLP in 43% of cases during their first decade.10
ERG: pathognomonic pattern characterized by slow insensitive scotopic responses.29
Nyctalopia. Mutations in AIPL1 also cause phenotypes of cone rod dystrophy (CRD) and juvenile arRP.
|
Maculopathy that is specific for drusen-like deposits and variable pigmentary atrophic changes with patches of hypopigmentation and pigmentary clumping.10
|
None
|
|
LCA5 (LCA5)
OMIM# 604537
. AR
. 6q11-q16
. 1-2%23
. 1230
|
Lebercillin
(Photoreceptor ciliary transport)
Lebercillin is located at the cilium between the inner and outer segments of photoreceptors.31 It interacts with intraflagellar transport (IFT) machinery and is involved in microtubule transport. Inactivation of Lebercillin mutations result in photoreceptor degeneration due to failure in outer segment formation.
|
OCT: Preserved central island of outer nuclear layer (ONL) that decreases in thickness eccentrically with normal or reduced foveal ONL peak.34
Nystagmus. Hyperopic refractive error
|
Normal fundus in childhood progressing to various degree of atrophic changes32 including colobomatous macular changes.33
|
Other ciliary defect as LCA5 is also expressed in upper (nasopharynx and trachea) and lower respiratory systems (lungs).9
|
|
RPGRIP1 (LCA6)
OMIM# 613826
. AR
. 14q11.2
. 5%35
. 717,36
|
Retinitis pigmentosa GTPase regulator-interacting protein 1
(Photoreceptor ciliary transport)
Retinitis pigmentosa GTPase regulator (RPGR) and retinitis pigmentosa GTPase regulator-interacting protein (RPGRIP) are co-localized to outer segments of human rod and cone photoreceptors as well as neuronal cells such as amacrine cells.37 Both RPGR and RPGRIP proteins co-localize specifically to the photoreceptor connecting cilium, where they are essential for disk morphogenesis and regulation of actin cytoskeleton dynamic.38 Most mutations in RPGRIP1 result in protein truncation and complete functional loss.
|
There is an initial severe decline in visual function (LP to NLP) that is stable afterward without significant worsening.39
OCT: Preserved central foveal outer retinal structures surrounded by retinal laminar disorganization41
|
Normal retinal
appearance initially that progresses afterward to drusen-like pigmentary changes in 60% and 80% of cases.10,40
|
None
|
|
CRX (LCA7)
OMIM# 613829
. AR (AD rare42)
. 19q13.33
. 1%
5110
|
Cone–rod homeobox
(Photoreceptor morphogenesis)
CRX is a transcription factor essential for both photoreceptor outer segment elongation and phototransduction cascade as it activates a specific sequence related to upstream processing of photoreceptor-specific genes.43
|
Severe relatively stable vision loss (CF-NLP) in the first few months of life44,45
ERG: markedly reduced or nondetectable.
Infantile nystagmus; High hypermetropia; Photodysphoria; Keratoconus; Cataracts.9
|
Diffuse pigmentary retinal changes and atrophic macular changes.45,46
|
None
|
|
CRB1 (LCA8)
OMIM# 613835
. AR
. 1q31.3
. 10%17,39
. 1667,47
|
Protein crumbs homolog 1
(Photoreceptor morphogenesis)
The protein localizes to the inner segment of photoreceptors and is known to control the photoreceptor outer segment orientation and its adherens junctions with Muller cells by stabilizing the membrane-associated spectrin cytoskeleton.48
|
Variable VA with possible mild temporary improvement followed by noticeable decline.9
OCT: abnormally “unlaminated” thickened retina, almost resembling that of an immature state.49
High hypermetropia (+5.00 to +10.00).50 RP with and without a Coats-like vasculopathy.
Late-onset maculopathy, isolated AR foveal retinoschisis.51-54
|
Nummular pigmentation maculopathy with preservation of the para-arteriolar RPE.
|
None
|
|
NMNAT1 (LCA9)
OMIM# 608553
. AR
. 1p36.22
. Unknown prevalence and # of mutations
|
Nicotinamide/nicotinic acid mononucleotide adenylyl transferase 1
(Coenzyme NAD biosynthesis)
The protein catalyzes the conversion of nicotinamide mononucleotide (NMN) and ATP into NAD+ as well as the cleavage of the latter.56 NMNAT1 is essential for cell survival and is reported to affect NAD protein folding of the resultant structure.57
|
Overall thinned retina.34
|
Early onset extensive and progressive central maculopathy with colobomatous-like atrophic changes with outer hyperpigmented borders, as well as typical peripheral pigmentary changes, optic nerve pallor, and retinal blood vessel attenuation. This is possibly a developmental abnormality with lack of foveal formation and inner retinal laminae crossing the central retina.56
|
None
|
|
CEP290 (LCA10, NPHP6)
OMIM# 611755
. AR
. 12q21.32
. 15-20%57
. 1536,57
|
Centrosomal protein Cep290
(Photoreceptor ciliary transport)
CEP290 interacts with photoreceptors building blocks, including pericentriolar material 1 (PCM1), to properly ensure centromere localization.58
|
VA is a significant variable early and does not correlate with age in early infancy. Eventually, VA is reported to be extremely poor.57,59,60
OCT: Preserved central island of ONL that decreases in thickness eccentrically with normal or reduced foveal ONL peak34,61
|
Relatively normal in early childhood
|
Nephronophthisis, hypotonia, ataxia, impaired olfactory function.60,62
Joubert syndrome57,60
Senior-Løken Syndrome 663
|
|
IMPDH1 (LCA11)
OMIM# 613837
. AR
. 7q32.1
. 5%23
. 136
|
Inosine-5-prime-monophosphate dehydrogenase
(Guanine synthesis)
IMPDH1 is the rate-limiting enzyme for the de novo synthesis of guanine nucleotides.64
|
Variable visual acuity
|
Diffuse RPE mottling, no pigmentary deposits.65
|
None
|
|
RD3 (LCA12)
OMIM# 610612
. AR
. 1q32.3
. <1%23
. 86
|
Retinal degeneration 3
(Protein trafficking)
RD3 is a chaperone protein required for the exit of guanylate cyclase from the endoplasmic reticulum of photoreceptors during outer segments trafficking.66
|
Poor vision with progressive decrease.9,67
Initial hypermetropia progressing to myopia over the course of the disease.68
Nystagmus69
|
Atrophic macular lesions possibly progressing to a bull's-eye pattern with central yellow pigmentation.67,68
|
None
|
|
RDH12 (LCA13)
OMIM# 612712
. AR
. 14q24.1
. 10%17,69
. 657
|
Retinol dehydrogenase 12
(Retinoid visual cycle)
RDH12 localizes to the inner segment base of photoreceptors70 and directs the transformation of 11-cis-retinol into 11-cis-retinal.71
|
Variable visual acuity
OCT: Severely disorganized retinal architecture corresponding to macular excavation with variable thinning or thickening of retinal layers.34
Absent or very mild hypermetropia.72
Coats-like exudative vasculopathy9
|
Early childhood maculopathy characterized by pigmentary fishnet or reticular
pattern with diffuse RPE atrophy. Intraretinal bone-spicules pigmentation develops during the disease course.69
|
None
|
|
LRAT (LCA14)
OMIM# 613341
. AR
. 4q32.1
. <1%23
. 1010
|
Lecithin retinol acyltransferase
(Retinoid visual cycle)
LRAT catalyzes the esterification of all-trans-retinol in the RPE layer into all-trans-retinyl ester.
|
Phenotype is similar to RPE65 deficiency
Juvenile RP. Early-onset severe retinal dystrophy (EOSRD)
|
Normal-appearing posterior pole with sharp peripheral hypopigmenation outside the arcades.73
|
None
|
|
TULP1 (LCA15)
OMIM# 613843
. AR
. 6p21.31
. <1%23
. 3110
|
Tubby-like protein 1
(Photoreceptor ciliary transport)
TULP1 contributes to rhodopsin transport between the inner and outer segments of photoreceptor cells74 and stimulates the phagocytosis of RPE cells.
|
Reading vision in early stages75
OCT: Preserved central island of ONL that decreases in thickness eccentrically with normal or reduced foveal ONL peak34 FAF: hyperautofluorescent perifoveal ring75
Nystagmus, hemeralopia, mild myopia; no photophobia75
|
Funduscopic findings of patients with TULP1 LCA mutations vary significantly and include a normal fundus at early age, progressing to indistinct foveolar reflex and pronounced salt-and-pepper maculopathy later on with midperipheral RPE atrophy.75
|
None
|
|
KCNJ13 (LCA16)
OMIM# 614186
. AR
. 2q37.1
. Unknown prevalence
. 37
|
Kir7 inwardly rectifying potassium channel
(Phototransduction)
KCNJ13, which is expressed in the apical microvilli of retinal pigment epithelia, is a potassium channel involved in maintaining the resting membrane potential near the potassium equilibrium potential.
|
Poor night and reading visual function at an early age.76 Progressive visual deterioration affecting both peripheral and central vision. VA of CF reported by the first decade.76
OCT: loss of outer retinal structures with RPE/Bruch membrane thinning.76,77
Snowflake vitreoretinal degeneration4
|
Severe retinopathy with nummular pigment and retinal degeneration and thinning.76,77
|
None
|
|
IFT140
OMIM# 614620
. AR
. 16p13.3
. Unknown prevalence
. 1110
|
Intraflagellar transport 140 chlamydomonas homologue protein
(Photoreceptor ciliary transport)
IFT140 is specifically involved in retrograde ciliary transport78
|
Variable visual acuity
OCT: IS/OS disruption and increased foveal thickness.
FAF: hyperautofluorescent macular ring79
arRP79
|
Diffuse pigment clumps at posterior pole and mid-peripheral fundus79
|
None isolated
Mainzer-Saldino syndrome presents with retinal dystrophy (like LCA), severe renal disease, cerebellar ataxia, and skeletal dysplasia78
Cono-renal syndrome includes early-onset LCA-like retinal dystrophy, cone-shaped phalangeal epiphyses of the hands, chronic renal failure, and ataxia80,81
|
|
IQCB1 or NPHP5
OMIM# 609237
. AR
. 3q13.33
. Unknown prevalence
. 248
|
IQ motif containing B1 protein
(Photoreceptor ciliary transport)
IQCB1 has been reported to localize to retinal photoreceptors and to primary epithelial cells cilia.82
|
Visual acuity ranging between 20/70 and NLP83
ERG: markedly reduced or nondetectable83
hypermetropia. Coats-like changes83
|
Lobular pattern of hypo- and hyperpigmentation around the vascular arcades with retinal vessels straightening83
|
Nephronophthisis
Seniør-Loken syndrome 584
|
|
CLUAP1
OMIM# 616787
. AR
. 16p13.3
. Unknown prevalence
. 17
|
Clusterin-associated protein 1
(Photoreceptor ciliary transport)
CLUAP1 is found at the connecting cilium between the inner and outer segments of retinal photoreceptors.85 It is involved in IFT particle turnaround.
|
Early onset severe visual deterioration to NLP.86
ERG: extinguished.86
Nystagmus86
|
Normal88
|
None
|
|
PRPH2
OMIM# 179605
. AR
. 6p21.1
. Unknown prevalence
. 1127,87
|
Peripherin
(Photoreceptor morphogenesis)
Peripherin 2 localizes to the photoreceptor outer segment, where it serves as an adhesion molecule to stabilize the segment88
|
Variable visual acuity
OCT: Foveal globular lesions89
adRP, digenic RP, autosomal dominant macular degeneration
|
Peripheral and macular pigment deposits with central atrophic and chorioretinal sclerosis.89
|
None
|
|
ERG, electroretinogram; FAF, fundus autofluorescence; NLP, no light perception; OCT, optical coherence tomography; VA, visual acuity
|
LCA was defined in 1867 by Theodor Leber, and considered currently a pure ocular disease. Some patients, however, have a syndromic constellation of LCA-like ocular phenotype and systemic findings. These include:
Joubert syndrome with oculo-renal disease (JBTS2: OMIM# 608091, JBTS3: OMIM# 608629, JBTS4: OMIM# 609583, JBTS5: OMIM# 610188, JBTS7: OMIM# 611560, JBTS9: OMIM# 612285, JBTS14: OMIM# 614424, JBTS20: OMIM# 614970) is a genetically heterogeneous group of disorders that are all autosomally inherited. Classic phenotypic manifestations include cerebellar and brainstem malformations (molar tooth sign), as well as hypotonia and developmental delay.90 However, patients often present with other findings, including juvenile-onset cystic nephronophthisis, early-onset LCA-like retinal dystrophy and either nystagmoid eye movement, episodic hyperpnea and/or apnea, or both.90 Mutations in the following genes have been reported to cause Joubert syndrome with oculo-renal disease:
- TMEM216 (OMIM# 613277) causes JBTS2 (OMIM# 608091). Transmembrane protein 216 was found to localize to the ciliary base or adjacent basal body in the renal collecting duct and proximal renal tubular cells, as well as human retinal pigment epithelium.91 Functionally, TMEM216 is thought to be involved in cellular polarization and centrosomal apical docking.91 Mutations in TMEM216 can lead to ciliary shortening, disruption of vesicular trafficking92 and prevention of ciliogenesis.
- AHI1 gene (OMIM# 608894) causes JBTS3 (OMIM# 608629). AHI1, which encodes Abelson helper integration site 1, has been reported to control ciliogenesis by regulating the formation of primary nonmotile cilia.93
- NPHP1 gene (OMIM# 607100) causes JBTS4 (OMIM# 609583). NPHP1 (OMIM# 607100) which codes for nephrocystin 1. The latter belongs to a family of proteins involved in cellular polarization. Abnormal ciliary formation, delayed tight junction formation, and disorganized collagenous matrices were reported in the presence of dysfunctional nephrocystin proteins.94
- CEP290 gene (also aka NPHP6 OMIM# 610142) causes JBTS5 (OMIM# 610188). Centrosomal protein Cep290 interacts with photoreceptors building blocks, including pericentriolar material 1 (PCM1), to properly ensure centromere localization.58
- RPGRIP1L gene (NPHP8, OMIM# 610937) causes JBTS7 (OMIM# 611560). RPGRIP1L, which encodes for retinitis pigmentosa GTPase regulator-interacting protein 1-like protein or nephrocystin 8, localizes to the basal body and ciliary axoneme of primary cilia.95 It serves as a docking site for ciliary protein vesicular fusion-related processes.96
- CC2D2A gene (OMIM# 612013) causes JBTS9 (OMIM# 612285). Coiled-coil and C2 domain-containing protein 2A localizes to the basal body of ciliary cells.97 It interacts with CEP290 in mediating ciliary transport mechanisms. Specifically, it serves as a docking site for ciliary protein vesicular fusion-related processes.96
- TMEM237 gene (OMIM# 614423) causes JBTS14 (OMIM# 614424). Transmembrane protein 237 localizes to retinal photoreceptor outer segments and to the outer plexiform layer’s horizontal cells98 It regulates ciliogenesis by serving as a transition zone protein.99
- TMEM231 gene (OMIM# 614949) causes JBTS20 (OMIM# 614970). Transmembrane protein 231 is part of a ring-like proteinaceous structure that restricts diffusion across the ciliary membrane. A dysfunctional TMEM231 affects ciliogenesis by altering ciliary growth.100
Senior-Løken syndrome (SLSN3: OMIM# 606995, SLSN4: OMIM# 606996, SLSN5: OMIM# 609254, SLSN6: OMIM# 610189, SLSN7: OMIM# 613615, SLSN8: OMIM# 616307, SLSN9: OMIM# 616629) is a heterogeneous renal-retinal syndrome characterized by juvenile nephronophthisis (OMIM# 256100) and early-onset LCA-like retinal dystrophy.90,101,102 SLS1 is caused by mutations in NPHP1 (OMIM# 607100), which codes for nephrocystin 1. The latter belongs to a family of proteins involved in cellular polarization. Abnormal ciliary formation, delayed tight junction formation, and disorganized collagenous matrices were reported in the presence of dysfunctional nephrocystin proteins.94 Mutations in nephrocystin protein family other than NPHP1 were reported to cause SLS. These include:
- NPHP3 gene (OMIM# 608002) causes SLSN3 (OMIM# 606995). Nephrocystin 3 is involved in regulating the composition of ciliary complexes.103
- NPHP4 gene (OMIM# 607215) causes SLSN4 (OMIM# 606996). Nephrocystin 4 serves as a docking site for ciliary protein vesicular fusion-related processes.96
- IQCB1 gene (NPHP5, OMIM# 609237) causes SLSN5 (OMIM# 609254). IQCB1 has been reported to localize to retinal photoreceptors and to primary epithelial cell cilia.82
- CEP290 gene (NPHP6, OMIM# 610142) causes SLSN6 (OMIM# 610189). CEP290 gene (aka NPHP6 OMIM# 610142) causes JBTS5 (OMIM# 610188). Centrosomal protein Cep290 interacts with photoreceptors building blocks, including pericentriolar material 1 (PCM1), to properly ensure centromere localization.56
- SDCCAG8 gene (NPHP10, OMIM# 613524) causes SLSN7 (OMIM# 613615). Serologically defined colon cancer antigen 8, or nephrocystin 10, was localized to the retinal photoreceptor transition zone in mouse104 It was reported to be involved in DNA repair processes.105
- WDR19 gene (OMIM# 608151) causes SLSN8 (OMIM# 616307). WD repeat-containing protein 19, aka DYF-2 (in elegans) is expressed in retinal photoreceptors. It encodes for the intraflagellar transport 144 (IFT144) protein which is a subunit of IFT-A complex involved in retrograde ciliary transport.106,107
- TRAF3IP1 gene (OMIM# 607380) causes SLSN9 (OMIM# 616629). It encodes for TNF Receptor Associated Factor 3-Interacting Protein 1, which is a subunit of IFT-B complex involved in anterograde ciliary transport.108
Mainzer-Saldino Syndrome (MZSDS, OMIM# 266920) is an autosomal recessive syndrome characterized by renal and skeletal dysplasia, LCA-like retinal dystrophy, and cerebellar ataxia. MZSDS is part of the autosomal recessive skeletal ciliopathies known as short-rib thoracic dysplasia (SRTD) with or without polydactyly. The alternative designation of MZSDS is short-rib thoracic dysplasia 9 with or without polydactyly. MZSDS is caused by mutations in IFT40 (OMIM# 614620), which is a ciliary transport gene involved in retrograde transport (Table 1). MZSDS patients can display early onset LCA-like retinal dystrophy with visual impairment and undetectable ERG.78,109
Conorenal syndrome (OMIM# 266920) includes early-onset LCA-like retinal dystrophy, cone-shaped phalangeal epiphyses of the hands, chronic renal failure, and ataxia80,81 This syndrome has been associated with pathologic mutations in IFT40 (OMIM# 614620). Fundus findings were reported as nonpigmented atypical retinal degeneration progressing to bone spicules deposition.110
LCA Classifications
LCA can be classified based on disease pathway, OCT findings, or retinal histopathology.
LCA classification based on disease pathway
There are currently 7 known, distinct pathways that are affected by one of the 20 mutant LCA genes. LCA disease pathway 1 involves molecules necessary for the phototransduction cascade, and includes the following genes: GYCY2D, AIPL1, and RD3. Disease pathway 2 is in the retinoid cycle and includes the RPE65, LRAT, and RDH12 genes. Disease pathway 3 includes the retinal transcription factor, which includes CRX. Disease pathway 4 involves intra-flagellar transport (IFT), and includes mutations in SPATA7, LCA5, RPGRIP1, CEP290, TULP1, NPHP5, CLUAP1, and IFT140. CRB1 and PRPH2 are included in disease pathway 5, which is involved in photoreceptor morphogenesis. Disease pathway 6 is in metabolic enzymes for cellular survival and includes IMPDH1 and NMNAT1. Finally, disease pathway 7 is in photoreceptor ion channels and is represented by mutations in KCNJ13.
LCA classification based on optical coherence tomography (OCT) findings
According to Jacobson et al., 201634 there are four major retinal architecture categories of LCA:
Type 1: Normal in vivo retinal architecture: LCA patients with GUCY2D mutations can display subnormal retinal and outer nuclear layer (ONL) thicknesses within the central foveal degrees and normal parafoveal thickness.1
Type 2: Thickened retina: This pattern, which is specific to CRB1 mutations, is characterized by a reduced foveal ONL, limited extra-central ONL, and thickened dysplastic or “unlaminated” retinal layers across the rest of the retina.1
Type 3: Normal foveal architecture: This OCT laminar pattern is specific for a preserved central foveal island of ONL that decreases in thickness eccentrically. Mutations in RPE65 (LCA2), Lebercilin (LCA5), RPGRIP1 (LCA6), CEP290 (LCA10), and TULP1 (LCA15) have been associated with this phenotype.1
Type 4: Severe macular atrophy or foveal maldevelopment: This OCT pattern is associated with LCA mutations displaying severe maculopathies, namely AIPL1 (LCA4), NMNAT1 (LCA9) and RDH12 (LCA13). Note that the colobomatous-like maculopathy seen with NMNAT1 (LCA9) mutations is possibly due to a congenital lack of foveal formation and inner retinal layers. All 3 mutations show a reduced central ONL. The parafoveal retinal thickness varies in AIPL1 (LCA4) and RDH12 (LCA13) patients but is significantly thinned in NMNAT1 (LCA9).1
LCA classification based on retinal histopathology findings
There are 3 distinct categories that have been described2 based on “pre-molecular testing” histological studies of LCA eyes from cadavers or blind painful enucleated eyes. In type 1, there is an abnormal embryological formation of photoreceptors corresponding to an aplastic process. Type 2 is characterized by a degeneration process in which there is early and progressive photoreceptor loss. In type 3, there is retinal dysfunction due to absence or dysfunction of key retinal biochemical messages despite relatively normal architecture. Not surprisingly, these 3 histological categories correlate well with the OCT-based classification. Specifically, type 1 may be represented by CRB1 mutations; type 2 may correlate well with RPGRIP1, LCA5, and RPE65 mutations; and type 3 may correlate with GUCY2D mutations.
Therapeutic Considerations
As in all other retinal dystrophies, the visual deterioration in LCA cannot be prevented and/or halted yet, although several very exciting and novel treatments are showing great promise in human clinical trials, including gene augmentation, oral drug therapy, and intraocular drugs. Early and precise clinical and molecular genetic diagnosis is the first step in the appropriate management of this group of diseases. A multi-disciplinary approach by ophthalmologists, ocular geneticists, and counselors, as well as retinal dystrophy support groups is the best means to efficiently frame this disease in its numerous facets, and assist patients with vocational, educational, and family planning. Monitoring and treating possible clinical associations, such as renal failure and hearing loss, is primordial to maximize patients’ quality of life.
There have been significant advances in therapeutic modalities, including gene replacement, stem cell therapy, and pharmacologic therapies in a number of inherited retinal diseases. Gene replacement therapy using subretinal injection of genetically engineered adeno-associated virus (AAV) vectors has shown very promising results in several LCA animal models and humans: GUCY2D RetGC1 mouse models,111-113 RPE65 Briard dogs,114 and mouse models20,115-118 as well as AIPL1 knock-out mouse models,119 AAV-based gene replacement was shown to preserve and rescue photoreceptors (both rods and cones) and improve and restore retinal function. Based on this success, human clinical trials for LCA patients were undertaken globally and are at various stages of completion (Table 2).
|
Table 2: List of ongoing and completed human clinical trials in LCA.124
|
|
Identifier
|
|
Sponsor
|
Target Gene
|
Agent
|
Delivery
|
Phase
|
Status
|
|
NCT00516477
|
Safety Study in Subjects With Leber Congenital Amaurosis
|
Spark Therapeutics
|
RPE65
|
AAV2-hRPE65v2
|
Subretinal
|
I
|
Ongoing
|
|
NCT02781480
|
Clinical Trial of Gene Therapy for the Treatment of Leber Congenital Amaurosis (LCA) (OPTIRPE65)
|
MeiraGTx UK II Ltd
|
RPE65
|
AAV2/5 OPTIRPE65
|
Subretinal
|
I
|
Ongoing
|
|
NCT00481546
|
Phase I Trial of Gene Vector to Patients With Retinal Disease Due to RPE65 Mutations (LCA)
|
University of Pennsylvania
|
RPE65
|
rAAV2-CBSB-hRPE65
|
Subretinal
|
I
|
Ongoing
|
|
NCT02946879
|
Long-Term Follow-Up Gene Therapy Study for Leber Congenital Amaurosis OPTIRPE65 (Retinal Dystrophy Associated With Defects in RPE65)
|
MeiraGTx UK II Ltd
|
RPE65
|
AAV2/5-OPTIRPE65
|
Subretinal
|
I and II
|
Ongoing
|
|
NCT00749957
|
Phase 1/2 Safety and Efficacy Study of AAV-RPE65 Vector to Treat Leber Congenital Amaurosis
|
Applied Genetic Technologies Corp
|
RPE65
|
rAAV2-CB-hRPE65
|
Subretinal
|
I and II
|
Ongoing
|
|
NCT01208389
|
Phase 1 Follow-on Study of AAV2-hRPE65v2 Vector in Subjects With Leber Congenital Amaurosis (LCA) 2
|
Spark Therapeutics
|
RPE65
|
AAV2-hRPE65v2
|
Subretinal
|
I and II
|
Ongoing
|
|
NCT00643747
|
Safety Study of RPE65 Gene Therapy to Treat Leber Congenital Amaurosis
|
University College, London
|
RPE65
|
tgAAG76 (rAAV 2/2.hRPE65p.hRPE65)
|
Subretinal
|
I and II
|
Completed
|
|
NCT01496040
|
Clinical Gene Therapy Protocol for the Treatment of Retinal Dystrophy Caused by Defects in RPE65 (RPE65)
|
Nantes University Hospital
|
RPE65
|
rAAV2/4.hRPE65
|
Subretinal
|
I and II
|
Completed
|
|
NCT00999609
|
Safety Study in Subjects With Leber Congenital Amaurosis
|
Spark Therapeutics
|
RPE65
|
AAV2-hRPE65v2
|
Subretinal
|
III
|
Ongoing
|
|
NCT03140969
|
Study to Evaluate QR-110 in Subjects With Leber's Congenital Amaurosis (LCA) Due to the c.2991+1655A>G Mutation (p.Cys998X) in the CEP290 Gene
|
ProQR Therapeutics
|
CEP290
|
QR-110
|
Intravitreal
|
I and II
|
Approved, not yet started
|
|
NCT01521793
|
Repeated Treatments of QLT091001 in Subjects With Leber Congenital Amaurosis or Retinitis Pigmentosa (Extension of Study RET IRD 01)
|
QLT Inc.
|
RPE65, LRAT
|
QLT091001
|
Oral
|
I
|
Completed
|
|
NCT01014052
|
Safety/Proof of Concept Study of Oral QLT091001 in Subjects With Leber Congenital Amaurosis (LCA) or Retinitis Pigmentosa (RP) Due to Retinal Pigment Epithelial 65 Protein (RPE65) or Lecithin:Retinol Acyltransferase (LRAT) Mutations
|
QLT Inc.
|
RPE65, LRAT
|
QLT091001
|
Oral
|
I
|
Completed
|
Presently, 3 phase I, 3 phase I/II and 1 phase III clinical trials in humans are being undertaken for RPE65-related patients using subretinal AAV gene replacement (Table 2). Furthermore, 2 phase I/II clinical trials have been completed. The data collected from these clinical investigations showed that AAV gene replacement is definitely safe but variably efficacious. Some studies have shown that the proposed treatment may not be sustainable, due to persistent progression of the retinal degeneration despite functional improvement in selected participants.121-124
Oral substitutes of key components of the visual pathway have been clinically tested and have been shown to be safe and efficacious in children and adults with RPE65 and LRAT mutations.125,126 The 2 phases I/II clinical trials using a synthetic prodrug precursor to 9-cis-retinal, QLT091001, showed both significant enlargement of the kinetic visual field and improvement in visual acuity in 44% and 67% of participants, respectively.126 A phase III trial with the same agent is being planned.
Finally, human embryonic stem cell (hESCs) therapy holds future promise as it aims to regenerate dysfunctional RPE cells. Currently, 2 human phase I/II stem cell clinical trials have demonstrated successful RPE integration of injected hESCs.127,128 The integration and transplantation of stem cells to the photoreceptor layer, which is needed for LCA therapy, is still in the preclinical stage.
References
- Coussa RG, Lopez Solache I, Koenekoop RK. Leber congenital amaurosis, from darkness to light: An ode to Irene Maumenee. Ophthalmic Genet. 2017; 38(1):7-15.
- Koenekoop RK. An overview of Leber congenital amaurosis: a model to understand human retinal development. Surv Ophthalmol. 2004; 49:379–398.
- Stone EM. Leber congenital amaurosis - a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144:791–811.
- Weleber RG, Francis PJ, Trzupek KM, Beattie C. Leber Congenital Amaurosis. https://www.ncbi.nlm.nih.gov/books/NBK1298/. Accessed May 7, 2017.
- Heher KL, Traboulsi EI, Maumenee IH. The natural history of Leber’s congenital amaurosis. Age-related findings in 35 patients. Ophthalmology. 1992; 99:241-245.
- Perrault I, Rozet JM, Gerber S, et al. Spectrum of retGC1 mutations in Leber's congenital amaurosis. Eur J Hum Genet. 2000;8:578–582.
- The Human Gene Mutation Database (HGMD®): http://www.hgmd.cf.ac.uk. Accessed November 27, 2017.
- Duda T, Venkataraman V, Goraczniak R., Lange C, Koch KW, Sharma RK. Functional consequences of a rod outer segment membrane guanylate cyclase (ROS-GC1) gene mutation linked with Leber's congenital amaurosis. Biochemistry. 1999;38:509-515.
- Chung DC, Traboulsi EI. Leber congenital amaurosis: clinical correlations with genotypes, gene therapy trials update, and future directions. J AAPOS. 2009;13:587-592.
- Galvin JA, Fishman GA, Stone EM, Koenekoop RK. Evaluation of genotype-phenotype associations in leber congenital amaurosis. Retina. 2005;25:919-929.
- Boye SE. Leber congenital amaurosis caused by mutations in GUCY2D. Cold Spring Harb Perspect Med. 2014; 5:a017350.
- Alstrom CH. Heredo-retinopathia congenitalis monohybrida recessiva autosomalis: a genetical-statistical study in clinical collaboration with Olof Olson. Hereditas. 1957; 43: 1-178.
- Perrault I, Hanien S, Gerber S, et al. A novel mutation in the GUCY2D gene responsible for an early onset severe RP different from the usual GUCY2D-LCA phenotype. Hum Mutat. 2005;25:222.
- Jacobson SG, Cideciyan AV, Peshenko IV, et al. Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human Leber congenital amaurosis en route to therapy: residual cone-photoreceptor vision correlates with biochemical properties of the mutants. Hum Mol Genet. 2013;22:168–183.
- Pasadhika S, Fishman GA, Stone EM, et al. Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2010;51:2608–2614.
- Khan AO, Al-Mesfer S, Al-Turkmani S, Bergmann C, Bolz HJ. Genetic analysis of strictly defined Leber congenital amaurosis with (and without) neurodevelopmental delay. Br J Ophthalmol. 2014;98: 1724-1728.
- den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008; 27:391–419.
- Znoiko SL, Crouch RK, Moiseyev G, Ma JX. Identification of the RPE65 protein in mammalian cone photoreceptors. Invest Ophthalmol Vis Sci. 2002; 43:1604–1609.
- Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA. 2005; 102:13658-136663.
- Jacobson SG, Aleman TS, Cideciyan AV, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci U S A. 2005; 102:6177–6182.
- Maeda T, Cideciyan AV, Maeda A, et al. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug,9-cis-retinyl acetate. Hum Mol Genet. 2009; 18:2277–2287.
- Lorenz B, Wabbels B, Wegscheider E, Hamel CP, Drexler W, Preising MN. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology. 2004; 111:1585–1594.
- Kumaran N, Moore AT, Weleber RG, Michaelides M. Leber congenital amaurosis/early-onset severe retinal dystrophy: clinical features, molecular genetics and therapeutic interventions. Br J Ophthalmol. 2017; 101:1147-1154.
- Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Nat Acad Sci. 2005; 102:12413-12418.
- Mackay DS, Ocaka LA, Borman AD., et al. Screening of SPATA7 in patients with Leber congenital amaurosis and severe childhood-onset retinal dystrophy reveals disease-causing mutations. Invest Ophthal Vis Sci. 2011; 52: 3032-3038.
- Li Y, Wang H, Peng J, et al. Mutation survey of known LCA gene and loci in the Saudi Arabian population. Invest Ophthalmol Vis Sci. 2009; 50:1336-1343.
- Stockton DW, Lewis RA, Abboud EB, et al. A novel locus for Leber congenital amaurosis on chromosome 14q24. Hum Genet. 1998; 103: 328-333.
- van der Spuy J, Chapple JP, Clark BJ, Luthert PJ, Sethi CS, Cheetham ME. The Leber congenital amaurosis gene product AIPL1 is localized exclusively in rod photoreceptors of the adult human retina. Hum Molec Genet. 2002; 11: 823-831.
- Ramamurthy V, Roberts M, van den Akker F, Niemi G, Reh TA, Hurley JB. AIPL1, a protein implicated in Leber's congenital amaurosis, interacts with and aids in processing of farnesylated proteins. Proc Nat Acad Sci. 2003; 100:12630-12635.
- Oliveira L, Miniou P, Viegas-Pequignot E, Rozet JM, Dollfus H, Pittler SJ. Human retinal guanylate cyclase (GUC2D) maps to chromosome 17p13.1. Genomics. 1994; 22:478-481.
- Boldt K, Mans DA, Won J, et al. Disruption of intraflagellar protein transport in photoreceptor cilia causes Leber congenital amaurosis in humans and mice. J Clin Invest. 2011; 121:2169-2180.
- Dharmaraj S, Li Y, Robitaille JM., Silva E, et al. A novel locus for Leber congenital amaurosis maps to chromosome 6q. (Letter) Am JHum.Genet. 2000; 66: 319-326.
- Mohamed MD, Topping NC, Jafri H, Raashed Y, McKibbin MA, Inglehearn CF. Progression of phenotype in Leber's congenital amaurosis with a mutation at the LCA5 locus. Br J Ophthalmol. 2003; 87:473–475.
- Jacobson SG, Cideciyan AV, Huang WC, et al. Leber Congenital Amaurosis: Genotypes and Retinal Structure Phenotypes. Adv Exp Med Biol. 2016; 854:169-175.
- Gerber S, Perrault I, Hanein S, et al. Complete exon-intron structure of the RPGR-interacting protein (RPGRIP1) gene allows the identification of mutations underlying Leber congenital amaurosis. Eur J Hum Genet. 2001; 9:561–571.
- Dryja TP, Adams SM, Grimsby JL, et al. Null RPGRIP1 alleles in patients with Leber congenital amaurosis. Am J Hum Genet. 2001; 68:1295-1298.
- Mavlyutov TA, Zhao H, Ferreira PA. Species-specific subcellular localization of RPGR and RPGRIP isoforms: implications for the phenotypic variability of congenital retinopathies among species. Hum Molec Genet. 2002; 11:1899-1907.
- Roepman R, Bernoud-Hubac N, Schick DE, et al. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum Molec Genet. 2000; 9:2095-2105.
- Hanein S, Perrault I, Gerber S, et al. Leber congenital amaurosis: comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype-phenotype correlations as a strategy for molecular diagnosis. Hum Mutat. 2004; 23:306–317.
- Dryja TP, McGee TL, Reichel E, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990; 343:364-366.
- Jacobson SG, Cideciyan AV, Aleman TS, et al. Leber congenital amaurosis caused by an RPGRIP1 mutation shows treatment potential. Ophthalmology. 2007; 114:895–898.
- Nichols LL 2nd, Alur RP, Boobalan E, et al. Two novel CRX mutant proteins causing autosomal dominant Leber congenital amaurosis interact differently with NRL. Hum Mutat. 2010;31(6):E1472-83.
- Kimura A, Singh D, Wawrousek EF, Kikuchi M, Nakamura M, Shinohara T. Both PCE-1/RX and OTX/CRX interactions are necessary for photoreceptor-specific gene expression. J Biol Chem. 2000; 14;275:1152-1160.
- Freund CL, Wang QL, Chen S, et al. De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. (Letter) Nature Genet. 1998; 18: 311-312.
- Swaroop A., Wang QL, Wu W, et al. Leber congenital amaurosis caused by a homozygous mutation (R90W) in the homeodomain of the retinal transcription factor CRX: direct evidence for the involvement of CRX in the development of photoreceptor function. Hum Molec Genet. 1999; 8: 299-305.
- Akagi T, Mandai M, Ooto S, et al. Otx2 homeobox gene induces photoreceptor-specific phenotypes in cells derived from adult iris and ciliary tissue. Invest Ophthal Vis Sci. 2004; 45:4570-4575.
- Lotery AJ, Malik A, Shami SA, et al. CRB1 mutations may result in retinitis pigmentosa without para-arteriolar RPE preservation. Ophthalmic Genet. 2001; 22:163-169.
- Pellikka M, Tanentzapf G, Pinto M, et al. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002; 416: 143-149.
- Jacobson SG, Cideciyan AV, Aleman TS, et al. Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum Molec Genet. 2003; 12:1073-1078.
- Abouzeid H, Li Y, Maumenee IH, Dharmaraj S, Sundin O. A G1103R mutation in CRB1 is co-inherited with high hyperopia and Leber congenital amaurosis. Ophthalmic Genet. 2006; 27: 15-20.
- Henderson RH, Mackay DS, Li Z, et al. Phenotypic variability in patients with retinal dystrophies due to mutations in CRB1. Br J Ophthalmol. 2011; 95:811–817.
- Simonelli F, Ziviello C, Testa F, et al. Clinical and molecular genetics of Leber's congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol Vis Sci. 2007; 48:4284–4290.
- Tsang SH, Burke T, Oll M, et al. Whole exome sequencing identifies CRB1 defect in an unusual maculopathy phenotype. Ophthalmology. 2014; 121:1773–1782,
- Wolfson Y, Applegate CD, Strauss RW, Han IC, Scholl HP. CRB1-related maculopathy with cystoid macular edema. JAMA Ophthalmol. 2015; 133:1357–1360.
- Emanuelli M, Carnevali F, Saccucci F, et al. Molecular cloning, chromosomal localization, tissue mRNA levels, bacterial expression, and enzymatic properties of human NMN adenylyltransferase. J Biol Chem. 2001; 276:406–412.
- Koenekoop RK, Wang H, Majewski J, et al. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat Genet. 2012; 44:1035-1039.
- den Hollander AI, Koenekoop RK, Yzer S, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006; 79:556–61.
- Stowe TR, Wilkinson CJ, Iqbal A, Stearns T. The centriolar satellite proteins Cep72 and Cep290 interact and are required for recruitment of BBS proteins to the cilium. Molec Biol Cell. 2012; 23:3322-3335.
- McAnany JJ, Genead MA, Walia S, et al. Visual acuity changes in patients with leber congenital amaurosis and mutations in CEP290. JAMA Ophthalmol. 2013; 131:178–182.
- Perrault I, Delphin N, Hanein S, et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat. 2007; 28:416.
- Cideciyan, A. V., Aleman, T. S., Jacobson, S. G., et al. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Hum Mutat. 2007; 28: 1074-1083.
- McEwen DP, Koenekoop RK, Khanna H., et al. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Nat Acad Sci. 2007; 104: 15917-15922.
- The Online Mendelian Inheritance in Man website, www.omim.org. OMIM# 610189. Accessed August 15, 2017.
- Collart FR, Huberman E. Cloning and sequence analysis of the human and Chinese hamster inosine-5-prime-monophosphate dehydrogenase cDNAs. J Biol Chem. 1988; 263:15769-15772.
- Bowne SJ, Sullivan LS, Mortimer SE, et al. Spectrum and frequency of mutations in IMPDH1 associated with autosomal dominant retinitis pigmentosa and Leber congenital amaurosis. Invest Ophthal Vis Sci. 2006; 47: 34-42.
- Molday LL, Djajadi H, Yan P, et al. RD3 gene delivery restores guanylate cyclase localization and rescues photoreceptors in the Rd3 mouse model of Leber congenital amaurosis 12. Hum Mol Genet. 2013; 22: 3894-3905.
- Preising MN, Hausotter-Will N, Solbach MC, Friedburg C, Rüschendorf F, Lorenz B. Mutations in RD3 are associated with an extremely rare and severe form of early onset retinal dystrophy. Invest Ophthal Vis Sci. 2012; 53: 3463-3472.
- Friedman JS, Chang B, Kannabiran C, et al. Premature truncation of a novel protein, RD3, exhibiting subnuclear localization is associated with retinal degeneration. Am J Hum Genet. 2006; 79: 1059-1070. Note: Erratum: Am J Hum Genet. 2007; 80: 388.
- Mackay DS, Dev Borman A, Moradi P, et al. RDH12 retinopathy: novel mutations and phenotypic description. Mol Vis. 2011; 17:2706–2716.
- Haeseleer F, Jang GF, Imanishi Y, et al. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem. 2002; 277:45537-45546.
- McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001; 20:469–529.
- Janecke AR, Thompson DA, Utermann G, et al. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004; 36: 850-854,. Note: Erratum: Nat Genet. 2004; 36: 1024 only.
- den Hollander AI, Lopez I, Yzer S, et al. Identification of novel mutations in patients with Leber congenital amaurosis and juvenile RP by genome-wide homozygosity mapping with SNP microarrays. Invest Ophthal Vis Sci. 2007; 48:5690-5698.
- Xi Q, Pauer GJ, Marmorstein AD, Crabb JW, Hagstrom SA. Tubby-like protein 1 (TULP1) interacts with F-actin in photoreceptor cells. Invest Ophthalmol Vis Sci. 2005; 46:4754-4761.
- Mataftsi A, Schorderet DF, Chachoua L, et al. Novel TULP1 mutation causing Leber congenital amaurosis or early onset retinal degeneration. Invest Ophthalmol Vis Sci. 2007; 48: 5160-5167.
- Sergouniotis P. I., Davidson A. E., Mackay D. S., et al. Recessive mutations in KCNJ13, encoding an inwardly rectifying potassium channel subunit, cause leber congenital amaurosis. Am J Hum Genet. 2011; 89:183-190.
- Pattnaik BR, Shahi PK, Marino MJ, et al. A novel KCNJ13 nonsense mutation and loss of Kir7.1 channel function causes Leber congenital amaurosis (LCA16). Hum Mutat. 2015; 36:720-727.
- Perrault I., Saunier S., Hanein S., et al. Mainzer-Saldino syndrome is a ciliopathy caused by IFT140 mutations. Am J Hum Genet. 2012; 90:864-870.
- Xu M, Yang L, Wang F, et al. Mutations in human IFT140 cause non-syndromic retinal degeneration. Hum Genet. 2015; 134:1069-1078.
- Giedion, A. Phalangeal cone shaped epiphysis of the hands (PhCSEH) and chronic renal disease: the conorenal syndromes. Pediatr Radiol. 1979; 8: 32-38.
- Mendley SR, Poznanski AK, Spargo BH, Langman CB. Hereditary sclerosing glomerulopathy in the conorenal syndrome. Am J Kidney Dis. 1995; 25: 792-797.
- Otto EA, Loeys B, Khanna H, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005; 37: 282-288.
- Estrada-Cuzcano A, Koenekoop RK, Coppieters F, et al. IQCB1 mutations in patients with leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2011; 52:834-839.
- The Online Mendelian Inheritance in Man website, www.omim.org. OMIM# 609254. Senior-Loken Syndrome 5; SLSN5. Accessed August 15, 2017.
- Botilde Y, Yoshiba S, Shinohara K, et al. Cluap1 localizes preferentially to the base and tip of cilia and is required for ciliogenesis in the mouse embryo. Dev Biol. 2013; 381: 203-212.
- Soens ZT, Li Y, Zhao L, et al.Hypomorphic mutations identified in the candidate Leber congenital amaurosis gene CLUAP1. Genet Med. 2016; 18:1044-1051.
- The Online Mendelian Inheritance in Man website, www.omim.org. OMIM# 179605. Peripherin 2, Mouse, Homolog of PRPH2. Cytogenetic location. Accessed August 15, 2017.
- Bascom, R. A., Connell, G., Garcia-Heras, J., et al. Molecular and ultrastructural characterization of the products of the human retinopathy candidate genes ROM1 and RDS. (Abstract). Am J Hum Genet. 1990; 47 (suppl.): A101 only.
- Wang X, Wang H, Sun V, et al. Comprehensive molecular diagnosis of 179 Leber congenital amaurosis and juvenile retinitis pigmentosa patients by targeted next generation sequencing. J Med Genet. 2013; 50: 674-688.
- Parisi M, Glass I. Joubert Syndrome. https://www.ncbi.nlm.nih.gov/books/NBK1325. Accessed August 7, 2017.
- Valente EM, Logan CV, Mougou-Zerelli S, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. (Letter). Nat Genet. 2010; 42: 619-625.
- Lee JH, Silhavy JL, Lee JE, et al. Evolutionarily assembled cis-regulatory module at a human ciliopathy locus. Science. 2012; 335: 966-969.
- Hsiao YC, Tong ZJ, Westfall JE, et al. Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking. Hum Molec Genet. 2009; 18: 3926-3941.
- Delous M, Hellman NE, Gaudé HM, et al. Nephrocystin-1 and nephrocystin-4 are required for epithelial morphogenesis and associate with PALS1/PATJ and Par6. Hum Molec Genet. 2009; 18:4711-4723.
- Arts HH, Doherty D, van Beersum SE, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome.Nat Genet. 2007; 39:882-888.
- Williams CL, Li C, Kida K, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011; 192:1023-1041.
- Gorden NT, Arts HH, Parisi MA, et al. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet. 2008; 83:559-571.
- Zuniga FI, Craft CM. Deciphering the structure and function of Als2cr4 in the mouse retina. Invest Ophthal Vis Sci. 2010; 51: 4407-4415.
- Huang L, Szymanska K, Jensen VL, et al. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet. 2011; 89: 713-730.
- Chih B, Liu P, Chinn Y, et al. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol. 2012; 14: 61-72.
- Loken AC, Hanssen O, Halvorsen S, et al. Hereditary renal dysplasia and blindness. Acta Paediat. 1961; 50:177-184.
- Fairley KF, Leighton PW, Kincaid-Smith P. Familial visual defects associated with polycystic kidney and medullary sponge kidney. Brit Med J. 1963; 1:1060-1063.
- Hoff S, Halbritter J, Epting D, et al. ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3. Nat Genet. 2013; 45: 951-995.
- Otto EA, Hurd TW, Airik, R, et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet. 2010; 42: 840-850.
- Chaki, M., Airik, R., Ghosh, A. K., et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012; 150:533-548.
- Coussa RG, Otto EA, Gee HY, et al. WDR19: an ancient, retrograde, intraflagellar ciliary protein is mutated in autosomal recessive retinitis pigmentosa and in Senior-Loken syndrome. Clin Genet. 2013 ; 84:150-159.
- Bredrup C, Saunier S, Oud MM., et al. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am J Hum Genet. 2011;89:634-643.
- Bizet AA, Becker-Heck A, Ryan R, et al. Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization. Nat Commun. 2015; 6:8666.
- Schmidts M, Frank V, Eisenberger T, et al. Combined NGS approaches identify mutations in the intraflagellar transport gene IFT140 in skeletal ciliopathies with early progressive kidney disease. Hum Mutat. 2013; 34: 714-724.
- Beals RK, Weleber RG. Conorenal dysplasia: a syndrome of cone-shaped epiphysis, renal disease in childhood, retinitis pigmentosa and abnormality of the proximal femur. Am J Med Genet. 2007; 143: 2444-2447.
- Haire SE, Pang J, Boye SL, et al. Light-driven cone arrestin translocation in cones of postnatal guanylate cyclase-1 knockout mouse retina treated with AAV-GC1. Invest Ophthalmol Vis Sci. 2006; 47:3745–3753.
- Boye SE, Boye SL, Pang J, et al. Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PLoS One. 2010; 5:e11306.
- Mihelec M, Pearson RA, Robbie SJ, et al. Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency. Hum Gene Ther. 2011; 22:1179–1190.
- Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001; 28:92–95.
- Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998; 20:344–350.
- Pang JJ, Chang B, Hawes NL, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Mol Vis. 2005; 11:152–162.
- Pang JJ, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006; 13:565–572.
- Roman AJ, Boye SL, Aleman TS, et al. Electroretinographic analyses of Rpe65-mutant rd12 mice: developing an in vivo bioassay for human gene therapy trials of Leber congenital amaurosis. Mol Vis. 2007; 13:1701–1710.
- Tan MH, Smith AJ, Pawlyk B, et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum Mol Genet. 2009; 18:2099–2114.
- ClinicalTrials.gov. Studies found for Leber Congenital Amaurosis https://clinicaltrials.gov/ct2/results?cond=Leber+Congenital+Amaurosis&term=&cntry1=&state1=&recrs=. Accessed August 1, 2017.
- Weleber RG, Pennesi ME, Wilson DJ, et al. Results at 2 years after gene therapy for RPE65-deficient leber congenital amaurosis and severe early-childhood-onset retinal dystrophy. Ophthalmology. 2016; 123:1606–1620.
- Bainbridge JW, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med. 2015; 372:1887–1897.
- Testa F, Maguire AM, Rossi S, et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology. 2013; 120:1283–1291.
- Jacobson SG, Cideciyan AV, Roman AJ, et al. Improvement and decline in vision with gene therapy in childhood blindness. N Engl J. Med. 2015; 372:1920–1926.
- Koenekoop RK, Sui R, Sallum J, et al. Oral 9-cis retinoid for childhood blindness due to Leber congenital amaurosis caused by RPE65 or LRAT mutations: an open-label phase 1b trial. Lancet. 2014; 25: 384 (9953):1513-1520.
- Scholl HP, Moore AT, Koenekoop RK, et al. Safety and Proof-of-Concept Study of Oral QLT091001 in Retinitis Pigmentosa Due to Inherited Deficiencies of Retinal Pigment Epithelial 65 Protein (RPE65) or Lecithin:Retinol Acyltransferase (LRAT). PLoS One. 2015; 10:e0143846.
- Schwartz SD, Tan G, Hosseini H, et al. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Invest Ophthalmol Vis Sci. 2016; 579:ORSFc1-9.
- Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017; 376:1038–1046.