This chapter was reviewed for currency and updated by its author in August 2020.
Etiology and Epidemiology of Disease
Aicardi syndrome (AS) is a triad of features: agenesis of corpus collosum, chorioretinal lacunae, and infantile spasms. The etiology of Aicardi syndrome is unknown; however, the disorder is almost always seen in females and is thought to be a de novo mutation on the X-chromosome with hemizygous lethality in males.1 There are reports of males affected with Klinefelter syndrome (XXY genotype)2 and an isolated case of a XY genotype with Aicardi syndrome,3 although with atypical findings on exam. The syndrome is rare in all ethnicities and an age-adjusted prevalence of 0.63 in 100,000 female births was reported in the Norway population,4 and estimates in the United States have been reported as 1 per 105,000-167,000 live births (including female and males).5 There are no identified maternal risk factors.
Pertinent Elements of the History
The females are identified early in life, usually during work up for refractory infantile spasms or epilepsy, nystagmus, or roving eye movements with chorioretinal lacunae (Figures 1 and 2), or profound developmental delay in the first year of life.
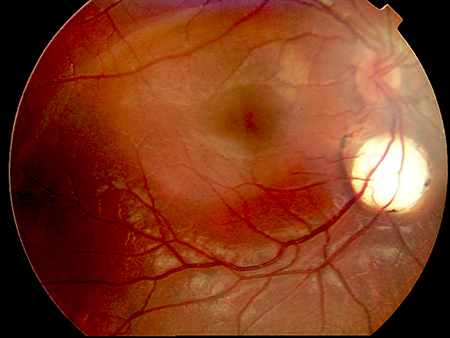
Figure 1.

Figure 2.
Figures 1 and 2. Chorioretinal lacunae in two girls with Aicardi syndrome. (Courtesy of Bart Leroy, MD).
Clinical Features
The 3 cardinal features of agenesis of the corpus callosum, chorioretinal lacunae, and infantile spasms describe the majority of the cases; however, major and supporting features were defined in 1999 to include those without the complete classic triad.6 Aicardi syndrome remains a clinical diagnosis defined by all 3 of the classic triad or > 2 of the classic triad plus ≥ 2 of the major or supporting features. Chorioretinal lacunae, cortical malformations, and periventricular heterotopias are features present in almost all cases.1
Classic Triad
- Infantile spasms
- Chorioretinal lacunae
- Agenesis of the corpus callosum (may be partial)
Major Features
- Coloboma of the optic disc (and nerve), often unilateral
- Cortical malformations (mostly microgyria)
- Periventricular (and subcortical) heterotopia
- Intracranial cysts: interhemispheric or around the third ventricle
- Papillomas of the choroid plexus
Supporting Features
- Vertebral and costal abnormalities
- Microphthalmia and/or other eye abnormalities
- ‘Split-brain’ EEG (dissociated suppression-burst tracing)
- Gross cerebral hemispheric asymmetry
Ophthalmic characteristics
The chorioretinal lacunae are considered pathognomonic for AS. The abnormal development of the eye, especially the choriocapillaris, occurs during the fourth to eighth week of gestation (even up to twelve weeks), at the same time as the corpus callosum development.7-9 On fundus exam, thinning of the choroid and sclera with degeneration of the rods and cones appears as hypopigmented or depigmented regions that are whitish or pink in color.8 Commonly, there are pigmented deposits in the center or along the lacunae borders. The lacunae are always multiple and usually bilateral, with varying sizes from < 1 to multiple disc diameters. The peripapillary lacunae tend to be the largest.7 Vessels do not typically bend or cross over the lacunae. The appearance of the lacunae remain stable over years;10 however, some reports suggest change in appearance and pigmentation over time.11,12
On pathologic examination, the lacunae are described as having features typical of optic nerve colobomas, but at the edges there are unique convolutions of tubular-like structures lined by pigmented and nonpigmented epithelial cells in close association with the capillaries8 and photoreceptor folds. The overlying retina remains intact, but often with abnormal histology.13 The chorioretinal lacunae are different from a coloboma in that they have defined margins, poorly differentiated or absent choriocapillaris, and a thin, but intact, Bruch membrane with attenuated and hypoplastic retinal pigment epithelium.8 In addition, there can be retinal cysts as seen on optical coherence tomography.14
It is very common for there to be other ophthalmic abnormalities associated with the lacunae. The most common is a coloboma of the optic disc that may even be seen in the fellow eye of a unilateral chorioretinal lacunae. Ring-like pigment deposits, often surrounding a colobomatous papilla, can resemble the morning glory appearance. Microphthalmia can be seen in up to half of the patients and is usually unilateral. The right eye is more often affected and more severe when the condition is bilateral, which is a similar finding to more frequent and severe brain malformations seen in the right hemisphere.15 Various other anomalies such as remnants of fetal pupillary membrane, remnants of the primary vitreous, retinal detachment, iris synechiae, posterior staphylomas of the iris or choroid, and cataracts are seen with varying frequency in AS.12,16
Non-ophthalmic characteristics
Common facial features include prominent premaxilla, upturned nasal tip, decreased angle of the nasal bridge, and sparse lateral eyebrows.17
Brain magnetic resonance imaging (MRI) findings can include polymicrogyria or pachygyria, periventricular and intracortical grey matter heterotopia, gross cerebral asymmetry, choroid plexus papilloma, ventriculomegaly, and intracerebral cysts that are often located at the third ventricle and in the choroid plexus.
Common electroencephalogram (EEG) findings include asynchronous multifocal epileptiform abnormalities with burst suppression and dissociation between the two hemispheres. If there is onset of infantile spasms, hypsarrhythmia may also be present.
Many small series and case reports describe skeletal anomalies, with costovertebral defects that can lead to scoliosis becoming problematic after the first year of life. There appears to be an increase in malignant tumors (medulloblastoma) and benign tumors (lipomas, angiosarcomas, hepatoblastomas, intestinal polyposis, and embryonal carcinoma), with the most common being choroid plexus papillomas.17-20
Diagnostic evaluation and testing
The diagnosis of Aicardi syndrome is based on clinical presentation, brain imaging, and EEG findings. However, pathognomonic chorioretinal lacunae seen on dilated fundus exam is often the final step to confirmation. Prenatal ultrasonography examination or fetal MRI may detect some features that can raise the possibility for the diagnosis.
Genetics
There are no familial reported cases of AS. Candidate genes on the X chromosome have been favored, given the presentation in only females; however no disease-causing variants have been found.21,22 Most recently with the use of next-generation sequencing technology, de novo variants were reported in 2 girls with nonsense variants in TEAD1 and a missense variant in OCEL1.23 However, further evaluation in 38 girls with AS was unable to find these mutations, and these genes are less favored to be a major cause of AS.24
Differential Diagnosis
The seizure disorder can be prominent and may appear to be associated with Dandy-Walker syndrome, agenesis of the corpus callosum, neuronal migration disorders, Lennox-Gastaut syndrome (LGS), lissencephaly, West syndrome, and cyclin-dependent kinase-like 5 (CDKL5) disorder. Small or peripheral lacunae can appear in ocular toxoplasmosis or the chorioretinopathy seen in microcephaly with or without chorioretinopathy, lymphedema, or mental retardation (MCLMR). Chorioretinal lacunae have been described in orofaciodigital syndrome type IX (OFD 9); however, this syndrome is different from AS clinically. If there is microphthalmia, microphthalmia with linear skin defects (MLS) syndrome can be considered.
Patient Management
There is no treatment for the visual impairment. Depending on cognitive ability, the child may need low-vision assistance. The seizure management can be refractory and lifelong.
Disease-related complications
Retinal detachment is an ophthalmic complication that presents as sudden onset vision loss. This may be unrecognized in children with limited expressive language and may present either on routine ophthalmic evaluation or with behavior changes. There is morbidity from general deconditioning or spasticity, increased risk of malignancies, and scoliosis requiring multiple surgeries. Sudden unexplained death in epilepsy (SUDEP) has also been reported.
Prognosis
The visual outcome is generally poor. Total blindness is rare despite extensive retinal involvement with chorioretinal lacunae, and most children with Aicardi syndrome have some useful visual behavior. The burden of retinal abnormality from the lacunae may have a positive correlation with the severity in mobility and language skills, but is unlikely to predict visual outcome.10 The amount of involvement of the fovea, rather than the overall burden, is likely more predictive of severe vision loss.
Survival is highly variable and depends on the severity of seizures and other organ system involvement. The ages of highest mortality risk are in the first few years of life and in adolescence; however, the majority live into early adulthood. The common causes of death include respiratory infections, systemic infections, malignancy, and SUDEP.
Children with AS have moderate to severe developmental and intellectual disability, although some can have a milder form of disability. Most will communicate with gestures, sounds, and other nonverbal means. It is rare for a child to form short sentences. Most children with AS are able to sit independently and feed themselves and a few can walk independently.
References
- Aicardi J. Aicardi Syndrome. Brain & Development. 2005; 27:164-171.
- Hopkins IJ, Humphrey I, Keith CG, Susman M, Webb GC, Turner EK. The Aicardi syndrome in a 47,XXY male. Aust Paediatr J. 1979; 15(4):278-280.
- Aggarwal KC, Aggarwal A, Prasad MS, Salhan RN, Upadahaya A. Aicardi’s syndrome in a male child: An unusual presentation. Indian Pediatr. 2000; 37(5):542-545.
- Lund C, Bjornvold M, Tuft M, Kostov H, Rosby O, Selmer K. Aicardi Syndrome: An epidemiologic and clinical study in Norway. Pediatric Neurology. 2015; 52:182-186.
- Kroner B, Preiss L, Ardini M, Gaillard W. New Incidence, Prevalence, and Survival of Aicardi Syndrome From 408 Cases. J Child Neurol. 2008; 23(5):531-535.
- Aicardi J. Aicardi Syndrome: Old and New Findings. Int Pediatr. 1998; 14(1):5-8.
- Hoyt CS, Billson F, Ouvrier R, Wise G. Ocular features of Aicardi's syndrome. Arch Ophthalmol. 1978; 96(2):291-295.
- McMahon R, Bell R, Moore G, Ludwin S. Aicardi's Syndrome: A Clinicopathologic Study. Arch Ophthalmol. 1984; 102(2):250-253.
- Ganesh A, Mitra S, Koul RL, Venugopalan P. The full spectrum of persistent fetal vasculature in Aicardi syndrome: an integrated interpretation of ocular malformation. Br J Ophthalmol. 2000; 84(2):227-228.
- Menezes A, Lewis T, Buncic J. Role of ocular involvement in the prediction of visual development and clinic prognosis in Aicardi syndrome. Br J Ophthalmol.1996; 80:805-811.
- Ospina L, Nayak H, MacCormick A. Progressive pigmentation of chorioretinal lesions in Aicardi syndrome Arch Ophthalmol 2004;122:790.
- Palmer L, Zetterlund B, Hard AL, Steneryd K, Kyllerman M. Aicardi syndrome: presentation at onset in Swedish children born in 1975-2002. Neuropediatrics 2006;37:154–158.
- Del Pero RA, Mets MB, Tripathi RC, Torczynski E. Anomalies of retinal architecture in Aicardi syndrome. Arch Ophthalmol. 1986; 104(11):1659-1664.
- Martel J, Rutar T, Lujan B, de Alba Campomanes A. Chorioretinal architecture in Aicardi syndrome: an optical coherence tomography and fluorescein angiography. J AAPOS. 2011;15(3):308-310.
- Cabrera M, Winn B, Porco T, et al. Laterality of brain and ocular lesions in Aicardi syndrome. Pediatr Neurol. 2011; 45:149–154.
- Fruhman G, Eble T, Gambir N, Sutton V, Van der Veyver I, Lewis R. Ophthalmologic findings in Aicardi syndrome. J AAPOS. 2012; 16(3):238-241.
- Sutton VR, Hopkins BJ, Eble TN, Gambhir N, Lewis RA, Van den VeVeyver IB. Facial and physical features of Aicardi syndrome: infant to teenagers. Am J Med Genet A. 2005; 138A:254-258.
- Palmer L, Nordborg C, Steneryd K, Aman P, Kyllerman M. Large-cell medulloblastoma in Aicardi syndrome. Case report and literature review. Neuropediatrics. 2004; 35:307–311.
- Pianetti Filho G, Fonseca LF, da Silva MC. Choroid plexus papilloma and Aicardi syndrome: case report. Arq Neuropsiquiatr. 2002; 60:1008–1010.
- Kamien BA, Gabbett MT. Aicardi syndrome associated with hepatoblastoma and pulmonary sequestration. Am J Med Genet A. 2009; 149A:1850–1852.
- Anderson, S., Menten, B., Kogelenberg, M., et al. Aicardi syndrome in a male patient. Neuropediatrics. 2009; 40(1), 39–42.
- Van den Veyver, I. B., Panichkul, P. P., Antalffy, B. A., Sun, Y., Hunter, J. V., & Armstrong, D. D. Presence of filamin in the astrocytic inclusions of Aicardi syndrome. Pediatric Neurology. 2004; 30(1), 7–15.
- Schrauwen, I., Szelinger, S., Siniard, A. L., et al. A De novo mutation in TEAD1 causes non-X-linked Aicardi syndrome. Investigative Ophthalmology & Visual Science. 2015; 56(6), 3896–3904.
- Wong, B. K., Sutton, V. R., Lewis, R. A., & Van den Veyver, I. B. Independent variant analysis of TEAD1 and OCEL1 in 38 Aicardi syndrome patients. Molecular Genetics & Genomic Medicine. 2017; 5(2), 117–121.