Establishing the diagnosis
A 6-year-old previously healthy female was referred to ophthalmology after a failed vision-screening test. On examination, she had reduced visual acuity (20/80) and color vision (control plate only) in the left eye. The visual acuity and color vision were normal in her right eye. She had a low amplitude, high frequency pendular horizontal nystagmus, which was bilateral but asymmetric (greater in the left eye compared to the right). On dilated fundus examination, she had bilateral diffuse optic nerve pallor (also greater in the left eye than the right) (Figure 1).
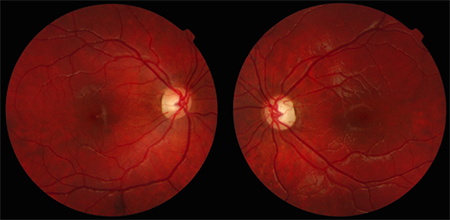
Figure 1. Color fundus photos showing bilateral diffuse optic nerve pallor.
Goldmann visual fields were performed and showed a right homonymous hemianopia with a central scotoma in the left eye (Figure 2). An urgent magnetic resonance imaging (MRI) scan of the brain and orbits was performed and revealed a large, lobulated enhancing mass in the sella and suprasellar region (Figure 3). The patient underwent an endoscopic biopsy (Figure 4), which confirmed the diagnosis of an optic pathway glioma (pilocytic astrocytoma, World Health Organization grade 1). The patient was referred to the genetics department to assess for other features of neurofibromatosis type 1 (NF1), but none were found. She was referred to the oncology department and started on chemotherapy consisting of weekly vincristine and carboplatin. During treatment, the patient was monitored with serial ophthalmologic exams and neuro-imaging. In addition to visual acuity and fundus examination, the patient was followed with serial Goldmann visual fields and optical coherence tomography (OCT) scans of the retinal nerve fiber layer (Figure 5).
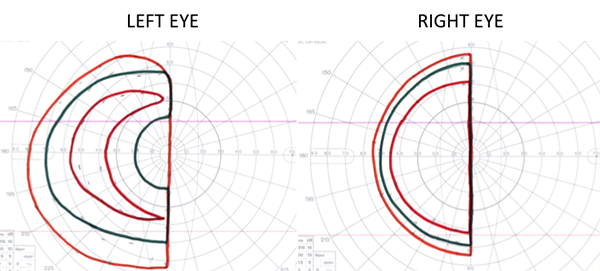
Figure 2. Goldmann visual fields showing a right homonymous hemianopia with a relative central scotoma in the left eye.
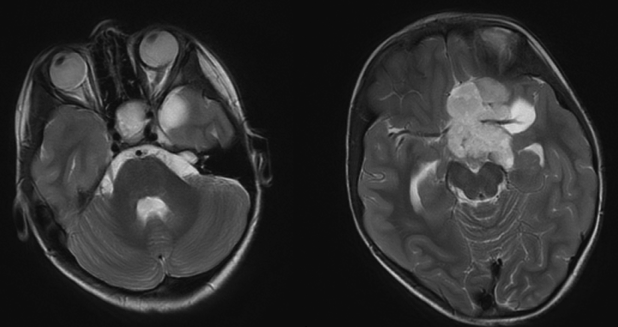
Figure 3. Axial T2-weighted MRI images showing a large, solid and cystic lobulated mass in the sella and suprasellar region.
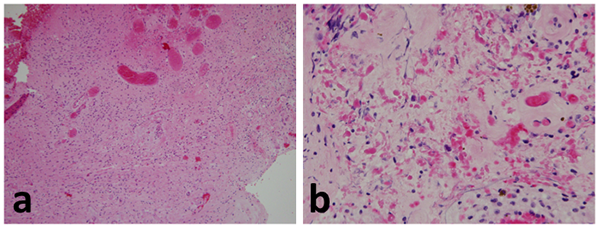
Figure 4. Histology of the optic pathway glioma. The lower power image (a) shows a moderately cellular tumor in a fibrillary background. Higher power image (b) illustrates Rosenthal fiber/eosinophilic granular bodies and hyalinized vessels.
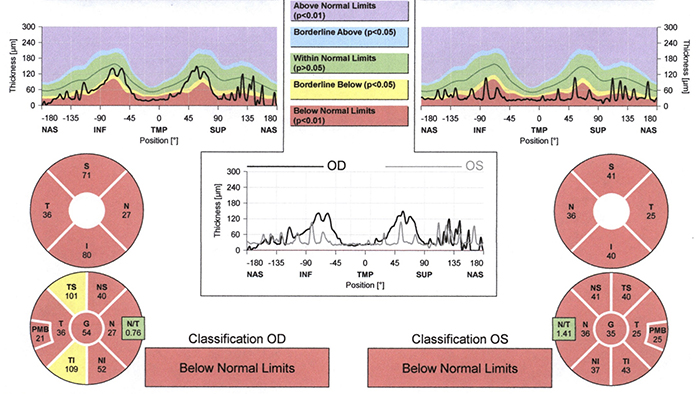
Figure 5. Optical coherence tomography (OCT) of the retinal nerve fiber layer showing diffuse loss of nerve fiber layer thickness.
During the primary course of chemotherapy with vincristine and carboplatin, her visual fields deteriorated on two serial ophthalmic exams and a repeat MRI showed an increase in tumor size. She was diagnosed with tumor progression and her chemotherapy regimen was changed to thioguanine, procarbazine, CCNU and vincristine. Repeat MRI showed further enlargement of the tumor with evidence of enlarging ventricles. The patient was referred to neurosurgery and underwent a subtotal tumor resection. She was stable for 6 months after surgery until a follow-up MRI revealed enlargement of the residual tumor, leading to a third course of chemotherapy with everolimus. Following completion of her treatment, she was followed with regular eye examinations and neuro-imaging and has remained stable for 5 years.
Epidemiology
Optic pathway gliomas (OPGs) are an important subtype of pediatric gliomas. Gliomas are the most common type of primary central nervous system (CNS) tumor in children, making up approximately 50% of all pediatric brain and CNS tumors1,2 with an overall population incidence rate of 3-4 per 100,000.3,4 Gliomas that specifically arise from the optic pathway represent approximately 2%-5% of intracranial tumors in children.5,6 7,8 These tumors principally occur in the first decade of life and the incidence decreases with increasing age.
Association with neurofibromatosis type 1 (NF1)
The most important risk factor for the development of an OPG is the presence of neurofibromatosis type 1 (NF1). NF1 is an autosomal dominant disorder with a high penetrance rate and birth prevalence of approximately 1 in 3,000.9,10 It has been estimated that 15%-20% of patients with NF1 will develop an OPG, although the incidence is difficult to determine precisely because a significant proportion of NF1-related OPGs never become symptomatic.3,6,11-15
Studies of large cohorts of patients with OPGs have reported that 50%-60% of affected patients have an underlying diagnosis of NF1.6,16,17 Given the strong association, when an OPG is diagnosed in a patient without a known diagnosis of NF1, it is imperative to assess for a family history of NF1 and other clinical features that would suggest the diagnosis. Recognizing the presence of NF1 is important because there are practical implications for patient management. NF1 is a systemic disease with diverse, multi-system manifestations, and affected patients need regular monitoring, ideally in a specialized clinic.18 The presence of NF1 also affects the visual prognosis of OPGs. Sporadic OPGs generally have a more aggressive course with a higher risk of tumor progression and more severe long-term visual impairment.6,11,17,19-22
In patients with known NF1, it is important to screen for early signs of visual dysfunction in order to detect clinically significant OPGs. In 1997, an NF1 optic pathway glioma task force performed an extensive literature review and recommended that screening of asymptomatic patients should consist of serial ophthalmology examinations by a pediatric ophthalmologist or neuro-ophthalmologist familiar with NF1.1,20,22 While the frequency of screening remains controversial, an updated review in 2007 recommended comprehensive eye examinations every year up to the age of 8, followed by examinations every 2 years until the age of 18.3,23 However, this has not been universally accepted and some authors have advocated for more frequent examinations for patients under the age of 6.24 If any unexplained abnormalities are detected on eye examination, an MRI of the head and orbits with contrast is necessary to assess for an OPG. However, in the absence of significant findings on eye examination, neuro-imaging is unnecessary. There is no conclusive evidence that early detection in asymptomatic children would reduce the rate of vision loss, so routine imaging in asymptomatic patients is not recommended. In addition, a normal MRI does not obviate the need for regular eye examinations, as there are several reported cases of clinically significant OPGs developing after normal neuro-imaging.25
Pathophysiology
Pediatric gliomas are a heterogeneous group of tumors that arise from the glial cells in the central nervous system. There are several subtypes of gliomas, including astrocytomas, oligodendrocytomas, ependymal tumors, and mixed glial-neuronal tumors. Tumors of the central nervous system, including gliomas, are graded by the World Health Organization (WHO) according to their histological features (Table 1).9 The tumor grade is designed to predict the biological behavior of the neoplasm and can be used clinically to help guide management decisions. OPGs are typically low-grade pilocytic astrocytomas (WHO Grade I).9 The majority of OPGs in children are indolent and rarely undergo malignant transformation.26 However, some patients have a histological variant of pilocytic astrocytomas called pilomyxoid astrocytomas (WHO Grade II), which may have a more aggressive clinical course including dissemination through the cerebrospinal fluid.27,28
|
Table 1. World Health Organization (WHO) grading system for tumors of the central nervous system.9
|
|
Grade
|
Histological Characteristics
|
Clinical Course
|
Astrocytic tumors
|
|
I
|
Circumscribed tumors without atypia
|
Slow-growing tumors with low proliferative potential and the possibility of cure with resection
|
Pilocytic astrocytoma (the majority of optic pathway gliomas)
|
|
II
|
Diffusely infiltrative tumors with cytological atypia
|
Relatively slow-growing tumors, but often recur and may progress to higher grades of malignancy
|
Pilomyxoid astrocytoma
Diffuse astrocytoma
|
|
III
|
Features of Grade II along with anaplasia and mitotic activity
|
Malignant tumors which often recur as higher grade tumors
|
Anaplastic astrocytoma
|
|
IV
|
Features of grade III along with microvascular proliferation and/or necrosis
|
Highly malignant, aggressive tumors
|
Glioblastoma
Gliosarcoma
|
On histology, optic pathway gliomas are characterized by moderately and variably cellular tumor nuclear cell density, in a fibrillary background (see Figure 4 for typical histology). The tumors are invariably GFAP-positive and Rosenthal fiber/eosinophilic granular bodies are common. Thickened/hyalinized small vessels may be seen. The proliferation index (Ki-67) can be somewhat elevated even in low-grade tumors. Unlike higher grade gliomas that are highly malignant and affect adults, they lack palisading necrosis, high mitotic indices, or pronounced nuclear atypia.7
Clinical presentation
In patients with NF1, the diagnosis of an OPG is often made on routine screening eye examinations.29 When OPGs present clinically, the list of potential signs and symptoms is long and variable. Broadly speaking, the list of presenting features can be divided into the four categories: ocular, neurological, endocrine, or other (Table 2).21,29
|
Table 2. Potential presenting features by category in pediatric patients with optic pathway gliomas
|
|
System
|
Presenting Feature
|
|
Ocular
|
Decreased visual acuity
Optic atrophy
Papilledema
Nystagmus
Proptosis
|
|
Neurological
|
Headache
Nausea/vomiting
Seizures
Cranial nerve palsy
Developmental regression
|
|
Endocrine
|
Precocious puberty
Short stature
Diabetes insipidus
|
|
Other
|
Diencephalic syndrome
Increasing head circumference
|
On presentation, it is important to take a careful history, focusing on the time course, character, and severity of presenting symptoms. One should also perform a detailed review of systems to assess for any associated ocular, neurological, endocrine, or systemic abnormalities. Finally, remember to ask about a family history of NF1 and other manifestations that would suggest an underlying diagnosis of NF1.
The eye examination should include age-appropriate visual acuity, pupillary examination, sensorimotor examination for strabismus, visual fields by confrontation, color vision, and dilated fundus examination looking for evidence of optic nerve pallor or edema. If available, Goldmann or Humphrey visual fields are useful to identify and characterize visual field deficits and potentially give valuable information about the anatomical location of a tumor. Other adjunctive testing such as optical coherence tomography (OCT) of the retinal nerve fiber layer30 and visual evoked potentials31 have shown promise in the diagnosis of OPGs but more studies are needed to further define their utility. Once the diagnosis of an OPG is suspected, the next step in the diagnostic algorithm is to obtain prompt neuro-imaging of the brain and orbits.
Imaging features
While neuro-imaging is essential in the diagnosis of OPGs, it can be logistically challenging in young patients and may necessitate sedation. As such, it is incumbent on the eye care provider to obtain an accurate history and examination to ensure that imaging is only ordered when indicated.
When neuro-imaging is needed, the preferred modality is an MRI with contrast of the brain and orbits. CT brain/orbits also has reasonable diagnostic accuracy for detecting an OPG16 and can be used if MRI is not readily available. However, MRI allows for better evaluation of the orbital apex, intracranial optic nerves, chiasm, and optics tracts.32 In addition, the exposure to ionizing radiation associated with CT scanning should be avoided if possible, especially in young children.33
MRI can effectively assess both the intraorbital and intracranial involvement of an OPG.26 With gadolinium, OPGs often display bright and uniform enhancement, which can be helpful in the diagnosis and in defining the extent of the tumor. For orbital sequences, fat and enhancing tumor are both bright on T1-weighted images, so fat-saturation sequences are helpful in differentiating an optic nerve glioma from surrounding orbital fat.
It may be difficult to differentiate an OPG from other entities that cause optic nerve enhancement, such as optic neuritis (inflammatory or infectious), idiopathic orbital inflammation syndrome, and optic nerve sheath meningioma. Usually an inflammatory or infectious process can be differentiated from an OPG by the clinical presentation. Optic nerve sheath meningioma can be more difficult to differentiate from OPGs, especially in older patients, because the clinical presentations often overlap. Classically, OPGs involving the optic nerve cause fusiform enlargement and kinking of the optic nerve with diffuse involvement of the substance of the nerve (Figure 6).34 In contrast, optic nerve sheath meningioma is characterized by tubular enlargement of the optic nerve with a post-contrast “tram-track” appearance, which results from the meningioma causing the nerve sheath to enhance brightly on either side of a hypointense optic nerve.35 Meningiomas are also much more likely to have evidence of calcification, although OPGs with calcification have been reported.36
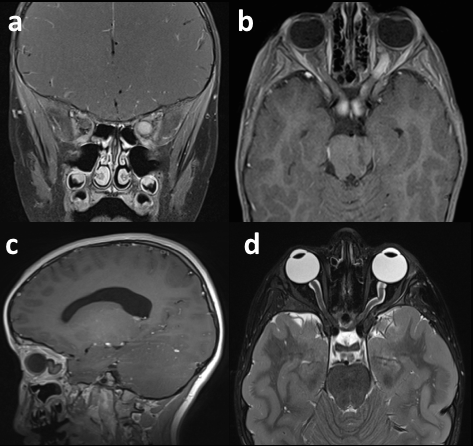
Figure 6. MRI images of two pediatric patients with optic pathway gliomas involving the optic nerve. Diffuse and uniform enhancement of an enlarged left optic nerve is shown on coronal (a) and axial (b) T1-weighted images. Kinking of an enlarged left optic nerve is shown on a sagittal T1-weighted image (c) and a coronal T2-weighted image (d).
There are some key differences in the radiological appearance of OPGs that are associated with NF1 compared to those that are not.37 NF1-associated OPGs are usually confined to the optic nerve (about 2/3) while non-NF OPGs usually involve the chiasm (over 90%). NF1-associated OPGs are more likely to present with bilateral optic nerve involvement while non-NF1 OPGs are more likely to extend beyond the optic pathway. Finally, non-NF1 OPGs are larger on average and often have cystic components.
Diagnostic Procedures
The diagnosis of an OPG can often be made based on the combination of clinical findings and radiological appearance of the tumor.38 In general, patients with known NF1 and typical findings of OPG on MRI do not need further diagnostic procedures, although biopsy may be useful in cases with atypical clinical or radiological findings.39 In patients without a known diagnosis of NF1, a surgical biopsy is often needed to firmly establish the diagnosis prior to starting treatment.26
Surgical biopsies can be performed through an open or endoscopic approach, depending on the location of the tumor and the preference of the surgeon. When deciding if a surgical biopsy is indicated, it is important to be mindful that biopsies are not innocuous and have been associated with serious complications including deterioration of cognition, worsened visual field loss, seizures, infection, and death.40,41
Management
The management of OPGs in children is complex and should be conducted in a center where there is significant experience in treatment of childhood cancer.3,42 The decision to initiate treatment is often not straightforward and can hinge upon several factors including patient age, tumor location and size, and visual symptoms.43,44 OPGs are typically low-grade tumors that do not metastasize and may even spontaneously regress.45 Therefore, OPGs do not always need treatment and nonprogressive tumors are usually monitored conservatively, and treatment is usually deferred until there is evidence of progression. Following the diagnosis of an OPG, patients should be monitored with serial neuro-imaging and ophthalmic exams. Eye examinations should be performed at least every 3 months for the first year, gradually increasing the interval between exams if visual function remains stable.3 Depending on the availability of neuro-imaging facilities, serial MR scans may be performed at the same interval or less frequently.
Treatment is indicated when there is evidence of progressive disease, although there are currently no universally accepted guidelines as to what exactly constitutes tumor progression. There is a general consensus that an OPG should be considered progressive when there is deterioration of visual function with a corresponding enlargement of the tumor on radiological imaging. The combination of functional deterioration and tumor enlargement is a definite indication for urgent treatment. However, it is more complicated when there is significant worsening of visual function with no change on MRI, or an enlargement of the tumor on MRI with no change in visual function. In these cases, the decision about whether to treat or monitor closely should be made by consensus between the oncology and ophthalmology services in consultation with the patient and the patient's family.
Surgery
Clinical studies have shown that surgery alone is rarely curative for OPGs.40 Even if the tumor appears well circumscribed and accessible to a conventional surgical approach on imaging, the borders of the tumor often extend beyond what the MRI findings suggest.46 In addition, surgery has significant adverse effects. For instance, OPGs of the optic nerve cannot be resected without sacrificing the entire optic nerve, causing complete loss of vision in the affected eye. OPGs that involve the chiasm are often closely associated with adjacent structures such as the hypothalamus, precluding any possibility of safely resecting the tumor in its entirety.
Although generally not curative, surgery does have an important role in the management of OPGs. As mentioned above, surgical biopsy may be crucial to confirm the diagnosis, especially in cases with atypical clinical or radiological findings. In addition, subtotal resection may be helpful for large suprasellar tumors that are causing obstructive hydrocephalus or mass effect on adjacent structures. Even if the entire tumor is not removed, OPGs generally grow very slowly and patients may remain stable for many years without need of further treatment after a subtotal resection.41 Finally, surgical resection continues to play an important role in advanced optic nerve gliomas with no visual potential to treat severe, disfiguring proptosis or painful corneal exposure.47
Radiotherapy
Radiotherapy was previously a common treatment for OPGs in children. However, long-term follow-up revealed that patients treated with radiotherapy had a significant risk of developing severe complications such as secondary CNS tumors48 and occlusive cerebral vasculopathy.49 The risk of severe complications was particularly high in younger patients and those with NF1. Therefore, radiation is now rarely used and should be avoided unless all other treatment options have been exhausted or for palliative reasons.
Chemotherapy
Chemotherapy is now generally considered the first-line treatment for OPGs in children. The typical primary chemotherapy treatment is weekly carboplatin and vincristine, which was shown to be effective in controlling newly diagnosed, progressive low-grade gliomas in children.15 This treatment regimen was subsequently found to improve the visual outcomes of patients with NF1-associated OPGs.11 There is a lack of evidence for the effectiveness of carboplatin and vincristine for OPGs in patients without NF1, but the first-line treatment is generally the same.50
If there is evidence of tumor progression during treatment with chemotherapy or afterward, the next step in therapy is usually a change to a different chemotherapeutic regimen. The treatment may also need to be changed if the patient develops a carboplatin hypersensitivity reaction, which occurs in approximately 40% of pediatric patients.51 Secondary chemotherapeutic treatments currently in use include temozolomide,52,53 bevacizumab,54 cisplatin-etoposide,55 and vinblastine,56 among others.57
Prognosis
Pediatric patients with OPGs have a good overall prognosis for survival.58 Several large studies have reported 5-year survival rates of over 95% and 10-year survival rates of over 90%.29,59,60 A single study has suggested that survival beyond 15 years after diagnosis may be significantly lower, but long-term data is limited.61 Death is usually due to tumor progression, although second tumors, vascular complications, and chemotherapy toxicity are also potential etiologies.61
In contrast to the low mortality rate of pediatric OPGs, the rate of visual impairment is high. Since vision loss is common and has a profound effect on quality of life, visual function has become a key outcome measure for research studies, for monitoring patients, and for guiding treatment decisions.20,22,23 The prognosis for visual function is significantly affected by the presence or absence of NF1. In long-term studies of visual outcomes, less than 50% of patients with NF1-associated OPGs had any evidence of visual impairment,62 42,43 compared to over 75% of patients with non-NF1 associated OPGs. Other reported risk factors for visual impairment include post-chiasmal involvement, younger age at diagnosis, and optic nerve pallor.11,12,14,42
Summary
Optic pathway gliomas (OPGs) constitute up to 5% of all intracranial tumors in children. Approximately 50% of OPGs arise in patients with neurofibromatosis type 1 (NF1) and these patients must be monitored regularly for early signs of visual dysfunction. While most OPGs are low-grade pilocytic astrocytomas, there are more aggressive variants that can disseminate through the cerebrospinal fluid. The potential presenting clinical features are highly variable, and neuro-imaging with MRI is essential in the workup of all suspected cases. Once identified, OPGs are often monitored for signs of progressive disease before treatment is instituted, usually in the form of chemotherapy. Although the overall survival rate is high for OPGs, there is a significant risk of visual impairment. Collaborative management by multidisciplinary teams is essential.
References
- Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol. 1997; 41(2):143–149.
- Ostrom QT, de Blank PM, Kruchko C, et al. Alex's Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro-oncology. 2015; 16 Suppl 10:x1–x36.
- Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007; 61(3):189–198.
- Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-oncology. 2014; 16 Suppl 4:iv1–63.
- Blazo MA, Lewis RA, Chintagumpala MM, Frazier M, McCluggage C, Plon SE. Outcomes of systematic screening for optic pathway tumors in children with Neurofibromatosis Type 1. Am J Med Genet A. 2004; 127A(3):224–229.
- Czyzyk E, Jóźwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. 2003; 18(7):471–478.
- Chen Y-H, Gutmann DH. The molecular and cell biology of pediatric low-grade gliomas. Oncogene. 2014; 33(16):2019–2026.
- Fried I, Tabori U, Tihan T, Reginald A, Bouffet E. Optic pathway gliomas: a review. CNS Oncol. 2013; 2(2):143–159.
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007; 114(2): 97-109.
- Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999; 89(1):1–6.
- Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro-oncology. 2012; 14(6):790–797.
- Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003; 28(4):262–270.
- Lewis RA, Gerson LP, Axelson KA, Riccardi VM, Whitford RP. von Recklinghausen neurofibromatosis. II. Incidence of optic gliomata. Ophthalmology. 1984; 91(8):929–935.
- Liu GT, Brodsky MC, Phillips PC, et al. Optic radiation involvement in optic pathway gliomas in neurofibromatosis. Am J Ophthalmol. 2004; 137(3):407–414.
- Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997; 86(5):747–754.
- Packer RJ, Bilaniuk LT, Cohen BH, et al. Intracranial visual pathway gliomas in children with neurofibromatosis. Neurofibromatosis. 1988; 1(4):212–222.
- Singhal S, Birch JM, Kerr B, Lashford L, Evans DGR. Neurofibromatosis type 1 and sporadic optic gliomas. Arch Dis Child. 2002; 87(1):65–70.
- Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007; 44(2):81–88.
- Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH. Sex is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol. 2014; 75(2):309–316.
- Avery RA, Ferner RE, Listernick R, Fisher MJ, Gutmann DH, Liu GT. Visual acuity in children with low grade gliomas of the visual pathway: implications for patient care and clinical research. J Neurooncol. 2012; 110(1):1–7.
- Astrup J. Natural history and clinical management of optic pathway glioma. Br J Neurosurg. 2003; 17(4):327–335.
- Avery RA, Hardy KK. Vision specific quality of life in children with optic pathway gliomas. J Neurooncol. 2014; 116(2):341–347.
- Fisher MJ, Avery RA, Allen JC, et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013; 81(21 Suppl 1):S15–24.
- Caen S, Cassiman C, Legius E, Casteels I. Comparative study of the ophthalmological examinations in neurofibromatosis type 1. Proposal for a new screening algorithm. Eur J Paediatr Neurol. 2015; 19(4):415–422.
- Listernick R, Charrow J, Greenwald M. Emergence of optic pathway gliomas in children with neurofibromatosis type 1 after normal neuroimaging results. J Pediatr. 1992; 121(4):584–587.
- Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009; 24(11):1397–1408.
- Fernandez C, Figarella-Branger D, Girard N, et al. Pilocytic astrocytomas in children: prognostic factors--a retrospective study of 80 cases. Neurosurgery. 2003; 53(3):544–553– discussion 554–555.
- Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999; 58(10):1061–1068.
- Nicolin G, Parkin P, Mabbott D, et al. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer. 2009; 53(7):1231–1237.
- Parrozzani R, Clementi M, Kotsafti O, et al. Optical coherence tomography in the diagnosis of optic pathway gliomas. Invest Ophthalmol Vis Sci. 2013; 54(13):8112–8118.
- Ng YT, North KN. Visual-evoked potentials in the assessment of optic gliomas. Pediatr Neurol. 2001; 24(1):44–48.
- Holman RE, Grimson BS, Drayer BP, Buckley EG, Brennan MW. Magnetic resonance imaging of optic gliomas. Am J Ophthalmol. 1985; 100(4):596–601.
- Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001; 176(2):289–296.
- Khan SN, Sepahdari AR. Orbital masses: CT and MRI of common vascular lesions, benign tumors, and malignancies. Saudi J Ophthalmol. 2012; 26(4):373–383.
- Mafee MF, Goodwin J, Dorodi S. Optic nerve sheath meningiomas. Role of MR imaging. Radiol Clin North Am. 1999; 37(1):37–58– ix.
- Pungavkar SA, Lawande MA, Patkar DP, Agrawal NV, Gadani S. Bilateral optic pathway glioma with intracranial calcification: magnetic resonance imaging and magnetic resonance spectroscopy findings. Australas Radiol. 2005; 49(6):489–492.
- Kornreich L, Blaser S, Schwarz M, et al. Optic pathway glioma: correlation of imaging findings with the presence of neurofibromatosis. AJNR Am J Neuroradiol. 2001; 22(10):1963–1969.
- Jakobiec FA, Depot MJ, Kennerdell JS, et al. Combined clinical and computed tomographic diagnosis of orbital glioma and meningioma. Ophthalmology. 1984; 91(2):137–155.
- Leonard JR, Perry A, Rubin JB, King AA, Chicoine MR, Gutmann DH. The role of surgical biopsy in the diagnosis of glioma in individuals with neurofibromatosis-1. Neurology. 2006; 67(8):1509–1512.
- Sawamura Y, Kamada K, Kamoshima Y, et al. Role of surgery for optic pathway/hypothalamic astrocytomas in children. Neuro-oncology. 2008; 10(5):725–733.
- Goodden J, Pizer B, Pettorini B, et al. The role of surgery in optic pathway/hypothalamic gliomas in children. J Neurosurg Pediatr. 2014; 13(1):1–12.
- Balcer LJ, Liu GT, Heller G, et al. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001; 131(4):442–445.
- Segal L, Darvish-Zargar M, Dilenge M-E, Ortenberg J, Polomeno RC. Optic pathway gliomas in patients with neurofibromatosis type 1: follow-up of 44 patients. J AAPOS. 2010; 14(2):155–158.
- Thomas RP, Gibbs IC, Xu LW, Recht L. Treatment options for optic pathway gliomas. Curr Treat Options Neurol. 2015; 17(2):333.
- Parsa CF, Hoyt CS, Lesser RL, et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch Ophthalmol. 2001; 119(4):516–529.
- Spicer GJ, Kazim M, Glass LR, et al. Accuracy of MRI in defining tumor-free margin in optic nerve glioma surgery. Ophthal Plast Reconstr Surg. 2013; 29(4):277–280.
- Shriver EM, Ragheb J, Tse DT. Combined transcranial-orbital approach for resection of optic nerve gliomas: a clinical and anatomical study. Ophthal Plast Reconstr Surg. 2012; 28(3):184–191.
- Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006; 24(16):2570–2575.
- Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999; 45(3):393–396.
- Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer. 2010; 46(12):2253–2259.
- Lafay-Cousin L, Sung L, Carret A-S, et al. Carboplatin hypersensitivity reaction in pediatric patients with low-grade glioma: a Canadian Pediatric Brain Tumor Consortium experience. Cancer. 2008; 112(4):892–899.
- Gururangan S, Fisher MJ, Allen JC, et al. Temozolomide in children with progressive low-grade glioma. Neuro-oncology. 2007; 9(2):161–168.
- Kuo DJ, Weiner HL, Wisoff J, Miller DC, Knopp EA, Finlay JL. Temozolomide is active in childhood, progressive, unresectable, low-grade gliomas. J Pediatr Hematol Oncol. 2003; 25(5):372–378.
- Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014; 132(1):111–114.
- Cardellicchio S, Bacci G, Farina S, et al. Low-dose cisplatin-etoposide regimen for patients with optic pathway glioma: a report of four cases and literature review. Neuropediatrics. 2014; 45(1):42–49.
- Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012; 30(12):1358–1363.
- Mishra KK, Squire S, Lamborn K, et al. Phase II TPDCV protocol for pediatric low-grade hypothalamic/chiasmatic gliomas: 15-year update. J Neurooncol. 2010; 100(1):121–127.
- Alvord EC, Lofton S. Gliomas of the optic nerve or chiasm. Outcome by patients' age, tumor site, and treatment. J Neurosurg. 1988; 68(1):85–98.
- Mishra MV, Andrews DW, Glass J, et al. Characterization and outcomes of optic nerve gliomas: a population-based analysis. J Neurooncol. 2012; 107(3):591–597.
- Stokland T, Liu J-F, Ironside JW, et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro-oncology. 2010; 12(12):1257–1268.
- Rakotonjanahary J, De Carli E, Delion M, et al. Mortality in Children with Optic Pathway Glioma Treated with Up-Front BB-SFOP Chemotherapy. PLoS ONE. 2015; 10(6):e0127676.
- Thiagalingam S, Flaherty M, Billson F, North K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology. 2004; 111(3):568–577.