By Margaret M. Reynolds, MD; Wendy M. Smith, MD
Introduction
The Standardization of Uveitis Nomenclature defines intermediate uveitis (IU) as the subset of uveitis where the major site of inflammation is the anterior vitreous and pars plana.1,2 Pars planitis is a term that is often used interchangeably with intermediate uveitis; however, it is more accurately classified as a type of intermediate uveitis that has no associated systemic disease or infectious etiology.1 IU is an important diagnosis to recognize in children, as studies have demonstrated that children are at risk for vision loss, and a shorter time period from symptom onset to diagnosis is correlated with improved visual outcomes.3
Epidemiology and risk factors
The annual incidence of pediatric uveitis is 4.3-6.9 per 100,000 children under the age of 16 years.4,5,6 Overall, intermediate uveitis affects between 15%-33% of uveitis patients less than 16 years old3,7,8.9.10.11 Younger age of onset has been observed to correlate with worse visual outcomes in some studies, although this risk might be mitigated by the use of systemic immunosuppressive medication.8,12
The majority of pediatric IU cases are bilateral with a slight male predominance.3,7,8,9
Risk factors
Systemic disease is rarely diagnosed in children with intermediate uveitis. Occasionally, IU is seen in patients with juvenile idiopathic arthritis (JIA); however, anterior uveitis is far more common in JIA. Although adult IU can be seen with sarcoidosis, multiple sclerosis (MS), or infectious diseases, pediatric cases are usually idiopathic and presumably primarily immune-mediated/autoimmune.8 An association has been reported between IU and HLA-DR2 and HLA-DR15, which have also been associated with MS.13,14 These HLA associations also suggest an immune predisposition and familial component. Overall, the vast majority of pediatric IU is idiopathic.8
Clinical findings
Symptoms
Pediatric IU is often asymptomatic. External signs of inflammation are absent, and children may not notice or may fail to report decreased vision. Cases have been diagnosed by chance during a routine eye exam, or when parents or teachers notice problems that could be attributed to decreased vision. Children may also present with altered red reflex or misaligned eyes.13 When children with IU do have symptoms, they may report painless decreased vision or floaters.8
Signs
Multiple clinical findings are associated with IU and may confirm the diagnosis. While the defining sign is vitritis, anterior segment involvement may also occur. Anterior uveitis is probably more common in pediatric IU compared to adult cases, but the anterior inflammation should not be the predominant area of inflammation in a case classified as IU.15 Anterior segment signs can include band keratopathy in 6% of affected eyes and endotheliopathy.8,13 Small keratic precipitates, peripheral anterior synechiae (approximately 3% of patients), and posterior synechiae (approximately 45%) have also been observed.13
Some degree of vitritis must exist in cases classified as IU. The vitreous inflammation can progress to condensations of coarse vitreous strands, which are termed snowballs and occur in nearly all cases of IU. Snowballs are composed of epithelioid cells and acellular components. They typically occur near the ora serrata and have a yellow-white, globular appearance. Up to 65% of IU cases have snowbanks, which are congregations of snowballs forming membranes of collagen, fibroblasts, and astrocytes. The snowbanks have a yellowish-white appearance and typically cover the pars plana, starting inferiorly and migrating superiorly. If snowbanking is chronic, it can evolve into a fibrotic, cyclitic membrane, leading to vitreous traction and retinal detachment (Figure 1). Finally, posterior vitreous detachment is common.13,16
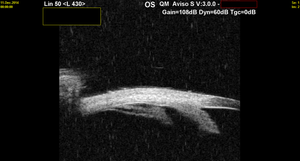
Figure 1. B-scan ultrasonography shows vitreous opacities along the inferior pars plana in a 7-year-old boy with bilateral intermediate uveitis.
Retinal manifestations include peripheral retinal vasculitis in approximately 90% of patients.13 Clinically, the retinal vasculitis is characterized by sheathing and irregularity of the vessels, primarily involving the venules.17 Up to 25% of pediatric patients also have periphlebitis.18 The sheathing observed on clinical exam often persists even when there is no longer active inflammation, so fluorescein angiography may be necessary to determine if the retinal vasculitis component is still present.
Less common manifestations of IU include retrolenticular cyclitic membrane. Very rarely, macular hole and macular ectopia have been reported; epiretinal membrane may also develop as a result of the inflammation.13,19,20
Finally, optic disc edema and hyperemia have been reported in 68%-71% of pediatric IU patients and might be more common than in adult cases.8,13,19
Diagnosis
The diagnosis of IU is based on clinical findings as defined by the International Uveitis Study Group and include inflammation of the anterior vitreous, peripheral retina, ciliary body, and/or anterior segment.1 Types of IU include pars planitis, which must be idiopathic by definition, with characteristic features of snowbanks and/or snowballs.2 Other types of IU include infections such as Lyme disease, HTLV-1, toxocariasis, syphilis, and bartonellosis, and associated systemic inflammatory conditions such as multiple sclerosis and sarcoidosis. Less commonly, necrotizing systemic vasculitis, Behçets disease, Eales disease, and collagen vascular disease can present as IU. In addition, surgery and trauma can result in vitritis. Masquerades of IU include endophthalmitis (consider in patients with a recent history of eye surgery, intraocular injection, immunocompromise, or parenteral nutrition), and malignancy (retinoblastoma, leukemia, lymphoma.).21
Ancillary testing
Laboratory tests or imaging are not necessary to diagnose IU, but ancillary testing should be performed to rule out associated systemic or infectious causes of inflammation. Typical testing may include a complete blood count with a differential (to evaluate for signs of malignancy and infection), chest x-ray (sarcoidosis and tuberculosis), serum angiotensin converting enzyme (sarcoidosis; although this enzyme is typically elevated in children), ANA (juvenile idiopathic arthritis), tuberculosis testing (skin or serum), and syphilis testing via monoclonal antibody testing for Treponema pallidum (syphilis IgG) or fluorescent treponemal antibody absorption test (FTA) with reflex RPR (rapid plasma reagin).13,21
Fluorescein angiography is helpful to assess the extent of vascular leakage and to identify retinal neovascularization. Characteristic findings include diffuse leakage from capillaries, diffuse or focal areas of leakage of larger venules, and disc and macular leakage (Figure 2). Ocular coherence tomography (OCT) can quantify macular thickening and intraretinal cystoid edema and is less invasive than fluorescein angiography (Figure 3).13 If inflammation obscures the view of the retina, ultrasonography can be useful to assess for cyclitic membranes, vitreous traction or retinal detachment.13 Children unable to tolerate a comprehensive retinal evaluation may require an examination under anesthesia.
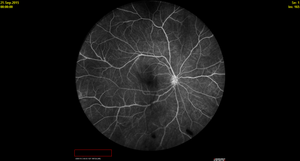
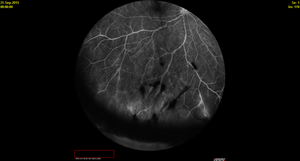
Figure 2. Fluorescein angiography showing focal large vessel (venule) inflammation and shadowing from peripheral vitreous snowballs in a 15-year-old girl diagnosed with intermediate uveitis and multiple sclerosis.

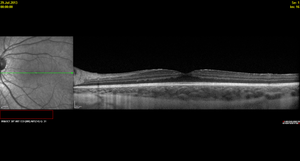
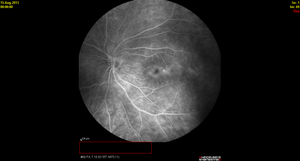
Figure 3. Spectralis OCT demonstrating diffuse macular thickening without frank intraretinal edema and fluorescein angiography showing diffuse small vessel leakage throughout the posterior pole in a 16-year-old girl diagnosed with pars planitis at age 13 years.
Management
If an underlying cause of IU is identified, appropriate treatment should be utilized (eg, antibiotics for infectious organisms, neuro-immunomodulating agents for demyelinating disease.) Since the majority of pediatric IU cases are idiopathic, it may be necessary to assess whether treatment of the uveitis is necessary. Indications for treatment include: significant bilateral disease and decreased visual acuity secondary to vitreous inflammation, macular edema, or vitreous hemorrhage from retinal neovascularization.8
First-line treatment may include periocular corticosteroid injections with triamcinolone; however, if the inflammation does not respond quickly, monotherapy with periocular steroid injections will lead to cataract formation and possibly glaucoma. Topical corticosteroids are not appropriate as monotherapy for IU, but they may be used as an adjunct to treatment in the presence of anterior uveitis, again with caution regarding cataract formation and the development of glaucoma. Alternatively, systemic corticosteroids can be utilized as a first-line treatment for bilateral or severe, vision-threatening inflammation, especially since children are at risk for amblyopia due to media opacity. If multiple periocular steroid injections are necessary in a short time period, then systemic corticosteroids and steroid-sparing medication should be considered. The typical systemic prednisone dose is 1-1.5 mg/kg/day for 2-3 weeks, followed by a slow taper if the inflammation decreases. The chronic use of steroids should be avoided, since children are at risk for multiple side effects including potentially irreversible effects on growth and development. If uveitis recurs when systemic steroids are tapered, then systemic steroid-sparing medication must be considered. The child should be evaluated by a uveitis specialist if this referral has not already occurred.8,22
Up to 15 percent of patients have persistent inflammation despite periocular and/or systemic corticosteroids. Systemic steroid-sparing immunosuppressives including cyclosporine, azathioprine, methotrexate, and mycophenolate mofetil may be used to treat pediatric IU. Methotrexate is often the first line steroid-sparing agent and is often well-tolerated in oral or subcutaneous injection formulations. In a study of pediatric anterior and intermediate uveitis patients, low-dose methotrexate over 4 to 40 months improved vision in all cases, and 60% had decreased inflammation.23
Azathioprine in combination with corticosteroids resulted in clinical improvement in 61% of patients.19 Mycophenolate mofetil plus corticosteroid therapy demonstrated similar results with decreased inflammation in 14 of 17 patients.24 Generally, mycophenolate is thought to be safer than azathioprine for children since it only impairs DNA synthesis in lymphocytes.
Cyclosporine, a calcineurin inhibitor, controlled inflammation in 13 of 14 pediatric uveitis patients, including 5 with intermediate uveitis.25 Although hypertension and renal insufficiency are the most serious adverse effects associated with cyclosporine, these complications are usually less common in children compared to adults. Tacrolimus is another calcineurin inhibitor, although there is less data regarding the use of this medication for pediatric intermediate uveitis.
Finally, biologic agents such as tumor necrosis factor inhibitors have been demonstrated to have efficacy as treatment for pediatric uveitis. Both infliximab and adalimumab have successfully controlled pediatric uveitis.26-30 In a study of 6 pediatric patients with uveitis, one of whom had IU, all patients had reduction in intraocular inflammation.27
Other therapies that have been utilized to treat pediatric IU include peripheral ablation of the pars plana snowbank with cryotherapy or panretinal photocoagulation (PRP) and pars plana vitrectomy. Cryotherapy and PRP are controversial; they might improve cystoid macular edema and decrease or stabilize inflammation, although fluorescein angiography may demonstrate that persistent inflammation simply moves more posterior to the ablated areas.31-33 Many patients who receive PRP or cryotherapy continue to require periocular steroids and/or systemic immunomodulatory medication. If retinal neovascularization is present, PRP to surrounding retina is appropriate. Tractional retinal detachments may also require laser barrier to prevent progression.
Finally, pars plana vitrectomy (PPV) has been reported to improve macular edema, possibly by decreasing the presumed “antigenic load” in the vitreous. This surgical treatment may be more beneficial for children than adults. Limited data in the literature includes 2 studies which reported improved visual acuity and resolution of macular edema after PPV.34,35 In general, PPV is not the first line treatment for pediatric IU and is not widely used. PPV may be indicated to treat a complication of IU, non-clearing vitreous hemorrhage due to retinal neovascularization.36
Complications
Complications stem from both the disease and the treatment. The most visually devastating complication of IU is cystoid macular edema, which has been observed in 44%-63% of patients.19,37 CME can be diagnosed by clinical exam. More subtle cases may require optical coherence tomography (OCT) and fluorescein angiography to confirm the diagnosis.
Other complications of uveitis include glaucoma, which has been reported in 7%-8% percent of patients. The inflammatory subtype of secondary glaucoma is rare in IU, but more likely if systemic disease such as sarcoidosis is present. Secondary angle-closure glaucoma can also develop as a result of anterior or posterior synechiae.9 Steroid-induced ocular hypertension occurs in 13%-23% of pediatric IU patients and may also result in secondary glaucoma.13
Retinal vasculitis can cause ischemia, leading to neovascularization of the retina, optic disc and/or snowbank. Tractional on neovascularization can result in vitreous hemorrhage.15 Younger age at diagnosis has been demonstrated to be a risk factor for vitreous hemorrhage.38.
Retinal detachment has been reported in 3%-5% of IU patients.13,19 Retinal neovascularization increases the risk of retinal detachment.17 Inflammatory exudates or larger fibrotic or neovascular membranes can lead to tractional retinal detachment.12,13
Inflammation and corticosteroids can cause cataract which occurs in 25%-48% of IU patients.13 Cataract surgery in the pediatric uveitis patient should not be performed unless the inflammation is very well controlled. Implantation of an intraocular lens is somewhat controversial, although it is becoming more common.39-41
Prognosis
Prognosis in pediatric IU varies, although good visual acuity is usually preserved in most types. Several patterns of IU typically occur. The first is a low-grade, self-limiting disease that gradually improves (10%). The second is a prolonged course without exacerbations (59%). The third is a chronic course with more than one exacerbation (31%).42 About 5% of patients have rapid, severe inflammation that is resistant to treatment. This form seems to be more common in children and is associated with pars plana exudates/snowbanks.12
It is difficult to predict which course an individual will take. Interestingly, visual prognosis is more linked to the severity of the inflammation rather than the duration of the disease; although both play a role.8,10 The most important risk factor for vision loss is the presence of macular edema. Although IU often “burns out” after 5-15 years, the inflammation can also persist for 15 to 20 years.13 It is not uncommon for the disease to last 15-20 years.
- Bloch-Michel E, Nussenblatt RB, International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. American Journal of Ophthalmology, 1987. 103(2): 234-235.
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. American Journal of Ophthalmology, 2005. 140(3): 509-516.
- Kump, LI, et al., Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology, 2005. 112(7): 1287-1292.
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004. 111(3): 491-500; discussion 500.
- Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in english primary and referral centers. Am J Ophthalmol. 2003. 135(5): 676-680.
- Païvönsalo-Hietanen T, Tuominen J, Saari KM. Uveitis in children: population-based study in Finland. Acta Ophthalmol Scand. 2000. 78(1): 84-88.
- Tugal-Tutkun I, Havrlikova K, Power WJ, Foster CS. Changing patterns in uveitis of childhood. Ophthalmology, 1996. 103(3): 375-383.
- de Boer J, Berendschot TT, van der Does P, Rothova A. Long-term follow-up of intermediate uveitis in children. Am J Ophthalmol, 2006. 141(4): 616-621.
- Rosenberg K., Feuer WJ, Davis JL, Ocular complications of pediatric uveitis. Ophthalmology. 2004. 111(12): 2299-2306.
- Jain R, Ferrante P, Reddy GT, Lightman S. Clinical features and visual outcome of intermediate uveitis in children. Clin Exp Ophthalmol. 2005. 33(1): 22-25.
- Giles CL. Pediatric intermediate uveitis. J Pediatr Ophthalmol Strabismus. 1989. 26(3): 136-139.
- Brockhurst RJ Schepens CL. Uveitis. IV. Peripheral uveitis: the complications of retinal detachment. Arch Ophthalmol. 1968. 80(6): 747-753.
- Arellanes-García L, Navarro-López P, Concha-Del Río LE, Unzueta-Medina JA. Idiopathic intermediate uveitis in childhood. Int Ophthalmol Clin. 2008. 48(3): 61-74.
- Tang WM, Pulido JS, Eckels DD, Han DP, Mieler WF, Pierce K. The association of HLA-DR15 and intermediate uveitis. Am J Ophthalmol. 1997. 123(1): 70-75.
- Hogan MJ, Kimura SJ, O'Connor GR. Peripheral retinitis and chronic cyclitis in children. Trans Ophthalmol Soc UK. 1965. 85: 39-52.
- Yoser SL, Forster DJ, Rao NA. Pathology of intermediate uveitis. Dev Ophthal. 1992. 23: 60-70.
- Arellanes-Garcia, L., L. Navarro-Lopez, and C. Recillas-Gispert, Pars planitis in the Mexican Mestizo population: ocular findings, treatment, and visual outcome. Ocul Immunol Inflamm. 2003. 11(1): 53-60.
- Malinowski SM, Pulido JS, Folk JC. Long-term visual outcome and complications associated with pars planitis. Ophthalmology. 1993. 100(6): 818-824; discussion 825.
- Capone A, T.M.A., Intermediate uveitis. In Albert DM, Jakobiec FA. Principles and practice of ophthalmology. Philadelphia: WB Saunders Company. 1994: 423–442.
- Lai HC, FitzSimmons SC, Allen DB, Kosorok MR, Rosenstein BJ, Campbell PW, Farrell PM. Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N Engl J Med. 2000. 342(12): 851-859.
- Malik AR, Pavesio, C. The use of low dose methotrexate in children with chronic anterior and intermediate uveitis. Br J Ophthalmol. 2005. 89(7): 806-808.
- Doycheva D, Deuter C, Stuebiger N, Biester S, Zierhut M. Mycophenolate mofetil in the treatment of uveitis in children. Br J Ophthalmol. 2007. 91(2): 180-184.
- Kilmartin DJ, Forrester JV, Dick AD. Cyclosporin A therapy in refractory non-infectious childhood uveitis. Br Ophthalmol. 1998. 82(7): 737-742.
- Ardoin SP, Kredich D, Rabinovich E, Schanberg LE, Jaffe GJ. Infliximab to treat chronic noninfectious uveitis in children: retrospective case series with long-term follow-up. Am J Ophthalmol. 2007. 144(6): 844-849.
- Rajaraman RT, Kimura Y, Li S, Haines K, Chu DS. Retrospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology. 2006. 113(2): 308-314.
- Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006. 113(5): p. 860-864 e2.
- Vazquez-Cobian LB, Flynn T, Lehman TJ. Adalimumab therapy for childhood uveitis. J Pediatr. 2006. 149(4): 572-575.
- Biester S, Deuter C, Michels H, et al. Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol. 2007. 91(3): 319-324.
- Aaberg TM, Cesarz TJ, Flickinger RR. Treatment of peripheral uveoretinitis by cryotherapy. Am J Ophthalmol. 1973. 75(4): 685-688.
- Devenyi RG, Mieler WF, Lambrou FH, Will BR, Aaberg TM. Cryopexy of the vitreous base in the management of peripheral uveitis. Am J Ophthalmol. 1988. 106(2): 135-138.
- Pulido JS, Mieler WF, Walton D, et al. Results of peripheral laser photocoagulation in pars planitis. Trans Am Ophthalmol Soc.1998. 96: 127-137; discussion 137-141.
- Kroll P, Romstöck F, Grenzebach UH, Wiegand W. [Early vitrectomy in endogenous juvenile uveitis intermedia--a long-term study]. Klinische Monatsblatter fur Augenheilkunde. 1995. 206(4): 246-249.
- Trittibach P, Koerner F, Sarra GM, Garweg JG. Vitrectomy for juvenile uveitis: prognostic factors for the long-term functional outcome. Eye (Lond). 2006. 20(2): 184-190.
- Potter MJ, Myckatyn SO, Maberley AL, Lee AS. Vitrectomy for pars planitis complicated by vitreous hemorrhage: visual outcome and long-term follow-up. Am J Ophthalmol. 2001. 131(4): 514-515.
- Henderly DE, et al., The significance of the pars plana exudate in pars planitis. Am J Ophthalmol. 1987. 103(5): 669-671.
- Lauer AK, Smith JR, Robertson JE, Rosenbaum JT. Vitreous hemorrhage is a common complication of pediatric pars planitis. Ophthalmology. 2002. 109(1): 95-98.
- Michelson JB, Friedlaender MH, Nozik RA. Lens implant surgery in pars planitis. Ophthalmology. 1990. 97(8): 1023-1026.
- Tessler HH, Farber MD. Intraocular lens implantation versus no intraocular lens implantation in patients with chronic iridocyclitis and pars planitis. A randomized prospective study. Ophthalmology. 1993. 100(8): 1206-1209.
- Ganesh SK, Babu K, Biswas J. Phacoemulsification with intraocular lens implantation in cases of pars planitis. J Cataract Refract Surg. 2004. 30(10): 2072-2076.
- Smith RE, Godfrey WA, Kimura SJ. Complications of chronic cyclitis. Am J Ophthalmol. 1976. 82(2): 277-282.