This chapter was reviewed for currency and updated in April 2021 by Sapna Gangaputra, MD, MPH, and H. Nida Sen, MD, MHS.
Introduction
The Standard of Uveitis Nomenclature defines posterior uveitis as inflammation primarily involving the retina and/or choroid. Panuveitis is diagnosed if the anterior chamber and vitreous are also inflamed.1 Uveitis accounts for 5% to 20% of blindness in the United States and Europe, and up to 25% of blindness in the developing world.2 Uveitis prevalence is lower in children, seen in about 30 cases per 100,000 compared to 93 per 100,000 in adults.3 In reports from tertiary uveitis referral centers, pediatric cases account for 5% to 30% of all uveitis.4 Despite a lower incidence of uveitis in children compared to adults, children may be more likely to suffer vision loss.5 In addition to amblyopia, in the case of posterior and panuveitis, vision loss in children may be caused by direct inflammatory involvement of the macula and optic nerve.6 Pan- and posterior uveitis are generally believed to be the least common categories of pediatric uveitis, representing 14% to 21% and 6% to 30% of pediatric uveitis respectively,7 although some reports rank it as high as 40%.8 Depending on geographic location, infectious etiologies, most commonly toxoplasmosis, account for 13% to 50% of pediatric uveitis are.2, 3, 9 Consistent with these data, a recent large case series and review of the literature from the Netherlands found 60 of 345 (17%) cases of pediatric uveitis to be infectious. The most prevalent pathogen was toxoplasmosis (60%), followed by viral infections (30%), most of which were varicella-zoster (VZV), almost one-third of which were acute retinal necrosis (ARN).10 If no infection or systemic association is found, the uveitis is deemed undifferentiated (also known as idiopathic), and assumed to be autoimmune in nature.
Table 1. Posterior and Pan- uveitis in Children
|
Noninfectious
|
Systemic Autoimmunity Syndromes
|
- Sarcoidosis, Familial Juvenile Systemic Granulomatosis
|
|
|
- Tubular Interstitial Nephritis and Uveitis Syndrome (TINU)
|
- Systemic Lupus Erythematosus (SLE)
|
- Systemic Necrotizing Vasculitides
|
Predominantly Ocular Diseases
|
- Vogt-Koyanagi-Harada Syndrome
|
|
|
- Inflammatory Chorioretinopathies (White Dot Syndromes)
|
Infectious
|
Viral
|
- Herpetic Retinitis (HSV, VZV, CMV)
|
- Lymphocytic Choriomeningitis Virus (LCMV)
|
|
|
|
|
|
|
|
|
Bacterial
|
|
|
|
|
- Cat Scratch Disease (Bartonella henselae)
|
- Lyme Disease (Borrelia burgdorferi)
|
|
|
|
|
Parasitic
|
|
|
|
|
- Diffuse Unilateral Subacute Neuroretinitis
|
|
|
Approach to the patient
Timely detection of uveitis in children may be difficult, especially in preverbal patients and in those with posterior-predominant uveitis without overt signs such as tearing or redness. Once uveitis is noted by the ophthalmologist, a thorough review of systems, inquiring specifically about trauma, environmental and dietary exposures, vaccination status, and systemic symptoms such as fever, rash, joint pain, and malaise should be elicited. It is also critical to obtain a full past medical history and birth history, including maternal infections and exposures. In addition to the ophthalmic exam, the physical examination should include vital signs (with weight for purposes of medication dosing), a basic constitutional and neurological surveillance, and inspection of skin for rashes and of joints for deformities. Ocular and systemic imaging and laboratory testing should be tailored to the patient based on level of clinical concern.
If clinical suspicion points to uveitis as a manifestation of a systemic disease, a team approach, with a pediatrician, infectious disease specialist, and/or rheumatologist is critical. Complete, prompt and sustained inflammatory control with treatment of amblyopia is vital to visual preservation and rehabilitation. In cases of undifferentiated (idiopathic) disease or in those with diagnostic uncertainty, the physician should remain cognizant of the possibility of masquerade syndromes, such as retinoblastoma, leukemia, juvenile xanthogranuloma, Coats disease, and inherited degenerative and/or vascular diseases of the retina, discussed elsewhere in this series.
Work up and diagnosis
There is no single standardized work up for pediatric posterior and panuveitis. The clinical features of uveitis guide the practitioner in determining the appropriate diagnostic testing. Occlusive retinal vasculitis with retinitis may guide the work up towards Behçet disease in a patient with oral and genital ulcers and neurological symptoms. Similar disease characteristics in an immunocompetent patient with unilateral disease may warrant consideration of acute retinal necrosis, with possible aqueous or vitreous sampling. Ruling out latent or chronic asymptomatic infections is critical prior to initiating long-term immunomodulatory therapy. Based on patient demographics and uveitis characteristics, the following diagnostic testing may be appropriate: For tuberculosis, interferon gamma release assay (IGRA) (QuantiFERON®-TB Gold In-Tube test or T-SPOT®.TB test), or tuberculin skin testing; for sarcoidosis, ACE, lysozyme and chest-x ray; for syphilis, treponemal (T pallidum EIA test, TP-PA test, and FTA-ABS test) and non-treponemal tests (RPR and VDRL); serum enzyme-linked immunosorbent assay (ELISA) testing, with or without Western blot confirmation. Disease-specific IgM and IgG may be appropriate when there is suspicion for toxoplasmosis, bartonella, herpesviridae, Lyme disease and others, although results must be interpreted with caution, as a positive IgG test indicates systemic exposure, but does not diagnose ocular disease. For systemic vasculitides and collagen vascular disease, cANCA (anti-PR3), pANCA (anti-mpo), ANA; for tubulointerstitial nephritis and uveitis (TINU), urinalysis and urine beta-2 microglobulin, with renal biopsy when indicated. Adjunctive testing with lumbar puncture and brain imaging may be useful in determining neurological involvement when warranted by clinical suspicion. Ocular inflammatory disease presents with reduced inflammation in patients who are immunocompromised (secondary to HIV or otherwise), as they are unable to mount an appropriate inflammatory response. Additionally, the differential diagnosis broadens in the context of the potential for unexpected opportunistic infections. Finally, HIV is frequently coexistent with syphilis, and the 2 should be considered concomitantly.
Diagnostic retinal imaging is often useful in further classification of the clinical features of posterior and panuveitis. Color fundus photographs may be helpful in documenting degree of vitreous haze, and extent and progression of chorioretinal lesions, particularly in children, in whom examination may be difficult. Handheld or wide-field photography is particularly useful in the pediatric population.
Optical coherence tomography (OCT) is a noninvasive imaging modality useful in refining posterior segment disease location and assessing prognostic factors for visual acuity outcome. Optic nerve atrophy and focal nerve fiber layer loss may be seen as long-term sequelae of hypertensive uveitis, papillitis or occlusive vasculitis at the nerve head. Disc edema may be present in many types of panuveitis. Diffuse retinal thickening, cystoid edema, selective layer loss, and inflammatory deposits are common features of many forms of panuveitis. OCT is also critical in the diagnosis of choroidal neovascular membrane and subretinal scarring, complications of many types of posterior uveitis. Subretinal fluid may be seen as a part of the uveitis syndrome or as a consequence of the treatment (central serous retinopathy from steroid exposure). Extended depth imaging and combined depth OCT imaging of the choroid is an emerging imaging modality that may show promise for monitoring disease activity in a variety of pediatric posterior uveitides, including VKH, SO, Behçet disease, toxoplasmosis and others.11 ,12, 13 (Figure 1)
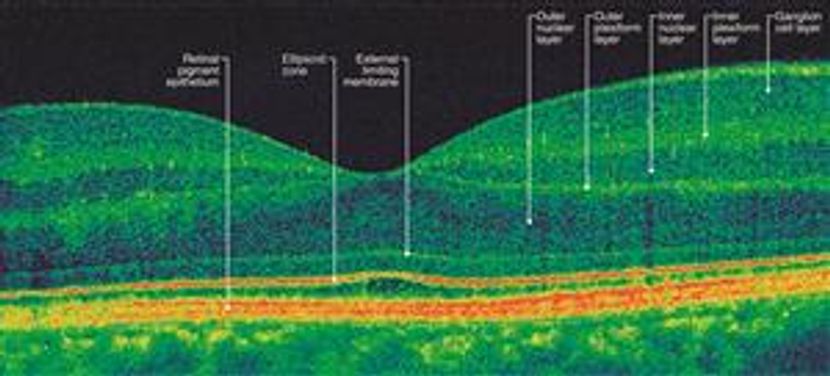
Figure 1A. OCT image of normal macular anatomy. Adapted, with permission, from Foster BS, et al. Optical Coherence Tomography in the Management of Retinal Disorders. Focal Points: Clinical Modules for Ophthalmologists, Module 11, 2006.
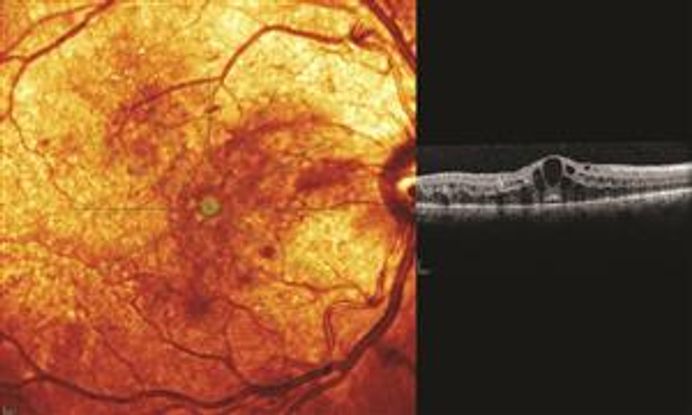
Figure 1B. OCT image of cystoid macular edema.
Fundus autofluorescence (FAF) is another noninvasive imaging modality that highlights lipofuscin accumulation within the RPE. Increased FAF is expected in the presence of increased RPE metabolic activity (active inflammation), and decreased FAF in the setting of loss of photoreceptors or the RPE (inactivity). It is a useful adjunct in multi-modal imaging of the posterior segment, with applications for many posterior uveitides, including white dot syndromes, VKH and infectious uveitis.14 (Figure 2)

Figure 2. FAF image of the white dot syndrome, Acute Zonal Occult Outer Retinopathy (AZOOR).
Fluorescein angiography (FA) and indocyanine green angiography (ICG) are specialized forms of posterior photography that rely on intravenous injection of the fluorescent molecules fluorescein sodium and indocyanine green dye, respectively. The molecular characteristics and emission spectrum make fluorescein useful in assessing retinal, and to a certain extent choroidal, vessels and structures. Frequently, the bulk of inflammatory cells in posterior uveitic lesions block background fluorescence in the early phases of the angiogram, and after recirculation and macrophage phagocytosis, late hyperfluorescence may be seen. Staining or blockage of retinal vasculature is diagnostic of retinal vasculitis. Attention should be paid to arterial versus venous involvement, as systemic collagen vascular diseases, ARN syndrome, and Behçet disease are known for occlusive arteritis. FA remains the gold standard for diagnosis of choroidal neovascular membrane. Indocyanine green (ICG) is a large, iodine-based molecule that remains within choroidal vasculature and is useful in highlighting choroidal circulation and choroidal inflammatory lesions. It should not be given to patients with iodine or shellfish allergies.
OCT angiogram is a newer noninvasive method of assessing for ocular blood flow through the retinal and choroidal circulation. It can be helpful for assessing choroidal lesions, superficial and deep retinal vasculature, and choroidal neovascular membranes.
Treatment options
Infectious posterior uveitic entities are treated with the appropriate anti-infectious agent, sometimes followed by judicious addition of corticosteroid to treat the post-infectious inflammatory component of disease. The gold standard for treatment in noninfectious uveitis is systemic or local corticosteroids. While corticosteroids are highly efficacious in controlling ocular inflammation, the ocular and systemic side effects can be significant. Among the known side effects of corticosteroids are hunger, hyperglycemia, weight gain, water retention, insomnia, systemic hypertension, osteopenia, hip necrosis, muscle weakness, immune suppression and poor wound healing, elevated intracranial pressure, mood changes, and adrenal suppression. Longer-term side effects include skin changes, development of diabetes mellitus, osteoporosis, Cushingoid changes of habitus (including ‘moonlike’ facies and the typical ‘buffalo hump’ on the neck), hyperlipidemia, and hypertension. Ocular adverse events may include posterior subcapsular cataract, ocular hypertension, and central serous retinopathy. Children are especially vulnerable to steroid-responsive ocular hypertension and cataract, so local steroids must be used judiciously. In the setting of systemic administration, children are particularly susceptible to growth retardation, osteoporosis, and adrenal suppression.15
The typical paradigm for the treatment of noninfectious uveitis follows a stepwise approach that starts with the use of local corticosteroids followed by the addition of systemic corticosteroids or immune-modulating agents in refractory cases. In select cases, systemic therapy is required at the initiation of treatment. The rationale for this approach has been outlined previously.16 Steroid-sparing immunomodulating agents are commonly grouped into the categories of antimetabolites (methotrexate, azathioprine mycophenolate mofetil), T-cell inhibitors (cyclosporine, tacrolimus, sirolimus), alkylating agents (cyclophosphamide, chlorambucil), and biologic agents (infliximab, adalimumab, rituximab, and others).
Noninfectious Posterior and Panuveitis
An appropriate infectious work-up must be performed prior to assuming a noninfectious etiology. Systemic immunomodulation and local or systemic corticosteroids can exacerbate infectious uveitis, leading to permanent vision loss or potentially life-threatening systemic disease dissemination. Even if the patient’s clinical presentation is typical of a systemic autoimmune disease, latent or asymptomatic infections must be ruled out prior to initiation of immunomodulatory therapy. Considerations of testing for secondary latent infections must be specific to the demographics of the patient and the region, exposure history, and past medical and social history, but basic considerations prior to initiation of immunomodulatory therapy may include testing for syphilis, tuberculosis, hepatitis, HIV, or other diseases.
Systemic Disease with Non-Infectious Posterior and Panuveitis
Sarcoidosis and Familial Juvenile Systemic Granulomatosis (Blau Syndrome)
Pediatric sarcoidosis is a rare chronic multisystem inflammatory granulomatous disorder of unknown origin. The disease may affect any part of the eye, and intraocular inflammation is typically granulomatous. While it may be isolated to the anterior segment, panuveitis, disc edema, multifocal chorioretinitis, choroidal granulomas and retinitis are reported features in pediatric patients. The characteristic candle-wax periphlebitis seen in adults is not as typical in children.17 Uveitis manifestations are protean, with reports of biopsy proven pediatric sarcoid simulating TINU,18 birdshot chorioretinopathy,19 and tuberculosis.20 Patients under the age of five years tend to have extrapulmonary manifestations of sarcoidosis, and the uveitis may be part of a triad with rash and arthritis. Lung involvement is seen in only one-third of this cohort. Older children (typically aged 8-15 years) with systemic involvement are more likely to show fatigue and multi-organ disease, often with hilar lymphadenopathy.21 Available data in the US suggests that there is no sex predilection, and African Americans are disproportionately affected.22
In a 21-year respective review of 460 pediatric uveitis patients at a tertiary care center in the American Midwest, 13 patients (2.8%) had probable, presumed, or definite sarcoidosis. The mean age was 11.6 years (range: 5 to 16 years). Elevated angiotensin-converting enzyme and lysozyme were elevated in 6 and 5 patients respectively. Five of 12 patients in whom chest imaging was performed had signs of sarcoidosis. Interestingly, in this series, anterior segment involvement was more often nongranulomatous than granulomatous. Seven patients had multifocal choroiditis and 4 patients had retinal periphlebitis.23
Due to nonspecific clinical features and the lack of a specific test, confirming the diagnosis of ocular or systemic sarcoidosis can be difficult in the pediatric population. Angiotensin converting enzyme (ACE), lysozyme and serum calcium may be elevated, and anemia, leukopenia, and eosinophilia are often reported. However, the serum ACE level is often physiologically elevated in children, making its significance in the diagnosis of sarcoidosis less meaningful. The presence of noncaseating epithelioid cell granulomas on tissue biopsy is diagnostic.17, 22
Familial Juvenile Systemic Granulomatosis, also known as Jabs-Blau syndrome is also a multi-system granulomatous inflammatory disorder. Inherited in an autosomal dominant fashion due to mutations in the NOD2CARD15 gene on chromosome 16q21.1 (OMIM 605956), it is marked by granulomatous polyarthritis and uveitis, often with rash and vasculopathy. Pulmonary involvement and adenopathy are absent. Patients usually present with a skin rash in the first year of life, polyarthritis within 24 to 48 months, and uveitis around 48 months.24 Patients commonly present with panuveitis and multifocal choroiditis, often severe. Isolated anterior uveitis, and ischemic optic neuropathy have also been described. Frequent ocular complications, including cataracts, glaucoma, band keratopathy, and macular edema are present.25 Expeditious referral to a uveitis specialist and/or pediatric rheumatologist to direct, systemic immunomodulatory therapy is crucial to disease management. Genetic counseling should be completed prior to genetic testing.
Behçet Disease
Behçet disease, with a worldwide distribution highest in Japan, the Middle East, and Mediterranean countries, is multisystemic autoinflammatory vasculitis characterized by mucocutaneous, articular, gastrointestinal ophthalmological and potentially life-threatening cerebral lesions.26 Although mean age of onset is 30 years, it also rarely occurs in infants, children and adolescents, with pediatric patients accounting for 3% to 7% of all cases.27 Recurrent oral and genital ulcers, positive pathergy test, and skin rash may be elicited on the review of systems. Despite the severity of pediatric uveitis in this group, systemic signs and symptoms are typically milder than in adults, resulting in retrospective Behçet diagnosis in many cases. Up to one-third of patients with pediatric Behçet disease have neurologic involvement, so the ophthalmologist must maintain a low threshold to order neuroimaging and consultation for symptoms.28 Ocular manifestations are variable, but hypopyon uveitis with occlusive retinal vasculitis is the classical description. Scleritis, episcleritis, and optic neuritis have also been reported in children.27 A retrospective review of 34 Turkish patients with pediatric Behçet uveitis showed a 70% male predominance, average age of onset at 14, with panuveitis in 53%, posterior uveitis in 32%, and anterior uveitis in 15% of patients. Vision-threatening complications were common.29 Presence of HLA-B51 is supportive but not diagnostic. Family history of Behçet disease is also common among younger children with BD. Diagnosis of Behçet is based on clinical criteria defined by the International Study Group for Behçet Disease.26 Figure 3.



Figure 3. Behcet disease. (Top) mucous membrane ulcers (oral aphthae). (Middle) Hypopyon. (Bottom) Retinal vasculitis.
Tubulointerstitial Nephritis and Uveitis Syndrome (TINU)
TINU is typically characterized by the sudden onset of bilateral anterior uveitis in the setting of inflammatory interstitial renal disease which may be out of phase with the onset of uveitis. Coincident constitutional signs such as fatigue, malaise, and fever are common. Definitive diagnosis is made with renal biopsy, although urinary abnormalities, particularly elevated b2 microglobulin, are common.30 HLA-DRB1*0102 has been found to have a strong association with TINU, but testing may not be practical in standard laboratory settings.31 Although there are some reports of TINU syndrome in elderly patients, it generally occurs in children and young adults. According to early reports females may be affected three times more often than males,32 but a male predominance has been reported more recently.30 Panuveitis and posterior uveitis may also be associated with TINU. A case series of 15 children with otherwise unexplained panuveitis with multifocal choroidal lesions found that 14 had HLA-DR, DQ class II DNA typing consistent with those found in confirmed TINU patients.33 Several other groups have reported similar multifocal choroidal lesions with posterior segment inflammation.34
Systemic Lupus Erythematosus (SLE)
Systemic lupus erythematosus is a multisystem autoimmune disease characterized by presence of circulating antibodies against cell nuclear components (ANA, Anti-dsDNA). It can occur from infancy to old age, with peak diagnosis between ages 15 and 40 years.35 Although adult and pediatric SLE share clinical features, children have more aggressive disease, and one-third of pediatric SLE patients have atypical presentations.36 Inflammatory posterior segment changes can affect the retina, choroid, retinal vasculature, and optic nerve, with retinal involvement second only to keratoconjunctivitis sicca in overall frequency of occurrence. Macular edema and vascular occlusive disease are particularly sight-threatening.37 In a cross-sectional study reporting eye exams in 52 consecutive children with SLE, nearly 35% (18) were found to have ocular abnormalities. Of these patients, microangiopathic retinopathy (4 /18) retinal vaso-occlusive disease (1 /18), optic neuropathy (3/18) and iridocyclitis (1/18) were reported, along with subcapsular cataract, dry eye, and visual field defects.38
Systemic Necrotizing Vasculitides (SNVs)
Childhood-onset ANCA-associated vasculitides (granulomatosis with polyangiitis [GPA], microscopic polyangiitis [MPA], and eosinophilic granulomatosis with polyangiitis [Churg-Strauss, EGPA]), and polyarteritis nodosa (PAN) are rare systemic necrotizing vasculitides of medium and small arteries. Childhood primary vasculitis is diagnosed in 3% of all children referred to US pediatric rheumatology clinics. 39 A large French study of 1,286 pediatric patients with SNVs found ophthalmologic involvement in 214 (16.6%). GPA had the highest degree of eye findings, with 34.1% in this study and a range of 29% to 57% in the literature. Posterior segment involvement (retinal vasculitis and optic neuropathy) is less common than anterior segment (conjunctivitis, episcleritis). Orbital involvement is fairly specific to GPA.40
Predominantly Ocular Noninfectious Posterior and Panuveitis
Sympathetic Ophthalmia (SO) and Vogt-Koyanagi-Harada Syndrome (VKH)
SO and VKH are bilateral granulomatous panuveitides thought to be due to a T-cell mediated autoimmune response to retinal/uveal antigens, resulting in inflammatory infiltration of the choroid and, to a lesser extent, the iris and ciliary body. Sympathetic uveitis occurs when penetrating ocular trauma or multiple surgeries in the exciting eye allows for systemic exposure to uveal antigens, resulting in inflammation in both the exciting injured eye and contralateral sympathizing eye; VKH requires no prior ocular injury, but is strongly associated with the human leukocyte antigen (HLA) class II, specifically HLA-DR4, and is typically seen in darker-pigmented individuals, predominantly Asians, South Asians, Hispanics/Latinos, and Native Americans. In a genetically predisposed individual, it may be triggered by environmental exposure or viral illness.41 Both entities are typified by iridocyclitis, vitritis, optic disc edema, and choroidal inflammation manifested as focal Dalen-Fuchs nodules or exudative retinal detachments,42 and/or with multiple areas of pinpoint hyper and hypofluorescence on fluorescein angiography. The differential diagnosis for Dalen-Fuchs nodules includes SO, VKH, and sarcoidosis. Dalen-Fuchs nodules are epithelioid cells containing pigment between the RPE and Bruchs membrane. VKH, much more than SO, is associated with central nervous system signs and symptoms, including CSF pleocytosis on lumbar puncture, meningismus, tinnitus, and dysacusis, and later in the disease course, cutaneous findings such as alopecia, vitiligo, and poliosis. Approximately 3% to 15% of VKH cases begin in childhood. Pediatric patients with VKH syndrome present with similar but more severe manifestations and complications than adults.43 Depending on geographic location, VKH comprises 0.7% to 3% of pediatric uveitis.44 SO is rare, affecting 0.03/100,000 persons per year.45 However, pediatric ocular injuries account for up to 43% to 50% of all eye injuries.46
A large study from India examined the records of 2511 pediatric ruptured globes, and found that 6 patients (0.24%) developed SO, mirroring the rates described in other series.47 Debate exists over whether eyes with no light perception should be enucleated as prophylaxis for SO. Traditional teaching suggests that enucleation within 2 weeks of penetrating ocular trauma in an eye with no visual potential may reduce the risk of SO. However, there has been at least one report of disease onset within 5 days of injury. There is also a report of SO presenting after enucleation, therefore the value of enucleation has been controversial. Standard of care is treatment with systemic corticosteroids and immunosuppressive therapy. Enucleation of the exciting eye is not considered effective once disease is present.48 Figure 4.
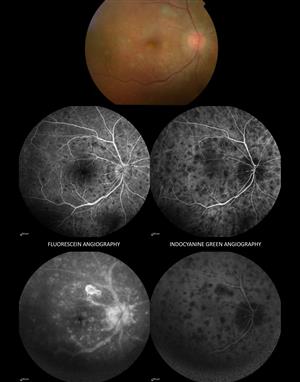
Figure 4. Sympathetic ophthalmia.
White dot syndromes
The term “white dot syndromes” describes a group of heterogeneous inflammatory disorders marked by multiple whitish-yellow lesions located at the level of the outer retina, retinal pigment epithelium, and choroid. With the exception of birdshot chorioretinopathy (BSCR) and serpiginous choroidopathy (SC), which typically occur in older patients, the white dot syndromes are usually seen in patients in early adulthood, though occasionally presentation dips into the pediatric population. The group that can affect children includes acute posterior multifocal placoid pigment epitheliopathy (APMPPE), multiple evanescent white dot syndrome (MEWDS), multifocal choroiditis with panuveitis (MFC), and punctate inner choroiditis (PIC). Despite their distinct presentations, several of the white dot syndromes share common clinical features. It has been speculated that MFC, PIC, and MEWDS are spectrum disorders of a single disease entity with female predominance, enlarged blind spot, photopsias, and reduced ERG.49, 50
Patients with MEWDS typically present with unilateral macular granularity and multiple subretinal white dots, which may form wreath-like patterns of early hyperfluorescence and late staining on fluorescein angiography. The patients are usually myopic, and a viral prodrome is present in up to one-third of cases. Prognosis without treatment is generally good. Similarly, patients with APMPPE also have a sudden onset; however, it is typically bilateral and symmetric, with minimal vitritis and larger, patchy placoid; white spots with early blockage; and late stain appearance on fluorescein angiography. Strict attention should be paid to any neurological symptoms, as CNS vasculitis has been reported. Resolution without treatment is typical, although corticosteroids are required in cases with CNS involvement, and in some cases of recalcitrant macular involvement. MFC and PIC are associated with myopia and may have a more insidious onset. Both are more likely to be bilateral, with discrete yellow or punched-out subretinal spots. MFC has moderate vitritis, while PIC has minimal or no vitreous cell. PIC, in particular, is strongly associated with choroidal neovascularization (CNV). In addition to treatment of CNV, immunosuppressive, or immunomodulatory inflammatory control may be necessary for progressive choroiditis.49
In a retrospective review of 407 consecutive pediatric patients with uveitis in Germany, white dot syndromes were found in 1% to 5% of patients: APMPPE in 4 patients, MEWDS in 4, MCP in 3 and SC in 1 patient.51 An Indian study of 70 patients with serpiginous choroiditis reported one 11-year-old child in their cohort.52 A 20-year study in the of white dot incidence in a US cohort showed overall low rates, with age- and gender-adjusted incidence rate per 100,000 persons measuring 0.22 for MEWDS, 0.15 for APMPPE, 0.03 for MCP, and 0.04 for PIC. Among the 12 incident cases total, pediatric cases included MEWDs in an 11-year-old child and a 13-year-old child, and APMPPE and MCP in two 19 year olds.53 There have been several case reports of MEWDS occurring in temporal association with human papilloma virus vaccine and others.5
Infectious Posterior and Panuveitis
Identification of infectious uveitis is most straightforward when there is an acute illness with systemic manifestations pointing to a known infectious etiology that can affect the eye. In this setting, blood testing may show seropositivity or seroconversion specific to the suspected infection. Diagnosis of infectious uveitis is also clear when an anterior chamber or vitreous tap or chorioretinal biopsy can provide tissue for culture, histology, or PCR diagnosis. Due to ocular morbidity, advanced requirements for specimen handling and laboratory interpretation, and low yields, tissue diagnosis in ophthalmology may not be appropriate, available, or meaningful. The course of diagnostic inquiry becomes more difficult in the setting of congenital or latent infections, or emerging, rare, or zoonotic infections. An additional confounding consideration is the fact that an occult infection or an infectious trigger has been proposed as an initiating factor for a number of noninfectious uveitides (eg, white dot syndromes, sarcoidosis, VKH, etc). Identification of characteristic ocular and systemic findings is critical in narrowing the differential and clinching the diagnosis.
Congenital Infections
Infections acquired in utero, during the birth process, or in the neonatal period may have devastating consequences for the infant. The original “TORCH” acronym described a group of clinically similar congenital infections caused by Toxoplasma gondii, rubella virus (salt and pepper fundus, no posterior or panuveitis), cytomegalovirus (CMV), and herpes simplex virus (HSV). Over the past two decades, the list expanded to include “other” infections that can cause posterior or panuveitis such as syphilis, varicella zoster, and lymphocytic choriomeningitis virus.55, 56
Toxoplasmosis
Toxoplasmosis, caused by Toxoplasma gondii, is a widespread obligate intracellular protozoan parasite. Its definitive host is cats, but it can infect almost all species of mammals and birds all over the world. A quarter to a third of the human population is infected with T gondii, 57. As mentioned previously, it may account for up to 50% of pediatric posterior uveitis. The encysted organism can migrate to muscular and neural tissue, including the retina. When the cysts rupture, the organisms are released and cause active disease.
Vertical transmission through the placenta results in a spectrum of clinical findings, which can range from absent signs and symptoms to retinochoroiditis, microphthalmia, cataract, retinal detachment, and optic atrophy, cerebral calcifications, hydrocephalus, micro- or macrocephaly, seizures, fevers, lymphadenopathy and hepatosplenomegaly, and developmental delay. The “classic” Sabin’s tetrad of hydrocephalus or microcephalus, intracranial calcifications, retinochoroiditis, and mental retardation, however, has been recorded in less than 10% of infants with congenital toxoplasmosis.58 Eighty-five percent of patients with subclinical congenital infections are reported to develop retinochoroiditis.59
It is now recognized that postnatally acquired infection is the more frequent cause of ocular toxoplasmosis; however, in the absence of other neurological features, it is difficult to distinguish between the clinical manifestations of congenital and acquired ocular lesions. Both forms of transmission can present with similar rates of recurrences, and delayed onset ocular activity. It has been suggested that bilateral disease and “wagon wheel” type scarring in the macular location are more common in congenital ocular toxoplasmosis.57 Several multi-year studies of pediatric uveitis suggest that ocular toxoplasmosis is present in about 1% to 15% of patients, depending on geographic region. It is more likely to be diagnosed due to symptoms in the active stage in older children, but is most commonly seen incidentally during the inactive stage. 60
In children identified through screening, the risk of retinochoroiditis rises from 10% in infancy to approximately 30% by 12 years of age in children.61 Lesions tend to be more common and more severe in South America because of the predominance of more virulent parasite strains.62 Active disease typically appears as a unilateral patch of necrotizing retinochoroiditis in the presence or absence of an adjacent chorioretinal scar. There is usually associated periphlebitis, and vitritis, often with papillitis and granulomatous anterior segment inflammation, with or without elevated intraocular pressure. The diagnosis is made on clinical (“headlight in the fog”) and serological grounds, but given the worldwide high seroprevalence, the presence of specific IgG antibodies confirms previous exposure to the parasite, thus only supporting the diagnosis. PCR of ocular fluids or Goldmann-Witmer testing, which compares intraocular antibody production to that of serum, may be helpful to establish a definitive diagnosis; however, obtaining these tests may be difficult as few centers have the capability or laboratories that perform these tests.58
Treatment modalities for ocular toxoplasmosis vary, and there is no single universally accepted regimen. The current drugs used in the treatment of T gondii act primarily against the tachyzoite. The encysted form (bradyzoite) is resistant to therapy, which is the reason the disease is prone to recurrences. The gold standard treatment is pyrimethamine, sulfadiazine and leucovorin (folinic acid) for 3-6 weeks, with prednisone 24-48 hours after commencing therapy. Duration of treatment varies by response and prednisone is typically indicated for significant intraocular inflammation (vitritis). There seems to be a correlation with lesion size and durations of active disease.63 Alternative treatments may include azithromycin, clarithromycin, atovaquone, dapsone, and trimethoprim-sulfamethoxazole. Sulfadiazine should be used with caution in renal impairment, and pyrimethamine should not be used in children with G6PD deficiency.64 In the absence of systemic disease, intravitreal clindamycin with dexamethasone may be effective. However, because active ocular toxoplasmosis is typically self-limited in nature, treatment efficacy can be difficult to study. Large scale randomized controlled trials are needed.65 Recurrent toxoplasmic retinitis, especially if threatening the fovea are managed with long-term prophylactic treatment (Figure 5).66
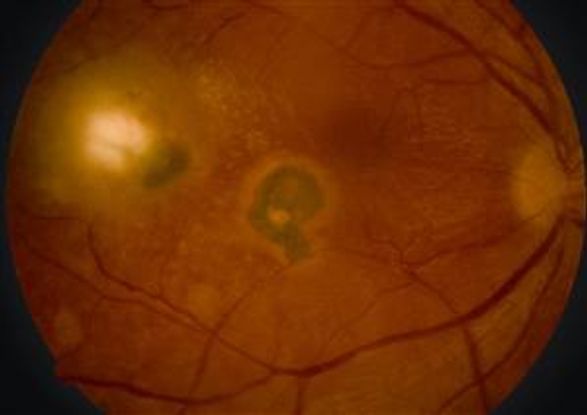
Figure 5. Color fundus photograph demonstrating paracentral and temporal hyperpigmented macular scars. Temporal scar has an area of active retinochoroiditis. Diffuse multifocal outer retinal yellow spots are seen between the two lesions. Segmental arterial sheathing known as Kyrieleis arterioitis is seen on the main arteriole of the inferior arcade.
Herpesviridae
Congenital infection of the double-stranded DNA viruses – cytomegalovirus (CMV), herpes simplex (HSV), and varicella zoster (VZV) – can occur in utero, peripartum, or postnatally, resulting in a variety of clinical presentations, ranging from asymptomatic to pronounced, with high mortality rates and significant long-term morbidity. If viruses are contracted transplacentally during maternal viremia, infants are at risk for developing severe systemic syndromes.67 All three can present with posterior and panuveitis, with necrotizing retinitis, retinal whitening, hemorrhage, vascular sheathing, or an inactive chorioretinal scar that can mimic congenital toxoplasmosis.59 Serologic tests and cultures from both the mother and neonate can help determine the infectious agent. Treatment is with acyclovir for herpes simplex and zoster, and with ganciclovir or foscarnet for cytomegalovirus.
Differentiating between primary acquired versus reactivation of viral retinitis can be difficult due to similarities of clinical presentation. In children beyond the neonatal period, viral retinitis can present as the acute retinal necrosis syndrome (ARN). Most pediatric cases are caused by reactivation of congenital HSV-2 disease. CMV retinitis is typically seen in children who are immunocompromised due to HIV, transplantation and other immunodeficiencies 68
CMV is the most common intrauterine infection, with reported rates ranging from 0.5% to 2.4% of live births.59 However, approximately 90% of congenitally infected newborns exhibit no clinical abnormalities at birth. Newborns with a syndromic presentation can have intrauterine growth retardation, thrombocytopenic purpura, microcephaly, hepatosplenomegaly, jaundice, pneumonia, and sensorineural deafness.59, 67 When CMV retinitis is present, it has a similar appearance to that seen in in immunocompromised adults with retinal whitening and predominant hemorrhage.69 Intravenous ganciclovir or valgancyclovir are used for retinitis, but carry the risk of toxicity and neutropenia. Intravitreal ganciclovir and foscarnet have also been used with success, with reports of late onset and/or reactivation being common.70-72 Figure 6.



Figure 6. . (Top) Classic fulminant retinitis. (Middle) Granular retinitis. (Bottom) Perivascular “frosted branch” cytomegalovirus retinitis.
The incidence of congenital varicella syndrome following maternal primary VZV infection in pregnancy may range from 0.4-9%. The syndrome may result in atrophic limbs, cicatricial skin lesions, cerebral atrophy, and seizures. It is relatively rare in the U.S.67 Funduscopic exam may reveal small or large discrete chorioretinal scars consisting of white, elevated, gliotic centers, each surrounded by an irregular ring of black pigment.69
Neonatal HSV infections are typically caused by maternal genital herpes, acquired by the infant in the perinatal, rather than the intrauterine period. Early studies from the US suggested that most (75%) neonatal HSV was due to HSV-2, however in many parts of the world, HSV-1 now predominates as the strain responsible for neonatal disease. The overall incidence in Western countries is 3-12.2 cases per 100,000 live births.73 However, the true incidence may be higher due to the asymptomatic nature of many neonatal infections. The risk of neonatal disease is more common in births when pregnant women contract primary HSV than in those who have a recurrence of a known past HSV infection (approximately 40% vs. 1%, respectively). The clinical presentation of neonatal HSV-2 disease has been classified into 3 categories: disseminated infection, encephalitis, and skin, eye, and mouth (SEM) infection. Although SEM is commonly recognized by the appearance of skin vesicles during the second week of life, the eye can be the sole site of infection. Often an isolated conjunctivitis is described, but when the retina is affected it may present with whitish-yellow punctate lesions in the posterior pole, with perivascular sheathing and hemorrhage, or with frank necrotizing retinitis and panuveitis. There are also typically atrophic and noninflamed scars with variable border pigmentation.59, 69 A dilated examination should always be performed on patients presenting with neonatal conjunctivitis or herpetic keratitis.
Lymphocytic Choriomeningitis Virus (LCMV)
LCMV is an enveloped RNA virus that is excreted in the feces, urine, and saliva of carrier rodents, predominantly mice. It is estimated that 5% of house mice throughout the United States carry LCMV; human infection has been reported in every continent.35, 30 Congenital infection has been linked to abnormalities of the central nervous system, including hydrocephaly and microcephaly, chorioretinitis and developmental delay. Postnatal infection may cause asymptomatic seroconversion or aseptic meningitis. Infection can produce chorioretinal lesions and scars that mimic ocular toxoplasmosis, in otherwise normal children infected by lymphocytic choriomeningitis virus.56, 59 Treatment is mostly supportive, with judicious use of corticosteroids.
Syphilis
Syphilis is a sexually transmitted disease caused by the spirochete, Treponema pallidum. The rate of congenital syphilis in the United States reached a low of 8.4 cases per 100,000 live births in 2012, but is again on the rise, and increased 38% in 2012–2014.74, 68 Systemic findings in patients with early congenital syphilis include rhinitis, hepatosplenomegaly, desquamative skin rash, low birth weight, pneumonia, and anemia. Manifestations seen later in childhood result from scarring during early systemic disease and include Hutchinson teeth, bony abnormalities, and deafness. Ocular inflammatory signs may present at birth or years later. In the posterior segment, multifocal chorioretinitis, optic disc inflammation, and retinal vasculitis have been described. Scarring from previously active disease is seen as a salt and pepper fundus, or secondary degeneration of the RPE with narrowing of the retinal vasculature and disc pallor, which can mimic retinitis pigmentosa.75 Acquired syphilis must be considered in the differential diagnosis for all type uveitis in a sexually active patient. In the posterior segment, acquired syphilis may present in with chorioretinitis, retinitis, vasculitis, vitritis and panuveitis. Chorioretinitis may be multifocal or placoid, and retinitis may take on a diaphanous, ground glass appearance.76 The rates of acquired syphilis more than doubled from 2000 to 2013, with young men being disproportionately affected, a trend surmised to be linked to unprotected sex in the era of effective HIV treatment. All patients should be tested for HIV, and ocular syphilis should always be treated as neurosyphilis, with patients undergoing lumbar puncture and CSF analysis.77 Treatment with parenteral penicillin for 2 weeks is the treatment of choice for ocular syphilis.78
Predominantly acquired infections
Lyme Disease (LD)
A variety of Ixodes tick species throughout the US, Europe, Asia and Australia transmits Borrelia burgdorferi, the spirochete bacterium responsible for Lyme disease. LD is a multisystem disorder that starts with a red target-like rash at the site of the bite, followed by a stage of relapsing migratory monoarthritis, cardiac conduction abnormalities, meningeal signs, cranial nerve palsies and disc edema, and a third stage of chronic arthritis, neurological symptoms, and chronic uveitis. Though perhaps most well-known for keratitis and intermediate uveitis, LD also can cause panophthalmitis, retinal vasculitis, chorioretinitis and choroiditis. The extreme clinical variability makes diagnosis difficult, and ELISA testing can yield false positives, and must be confirmed with Western blot. Patients treated promptly with antibiotics may never seroconvert. Intravenous ceftriaxone or penicillin for 2 to 3 weeks is a standard treatment to prevent neurologic sequellae.64, 79
Mycobacterium tuberculosis (TB)
TB uveitis poses diagnostic and therapeutic challenges. Given the fact that over 1/3 of the world’s population has been exposed to TB, with 9.6 million new cases in 2014,80 the presence of a positive tuberculin skin test or interferon gamma release assay in a uveitic patient does not imply a causative link. Diagnostic certainty may be obtained by ocular biopsy, but is difficult due to low yield and significant morbidity. In the setting of positive skin or IGRA testing, clinical features suggestive TB posterior or pan- uveitis include granulomatous uveitis, serpiginous or serpiginous-like uveitis, and choroidal granulomas. Treatment for active intraocular TB should be consistent with current WHO guidelines for systemic TB infection. The incidental finding of positive TB-testing (representative of latent TB) in a uveitis patient without classic clinical features or biopsy confirmation should be treated appropriately prior to initiating immunomodulatory therapy for non-infectious uveitis to avoid disseminated disease.81
Cat Scratch Disease
Cat scratch disease is the most common systemic infection related to Bartonella henselae species and usually occurs in immunocompetent patients younger than 20 years of age. The disease is transmitted from the bite or scratch of an infected cat or a kitten. Three to ten days after the inoculation, a papule or pustule may form at the site, and a flu-like illness may develop along with painful adenopathy. The classic intraocular manifestation is that of neuroretinitis, but vitritis, retinitis and choroiditis are also described. Diagnosis is made based on clinical suspicion, as well as serology. A positive IgM test is related to acute disease, but the timeframe of production is limited and may be missed. Bartonella IgG titers less than 1:64 are considered to be negative for infection. Titers between 1:64 and 1:256 characterize possible infection and the test should be repeated in 10–14 days. Titers greater than 1:256 suggest active or recent infection. Mild disease is self-limited and can be treated conservatively. Complicated, disseminated, atypical, or retinal-predominant disease can be treated with doxycycline (or erythromycin depending on patient age) and rifampicin, or azithromycin.82 Figure 7.
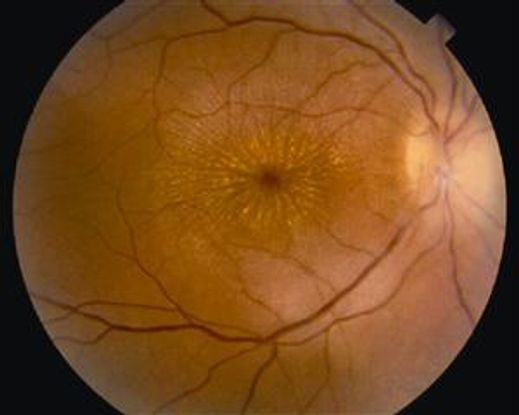
Figure 7. Color fundus photograph demonstrating disc edema and macular star in a patient with neuroretinitis due to cat-scratch disease.
Brucellosis
Human infection with the intracellular coccobacillus species, Brucella, typically occurs after ingestion of unpasteurized milk and dairy products, although dogs, bison, elk, caribou, moose wild hogs, and other animals are also carriers. Systemic disease is clinically classified as subclinical, acute, subacute, and chronic, commonly marked by hepatosplenomegaly, osteoarticular inflammation, and uveitis. Aggressive animal vaccination programs and milk pasteurization have reduced infection rates in industrialized countries, but more than half a million infections per year are reported worldwide. Pediatric disease comprises 3%-10% of reported cases. The uveitis may be anterior or posterior, without a single pathognomonic presentation. Posterior segment inflammation can present as multifocal choroiditis. Serological testing is the most commonly used method for diagnosing brucellosis and includes the agglutination test, ELISA, and polymerase chain reaction test. Treatment is with multi-drug antimicrobial regimens including doxycycline with rifampin or streptomycin. In children, trimethoprim –sulfamethoxazole is typically substituted for doxycycline.35, 64
Other Arthropod Vector-Borne Diseases
Rickettsia species are obligate intracellular gram-negative bacteria typically transmitted to humans by the bite of contaminated arthropods. Three groups comprise the species: spotted fever group, the typhus group, and the scrub typhus group. Systemic involvement is typically characterized by a triad of high fever, headache/malaise, and skin rash in a patient living in or traveling back from an endemic region. Most ophthalmic involvement is self-limited and asymptomatic, but occasionally the vitritis, retinal vasculitis, optic disc staining, white retinal lesions, serous detachments retinal hemorrhages, and choroiditis can become vision threatening. Treatment is typically with doxycycline or fluoroquinolones in older children, or macrolides in younger children.83
Ophthalmic manifestations of systemic West Nile Virus are most likely to occur in patients with neurological involvement. Active chorioretinitis, or inactive chorioretinal scars are usually bilateral, with target-like circular lesions along the course of the retinal vasculature.
Intraocular involvement in Dengue virus can cause bilateral retinal hemorrhages, retinitis, chorioretinitis, retinal vasculitis, or optic neuropathy.
Chikungunya virus is most well known for producing acute anterior uveitis, but posterior pole retinal whitening with macular edema and mild vitritis, have also been described.
Rift Valley fever, caused by the Bunyaviridae, produces fevers with a biphasic temperature curve, headaches, arthralgias and gastrointestinal disturbance. Uveitis with macular or paramacular necrotizing retinitis is the most common finding, with other reports of chorioretinitis, retinal vasculitis, or optic neuropathy. Treatment for these viruses is largely supportive.84
Other Parasitic Entities
Toxocara
Ocular toxocariasis is a manifestation of a parasitic infection by the roundworms, Toxocara canis or Toxocara cati, found in dogs and cats, respectively. A common source of transmission is from infected puppies to children via the fecal/oral route, often through soil contamination.
Ocular disease is caused by the migration of Toxocara larva through blood vessels in the circulatory system into the posterior segment of the eye. Ocular disease may be in the form of a peripheral or central granulomatous nodule with surrounding peripheral membranes, pigmentary scarring and retinal traction. Rarely, a painful red eye with no specific lesions but diffuse inflammation is seen. Testing is by clinical suspicion, and local immunodiagnosis when possible, as serologic tests may be negative when toxocara antigens localize exclusively in the vitreous cavity. Case reports have demonstrated improvement in visual acuity with treatment with corticosteroids and anthelmintics, such as albendazole or mebendazole, especially when signs of inflammation are present. Surgical management may be indicated for tractional retinal detachment.85 Figure 8.
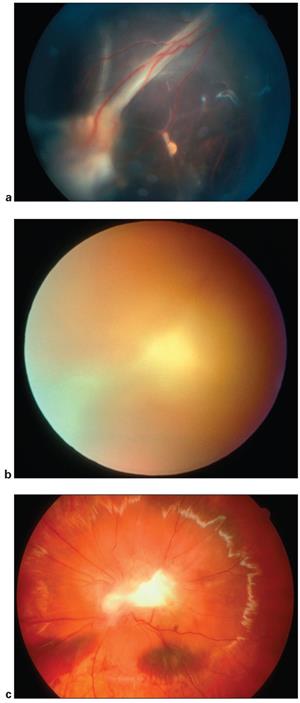
Figure 8. Toxocara infection. a. A 7‑year‑old child with vitreous folds from optic nerve to peripheral Toxocara lesion. b. A 14-year-old boy presents with severe vitreous inflammation. c. Fundus photograph of a 14-year-old boy after diagnostic vitrectomy reveals posterior pole Toxocara granuloma. Vitreous cytology revealed predominance of eosinophils and the Toxocara ELISA was positive. http://www.aao.org/image/itoxocarai-infection.
Diffuse Unilateral Subacute Neuroretinitis (DUSN)
A subretinal nematode, such as Ancylostoma canium, Baylisascaris procyonis, or others, can cause inflammation and retinal degeneration leading to profound vision loss. Early stage disease is characterized by mild to moderate vitritis, mild optic disc edema, and recurrent crops of evanescent, multifocal, yellow-white lesions at the level of the outer retina and choroid. Late, there is optic nerve atrophy, retinal vessel narrowing, and focal or diffuse retinal pigment epithelium degeneration. Identification of the worm followed by photocoagulation is the treatment of choice, but oral treatment with albendazole may be an alternative if the worm cannot be identified.86, 87
Onchocerciasis
Onchocerciasis, or river blindness, is an insect-borne disease caused by the nematode Onchocercas volvulus. It is endemic in about 30 countries in Africa and six countries in central and South America and in Yemen. Infection results in skin rash, subcutaneous nodules and chronic progressive eye disease. When the posterior segment is involved, typically inflammation is modest, but progressive chorioretinal and optic nerve atrophy are seen. Infection is treated with ivermectin in a single oral dose of 150 mg/kg/body weight/6 months.64, 87
Conclusion
Pediatric posterior and panuveitis is a vast topic, caused my numerous infectious and noninfectious etiologic entities. Taking a careful patient and maternal history, performing a complete review of systems, and a conducting a dedicated physical examination are critical in gaining a full understanding of the eye disease. A directed laboratory and diagnostic work up is an important component to refine the differential diagnosis. If the uveitis is thought to be idiopathic (undifferentiated), the ophthalmologist must remain alert to emerging systemic signs and symptoms, with frequent re-evaluation of his or her initial assumptions about the patient and the diagnosis. Additionally, uveitis that worsens with corticosteroid treatment may need to be reclassified as infectious or as a masquerade syndrome. A team approach with the pediatrician, infectious disease specialist and rheumatologist is often critical to the diagnosis and proper systemic or local treatment. Complications such as amblyopia, cataract, glaucoma, and retinal detachment should be swiftly identified and treated in order to minimize irreversible vision loss in this susceptible population.
References
- Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardizationof Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005; 140(3):509-516.
- Cunningham ET Jr. Uveitis in children. Ocul Immunol Inflamm 2000; 8(4):251-261.
- Nagpal A, Leigh JF, Acharya NR. Epidemiology of uveitis in children. Int Ophthalmol Clin 2008; 48(3):1-7.
- BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol 2005; 89(4):444-448.
- Edelsten C, Reddy MA, Stanford MR, et al. Visual loss associated with pediatric uveitis in english primary and referral centers. Am J Ophthalmol 2003; 135(5):676-680.
- Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996; 80(4):332-336.
- Engelhard SB, Bajwa A, Reddy AK. Causes of uveitis in children without juvenile idiopathic arthritis. Clin Ophthalmol 2015; 9:1121-1128.
- Okada AA, Foster CS. Posterior uveitis in the pediatric population. Int Ophthalmol Clin 1992; 32(1):121-152.
- Dhoot DS, Martin DF, Srivastava SK. Pediatric infectious posterior uveitis. Int Ophthalmol Clin 2011; 51(1):113-128.
- Hettinga YM, de Groot-Mijnes JD, Rothova A, de Boer JH. Infectious involvement in a tertiary center pediatric uveitis cohort. Br J Ophthalmol 2015; 99(1):103-107.
- Baltmr A, Lightman S, Tomkins-Netzer O. Examining the choroid in ocular inflammation: a focus on enhanced depth imaging. J Ophthalmol 2014; 2014:459136.
- Kim JS, Knickelbein JE, Jaworski L, et al. Enhanced Depth Imaging Optical Coherence Tomography in Uveitis: An Intravisit and Interobserver Reproducibility Study. Am J Ophthalmol 2016; 164:49-56.
- Trichonas G, Kaiser PK. Optical coherence tomography imaging of macular oedema. Br J Ophthalmol 2014; 98 Suppl 2:ii24-29.
- Samy A, Lightman S, Ismetova F, Talat L, Tomkins-Netzer O. Role of autofluorescence in inflammatory/infective diseases of the retina and choroid. J Ophthalmol 2014; 2014:418193.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol 2000; 11(6):478-483.
- Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000; 130(4):492-513.
- Hoover DL, Khan JA, Giangiacomo J. Pediatric ocular sarcoidosis. Surv Ophthalmol 1986; 30(4):215-228.
- Pepple KL, Lam DL, Finn LS, Van Gelder R. Urinary beta2-Microglobulin Testing in Pediatric Uveitis: A Case Report of a 9-Year-Old Boy with Renal and Ocular Sarcoidosis. Case Rep Ophthalmol 2015; 6(1):101-105.
- Sullu Y, Yildiran A, Sullu Y, Helek D, Ozkaya O Sarcoid uveitis simulating birdshot chorioretinopathy in a child. Retin Cases Brief Rep 2012; 6(1):7-10.
- Rejdak R, Pogorelov P, Mardin CY, Szkaradek M, Juenemann AG. Solitary sarcoid granuloma of the iris mimicking tuberculosis: a case report. J Ophthalmol 2014; 2014:656042.
- Deverrière G, Flamans-Klein A, Firmin D, Azouzi O, Courvill P, Le Roux P. [Early onset pediatric sarcoidosis, diagnostic problems]. Arch Pediatr 2012; 19(7):707-710.
- Gedalia A, Khan TA, Shetty AK, Dimitriades VR, Espinoza LR. Childhood sarcoidosis: Louisiana experience. Clin Rheumatol 2016 Jul;35(7):1879-1884.
- Choi DE, Birnbaum AD, Oh F, Tessler HH, Goldstein DA. Pediatric uveitis secondary to probable, presumed, and biopsy-proven sarcoidosis. J Pediatr Ophthalmol Strabismus 2011; 48(3):157-162.
- Rosé CD, Wouters CH, Meiorin S, et al. Pediatric granulomatous arthritis: an international registry. Arthritis Rheum 2006; 54(10):3337-3344.
- Latkany PA, Jabs DA, Smith JR, et al. Multifocal choroiditis in patients with familial juvenile systemic granulomatosis. Am J Ophthalmol 2002; 134(6):897-904.
- Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet 1990; 335(8697):1078-1080.
- Parentin F, Lepore L, Rabach I, Pensiero S. Paediatric Behcet's disease presenting with recurrent papillitis and episcleritis: a case report. J Med Case Rep 2011; 5:81.
- Metreau-Vastel J, Mikaeloff Y, Tardieu M, Koné-Paut I, Tran TA. Neurological involvement in paediatric Behcet's disease. Neuropediatrics 2010; 41(5):228-234.
- Citirik M, Berker N, Songur MS, Soykan E, Zilelioglu O. Ocular findings in childhood-onset Behcet disease. J aapos 2009; 13(4):391-395.
- Mackensen F, Billing H. Tubulointerstitial nephritis and uveitis syndrome. Curr Opin Ophthalmol 2009; 20(6):525-531.
- Mackensen F, David F, Schwenger V, et al. HLA-DRB1*0102 is associated with TINU syndrome and bilateral, sudden-onset anterior uveitis but not with interstitial nephritis alone. Br J Ophthalmol 2011; 95(7):971-975.
- Aguilar C, Lonngi M, de-la-Torre A. Tubulointerstitial Nephritis and Uveitis Syndrome: Case Report and Review of the Literature. Ocul Immunol Inflamm 2015:1-7.
- Reddy AK, Hwang YS, Mandelcorn ED, Davis JL. HLA-DR, DQ class II DNA typing in pediatric panuveitis and tubulointerstitial nephritis and uveitis. Am J Ophthalmol 2014; 157(3):678-686.e1-2.
- Ali A, Rosenbaum JT. TINU (tubulointerstitial nephritis uveitis) can be associated with chorioretinal scars. Ocul Immunol Inflamm 2014; 22(3):213-217.
- http://www.cdc.gov. Accessed August 1, 2015.
- Brunner HI, Gladman DD, Ibañez D, et al. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum 2008; 58(2):556-62.
- Read RW. Clinical mini-review: systemic lupus erythematosus and the eye. Ocul Immunol Inflamm 2004; 12(2):87-99.
- Al-Mayouf SM, Al-Hemidan AI. Ocular manifestations of systemic lupus erythematosus in children. Saudi Med J 2003; 24(9):964-6.
- Iudici M, Puechal X, Pagnoux C, et al. Brief Report: Childhood-Onset Systemic Necrotizing Vasculitides: Long-Term Data From the French Vasculitis Study Group Registry. Arthritis Rheumatol 2015; 67(7):1959-65.
- Rothschild PR, Pagnoux C, Seror R, et al. Ophthalmologic manifestations of systemic necrotizing vasculitides at diagnosis: a retrospective study of 1286 patients and review of the literature. Semin Arthritis Rheum 2013; 42(5):507-14.
- Rao NA. Mechanisms of inflammatory response in sympathetic ophthalmia and VKH syndrome. Eye (Lond) 1997; 11 ( Pt 2):213-6.
- Rathinam SR, Vijayalakshmi P, Namperumalsamy P, et al. Vogt-Koyanagi-Harada syndrome in children. Ocul Immunol Inflamm 1998; 6(3):155-61.
- Khalifa YM, Bailony MR, Acharya NR. Treatment of pediatric vogt-koyanagi-harada syndrome with infliximab. Ocul Immunol Inflamm 2010; 18(3):218-22.
- Ganesh SK, Bala A, Biswas J, et al. Pattern of Pediatric Uveitis Seen at a Tertiary Referral Center from India. Ocul Immunol Inflamm 2015:1-8.
- Kilmartin DJ, Dick AD, Forrester JV. Prospective surveillance of sympathetic ophthalmia in the UK and Republic of Ireland. Br J Ophthalmol 2000; 84(3):259-63.
- Thompson CG, Kumar N, Billson FA, et al. The aetiology of perforating ocular injuries in children. Br J Ophthalmol 2002; 86(8):920-2.
- Kumar K, Mathai A, Murthy SI, et al. Sympathetic ophthalmia in pediatric age group: clinical features and challenges in management in a tertiary center in southern India. Ocul Immunol Inflamm 2014; 22(5):367-72.
- Savar A, Andreoli MT, Kloek CE, et al. Enucleation for open globe injury. Am J Ophthalmol 2009; 147(4):595-600.e1.
- Quillen DA, Davis JB, Gottlieb JL, et al. The white dot syndromes. Am J Ophthalmol 2004; 137(3):538-50.
- Jampol LM, Wiredu A. MEWDS, MFC, PIC, AMN, AIBSE, and AZOOR: one disease or many? Retina 1995; 15(5):373-8.
- Spital G, Heiligenhaus A, Scheider A, et al. ["White dot syndromes" in childhood]. Klin Monbl Augenheilkd 2007; 224(6):500-6.
- Abrez H, Biswas J, Sudharshan S. Clinical profile, treatment, and visual outcome of serpiginous choroiditis. Ocul Immunol Inflamm 2007; 15(4):325-35.
- Abu-Yaghi NE, Hartono SP, Hodge DO, et al. White dot syndromes: a 20-year study of incidence, clinical features, and outcomes. Ocul Immunol Inflamm 2011; 19(6):426-30.
- Cohen SM. Multiple Evanescent White Dot Syndrome After Vaccination for Human Papilloma Virus and Meningococcus. J Pediatr Ophthalmol Strabismus 2009.
- Shet A. Congenital and perinatal infections: throwing new light with an old TORCH. Indian J Pediatr 2011; 78(1):88-95.
- Bechtel RT, Haught KA, Mets MB. Lymphocytic choriomeningitis virus: a new addition to the TORCH evaluation. Arch Ophthalmol 1997; 115(5):680-1.
- Maenz M, Schlüter D, Liesenfeld O, et al. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retin Eye Res 2014; 39:77-106.
- Vasconcelos-Santos DV, Dodds EM, Orefice F. Review for disease of the year: differential diagnosis of ocular toxoplasmosis. Ocul Immunol Inflamm 2011; 19(3):171-179.
- Mets MB, Chhabra MS. Eye manifestations of intrauterine infections and their impact on childhood blindness. Surv Ophthalmol 2008; 53(2):95-111.
- Garza-Leon M, Garcia LA. Ocular toxoplasmosis: clinical characteristics in pediatric patients. Ocul Immunol Inflamm 2012; 20(2):130-138.
- Freeman K, Tan HK, Prusa A, et al. Predictors of retinochoroiditis in children with congenital toxoplasmosis: European, prospective cohort study. Pediatrics 2008; 121(5):e1215-222.
- Tan HK, Schmidt D, Stanford M, et al. Risk of visual impairment in children with congenital toxoplasmic retinochoroiditis. Am J Ophthalmol 2007; 144(5):648-653.
- Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol 2004; 137(1):1-17.
- Madigan WP, Raymond WR, Wroblewski KJ, et al. A review of pediatric uveitis: Part I. Infectious causes and the masquerade syndromes. J Pediatr Ophthalmol Strabismus 2008; 45(3):140-149.
- Baharivand N, Mahdavifard A, Fouladi RF. Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: a prospective randomized clinical trial. Int Ophthalmol 2013; 33(1):39-46.
- Fernandes Felix JP, Cavalcanti Lira RP, Grupenmacher AT, Assis Filho HLG, Cosimo AB, Nascimento MA, Leite Arieta CE. Long-term Results of Trimethoprim-Sulfamethoxazole Versus Placebo to Reduce the Risk of Recurrent Toxoplasma gondii Retinochoroiditis. Am J Ophthalmol. 2020 May;213:195-202.
- Enright AM, Prober CG. Herpesviridae infections in newborns: varicella zoster virus, herpes simplex virus, and cytomegalovirus. Pediatr Clin North Am 2004; 51(4):889-908, viii.
- Grose C. Acute retinal necrosis caused by herpes simplex virus type 2 in children: reactivation of an undiagnosed latent neonatal herpes infection. Semin Pediatr Neurol 2012; 19(3):115-118.
- Yoser SL, Forster DJ, Rao NA. Systemic viral infections and their retinal and choroidal manifestations. Surv Ophthalmol 1993; 37(5):313-52.
- Tawse KL, Baumal CR. Intravitreal foscarnet for recurring CMV retinitis in a congenitally infected premature infant. J AAPOS 2014; 18(1):78-80.
- Shoji K, Ito N, Ito Y, et al. Is a 6-week course of ganciclovir therapy effective for chorioretinitis in infants with congenital cytomegalovirus infection? J Pediatr 2010; 157(2):331-333.
- Lalezary M, Recchia FM, Kim SJ. Treatment of congenital cytomegalovirus retinitis with intravitreous ganciclovir. Arch Ophthalmol 2012; 130(4):525-527.
- Jones CA, Raynes-Greenow C, Isaacs D, et al. Population-based surveillance of neonatal herpes simplex virus infection in Australia, 1997-2011. Clin Infect Dis 2014; 59(4):525-531.
- Morbidity and Mortality Weekly Report (MMWR), http://www.cdc.gov.Increase in Incidence of Congenital Syphilis — United States, 2012–2014. November 13, 2015 / 64(44);1241-1245 Accessed November 2015.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am 2013; 27(4):705-722.
- Aldave AJ, King JA, Cunningham ET. Ocular syphilis. Curr Opin Ophthalmol 2001; 12(6):433-441.
- Oliver GF, Stathis RM, Furtado JM, Arantes TE, McCluskey PJ, Matthews JM; International Ocular Syphilis Study Group, Smith JR. Current ophthalmology practice patterns for syphilitic uveitis. Br J Ophthalmol. 2019 Nov;103(11):1645-1649.
- Davis JL. Ocular syphilis. Curr Opin Ophthalmol 2014; 25(6):513-518.
- Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin 1997; 37(2):13-28.
- World Health Organization. Global tuberculosis report 2015. http://www.who.int/tb/publications/global_report/en/ Accessed August 15, 2016.
- Malalis JF, Goldstein DA. Advances in tuberculosis-associated uveitis. Int Ophthalmol Clin 2015; 55(2):37-46.
- Biancardi AL, Curi AL. Cat-scratch disease. Ocul Immunol Inflamm 2014; 22(2):148-154.
- Kahloun R, Gargouri S, Abroug N, et al. Visual loss associated with rickettsial disease. Ocul Immunol Inflamm 2014; 22(5):373-378.
- Khairallah M, Kahloun R. Ocular manifestations of emerging infectious diseases. Curr Opin Ophthalmol 2013; 24(6):574-580.
- Woodhall D, Starr MC, Montgomery SP, et al. Ocular toxocariasis: epidemiologic, anatomic, and therapeutic variations based on a survey of ophthalmic subspecialists. Ophthalmology 2012; 119(6):1211-1217.
- de Amorim Garcia Filho CA, Gomes AH, de AGSAC, et al. Clinical features of 121 patients with diffuse unilateral subacute neuroretinitis. Am J Ophthalmol 2012; 153(4):743-749.
- Rathinam SR, Cunningham ET. Infectious causes of uveitis in the developing world. Int Ophthalmol Clin 2000; 40(2):137-152.