Incidence
Retinal detachments (RDs) in the pediatric population are relatively uncommon. Their etiology includes both congenital and acquired disorders. Their management can be challenging and varies significantly from traditional management in the adult population.
Retinal detachments in the pediatric population account for 3.2% to 6.6% of all retinal detachments,1 and their incidence ranges from 0.38 to 0.69 per 100,000 children. RDs in children are most commonly seen secondary to trauma (44%), myopia (15%), aphakia (10%), retinopathy of prematurity (8%), and other, less common etiologies (23%). The relative rates of each underlying condition vary by geographic location.2,3 Approximately 70% of patients are male, and the majority of patients present between the ages of 9 and 12 years.4
History and Clinical Presentation
In pediatric patients with retinal detachment, a detailed history is very important. Of particular importance is a history of prematurity, infection, prior ocular surgery, genetic syndromes, and prior trauma. Children with nontraumatic retinal detachments typically present later than adults and have retinal detachments of undetermined duration. Visual acuity at presentation is typically poor, with a mean visual acuity of 20/400, and decreased visual acuity is the presenting symptom in 83% of patients.4 The presence of nystagmus can be seen in RDs associated with retinopathy of prematurity, persistent pupillary membrane (PHPV) and congenital hereditary retinal diseases, such as familial exudative vitreoretinopathy (FEVR), Norrie disease and other disorders.5 Younger children may present with esotropia, while older children may present with exotropia; the presence of strabismus may indicate a chronic retinal detachment. RDs can also present as leukocoria in 10% to 15% of patients; in these cases, retinoblastoma should be excluded first, before considering other causes of detachment. Patients can also develop hypotony with proliferative vitreoretinopathy.6,7
The contralateral eye in 89% of patients with RD has pathologic changes, including lattice degeneration, high myopia, retinopathy of prematurity, or congenital abnormalities.6 Bilateral retinal detachment at presentation is not uncommon, with up to one-quarter of patients presenting with a retinal detachment in both eyes. A history of retinal detachment in one eye predisposes the risk of developing a detachment in the contralateral eye.8–10
Younger children may be unable to cooperate with a depressed retinal examination in clinic, and thus an examination under anesthesia should be performed to avoid missing peripheral pathology. In patients with poor pupillary dilation or opacified media, ultrasound B-scan should be performed.
Macular Attachment and Proliferative Vitreoretinopathy
At presentation, pediatric retinal detachments are more likely to have a macula-off detachment, portending a worse prognosis. The rates of macula-off detachment at presentation are higher in patients less than 10 years of age.8 Total retinal detachment at presentation is also much more common in the pediatric population, likely related to delays in diagnosis and subsequent prolonged detachment.11 Pediatric patients are also more likely to have proliferative vitreoretinopathy (PVR) as compared to adults. At presentation, 40%-45% of patients have PVR of grade C or worse.6,8,9 The presence of PVR heralds a decreased rate of anatomical surgical success and is associated with a higher incidence of surgical reintervention and poor visual prognosis.9 Table 1 discusses the classification of PVR. Additionally, the development of amblyopia in young patients also contributes to the poor visual outcomes.
|
Table 1: Grading of Proliferative Vitreoretinopathy12
|
|
Grade of PVR
|
Description
|
|
A
|
Vitreous haze, pigment clumping, and clusters of pigment in the inferior retina
|
|
B
|
Wrinkling of retinal surface, tortuous vessels, retinal stiffness, rolled or irregular edges of the retinal defect, and decreased vitreous mobility
|
|
CP
|
Full-thickness retinal folds or subretinal strands posterior to the equator. Further subclassified by the clock-hours of involvement.
|
|
CA
|
Full-thickness retinal folds, subretinal strands, anterior displacement, or condensed vitreous strands anterior to the equator. Further subclassified by the clock-hours of involvement.
|
Etiology and Special Surgical Techniques
The underlying etiology for pediatric retinal detachments varies by geography. East Asia has a higher rate of myopia, and thus relatively more patients present with retinal detachments related to myopic degeneration.8,13 In other areas of the globe, congenital and genetic abnormalities account for a much higher rate of pediatric retinal detachments.9 Trauma remains a major risk factor for RD in the pediatric population regardless of geography, especially in young males.
Genetic and Vascular Causes of Retinal Detachment
Family Exudative Vitreoretinopathy
Family exudative vitreoretinopathy (FEVR) (Figures 1-4) is a bilateral disorder typically inherited in an autosomal dominant fashion, although X-linked and autosomal recessive inheritance patterns have been described.14,15 The specific gene involved depends on the mode of inheritance. Patients with this disease have abnormalities in the Wnt receptor/ß-catenin pathway, resulting in an increase in vascular endothelial growth factor (VEGF). This consequently increases vascular activity, prompting exudation. Staging is similar to that of Coats disease, which is discussed later in this chapter (Table 2).16
|
Table 2: Staging of Familial Exudative Vitreoretinopathy16
|
|
Stage
|
Examination Findings
|
|
1
|
Peripheral avascularity
|
|
2
|
Retinal neovascularization
A: without exudation
B: with exudation
|
|
3
|
Extramacular retinal detachment
A: without exudation
B: with exudation
|
|
4
|
Macula-involving retinal detachment
A: without exudation
B: with exudation
|
|
5
|
Total retinal detachment
|
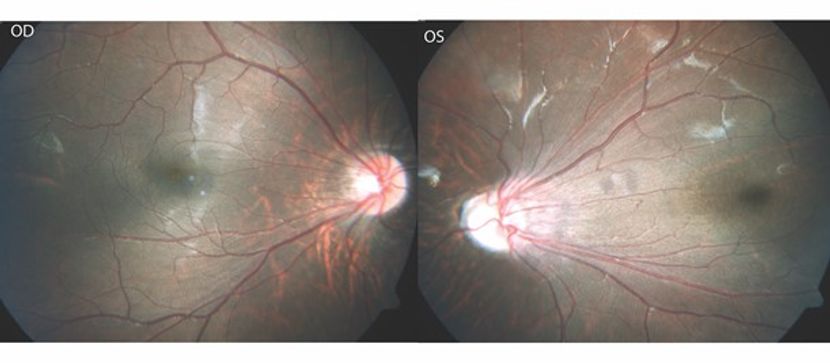
Figure 1. Color fundus pictures of right and left eye with FEVR. Both eyes show straightening of the retinal vasculature with dragging of the optic disc and macula though more significant on the left eye. (This image was provided by Vikas Khetan, MS, Senior Retinal Consultant, Sankara Nethralaya, Chennai, India.)
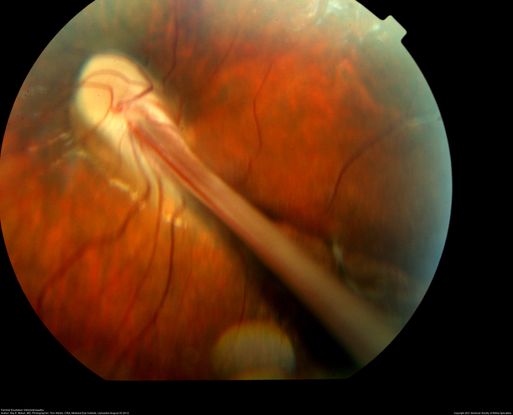
Figure 2. Color fundus picture of the left eye showing the optic disc dragging with a falciform retinal fold extending through the macula to the retinal periphery. (This image was originally published in the Retina Image Bank® website. Author: Raj K Maturi, MD; Photographer: Tom Steele, CRA, Midwest Eye Institute. Title: Familial Exudative Vitreoretinopathy. Retina Image Bank. Year 2012; Image Number 543 © the American Society of Retina Specialists.)
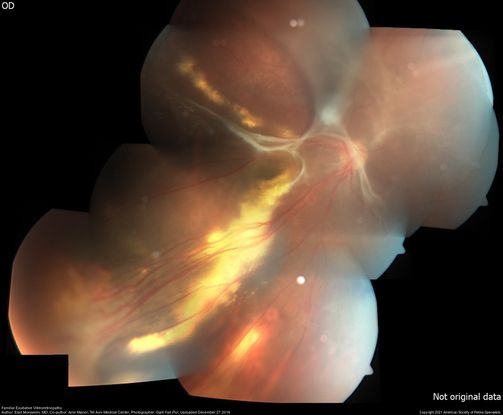
Figure 3. Color fundus montage of the right eye with FEVR. It shows long-standing exudative retinal detachment with extensive exudates at the macula and in the periphery and straightening of the retinal vasculature inferotemporally. In the far temporal periphery tractional fibrous proliferation with avascular retina can be seen. (This image was originally published in the Retina Image Bank® website. Author: Elad Moisseiev, MD; Photographer: Galit Yair-Pur, Tel Aviv Medical Center. Title: Familial Exudative Vitreoretinopathy. Retina Image Bank. Year 2016; Image Number 26879 © the American Society of Retina Specialists.)
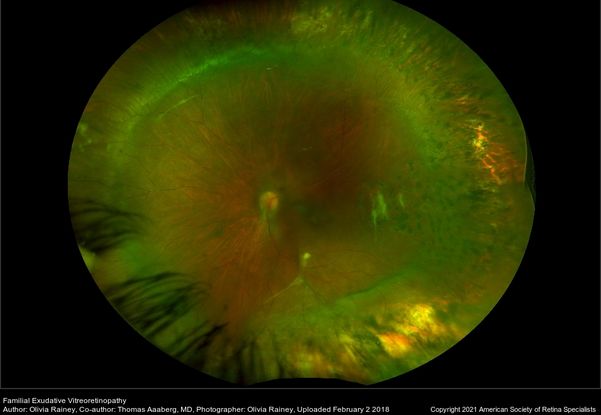
Figure 4. Ultrawide-field color picture of the left eye of a patient with familial exudative vitreoretinopathy post surgery. Cryotherapy, laser scars in periphery and scleral buckle effect is seen in the retinal periphery with attached retina. (This image was originally published in the Retina Image Bank® website. Author: Thomas Aaberg, MD; Photographer: Olivia Rainey, Retina Specialists of Michigan. Title: Familial Exudative Vitreoretinopathy. Retina Image Bank. Year 2018; Image Number 27801 © the American Society of Retina Specialists.)
FEVR is the most common congenital or developmental causes for retinal detachments in childhood.17 All forms of retinal detachment can occur in these patients, with exudative and tractional detachments forming from fibrovascular proliferation and rhegmatogenous detachments from atrophic holes. Laser photocoagulation and cryotherapy are the mainstays of treatment.18 Additionally, given the underlying abnormality in VEGF levels, anti-VEGF agents can be helpful in management.19 When surgical treatment is required, careful dissection in the peripheral avascular retina is critical, although extremely challenging.20
Retinopathy of Prematurity
Retinopathy of prematurity (ROP) is a vasoproliferative disease that occurs exclusively in premature infants and is a common cause of vision loss in the pediatric population.21 (See also International Classification of Retinopathy of Prematurity, Third Edition) ROP is graded in stages:22–24
-
Stage 0: immature retinas, no ROP
-
Stage 1: demarcation line between vascular and avascular retina
-
Stage 2: ridge of tissue arising at the demarcation line that extends above the plane of the retina
-
Stage 3: extraretinal fibrovascular proliferation (neovascularization) extending from the ridge into the vitreous cavity
-
Stage 4: partial retinal detachment. 4a denotes a partial extrafoveal detachment and 4b denotes foveal involvement
-
Stage 5: total retinal detachment (usually tractional)
Classification of ROP is also noted by location (Figure 5):25
-
Zone I: defined by a circle with radius twice the estimated distance from the optic disc center to the foveal center.
-
Posterior Zone II: region that begins at the margin between zone I and zone II and extends into zone II for 2 disc diameters.
-
Zone II: is a ring-shaped region extending nasally from the outer limit of zone I to the nasal ora serrata and with a similar distance temporally, superiorly, and inferiorly.
-
Zone III: the residual temporal crescent of retina anterior to zone II. By convention, zones II and III are considered to be mutually exclusive.
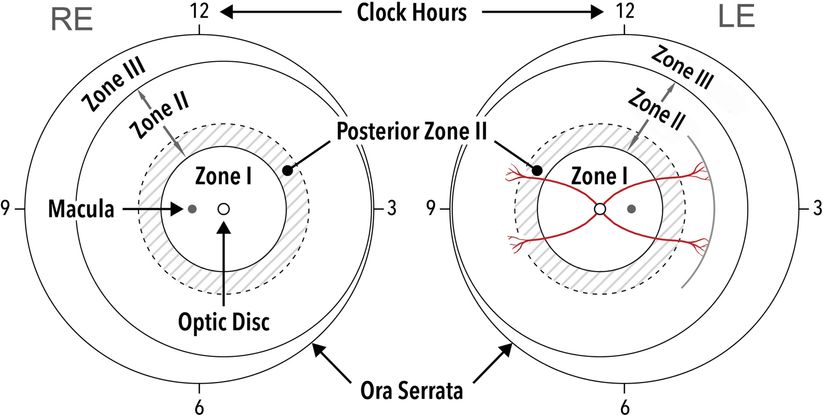
Figure 5. Zones of retinopathy of prematurity. Zone I corresponds to a circle centered on the optic disc, with a radius of twice the distance from the optic disc to the center of the fovea. A helpful clinical correlate for estimating the temporal extent of zone I is to place the nasal edge of the optic disc in the field of view of a 25- or 28-diopter lens, with the limit of zone I being the temporal field of view. Zone II forms a circle peripheral to zone I with a radius from the optic nerve to the nasal ora serrata. Posterior Zone II: region that begins at the margin between zone I and zone II and extends into zone II for 2 disc diameters. Zone III is made up of the remaining temporal crescent of retina outside zone II. (Ophthalmology 2021 128e51-e68DOI: (10.1016/j.ophtha.2021.05.031.)
Finally, the need for treatment of ROP is further classified into 2 categories, Type 1 and Type 2 ROP.26
-
Type 1 high risk prethreshold ROP is defined as zone 1 any stage with plus disease, zone 1 stage 3 with no plus disease, and zone 2 stage 2 or 3 with plus disease. All eyes with type 1 prethreshold ROP are currently recommended for immediate treatment.
Interventional treatment is currently recommended for Type 1 ROP. Current treatment options include laser panretinal photocoagulation (PRP) of the avascular retina, intravitreal injection of an anti-VEGF medication (such as bevacizumab or ranibizumab) or a combination of the 2.27 Guidance on which treatment to choose is evolving and there are disparate opinions about the best primary treatment.
Timing is critical in treating retinal detachments associated with ROP, with the ideal time to pursue surgical intervention being when the neovascular drive is decreased and the retinal detachment is still in an early phase. Typically, this occurs around 40 weeks gestational age.28 Both scleral buckling and vitrectomy have been pursued. Scleral buckling decreases the release of VEGF, which is advantageous, but it does not remove the vitreous laden with angiogenic signaling molecules. Scleral buckling can also induce strabismus and typically requires a second surgery to remove the buckle months later.29 Vitrectomy does restore normal retinal anatomy and, with appropriate delamination, can decrease the tractional forces seen in ROP. However, this surgery raises the risk of the creation of iatrogenic breaks and subsequent proliferative vitreoretinopathy. Recent studies have found that the addition of a scleral buckle to vitrectomy does not lead to an increase in anatomic success rates in patients with stage 4 ROP, and thus combining the 2 procedures is of no benefit.30 Administering anti-VEGF agents preoperatively can decrease fibrovascular proliferation, but can also cause contraction of existing fibrovascular bands, resulting in further tractional forces on the retina.31 Thus, some surgeons combine preoperative anti-VEGF treatment (namely, bevacizumab) with scleral buckling as a bridge to eventual vitrectomy.32 Even with advanced surgical techniques for stage 4 and or stage 5 ROP, outcomes are largely unsuccessful, with poor visual prognosis.33
Norrie Disease
Norrie disease (Figure 6) is a rare, X-linked recessive disease of the retina. Its underlying cause is a mutation in the NDP gene on the short arm of the X chromosome.34 The resultant protein, norrin, binds specifically to Frizzled 4 (Fz4), a Wnt receptor, and Lrp5/6, a Wnt co-receptor, and activates the canonical Wnt/ß-catenin signaling pathway inside the cell.35 This pathway is critical in regulating retinal development and in signaling for the hyaloid vessels to regress.
In Norrie disease, patients are typically severely visually limited due to retinal dysplasia. Retrolental fibroplasia, leukocoria, iris atrophy, and vitreous hemorrhage are common in this disease. Tractional retinal detachments often form due to stalk tissue becoming adherent between the retina and the lens; retinal detachments are frequently hemorrhagic.
Management of Norrie disease has typically been palliative, with most eyes becoming phthisical in childhood. However, there have been some reports of early vitrectomy, often combined with lensectomy, preventing phthisis bulbi and helping preserve light-perception vision. The retrolental stalk causing traction on the retina is released during vitrectomy, thus preventing the adherence between stalk, retina, and lens, and minimizing the hemorrhaging that can lead to more aggressive vitreoretinopathy. However, even with aggressive intervention, the prognosis in this disease is grim.36

Figure 6. Fundus photograph of 34-week-old infant with Norrie Disease. (https://www.aao.org/disease-review/norrie-disease)
Incontinentia Pigmenti
Incontinentia pigmenti (see also: https://eyewiki.aao.org/Incontinentia_Pigmenti. The 4 stages of the disease are depicted there), also known as Bloch-Sulzberger or Bloch-Siemens-Sulzberger syndrome, is a rare condition that affects the eyes in approximately 35% of cases. It is characterized by skin abnormalities (Figure 7) that evolve throughout childhood and adolescence. It is caused by a mutation in the IKBKG gene and is inherited in an X-linked dominant fashion. It is lethal to hemizygous males; thus only females survive to inherit the disease.37 Blindness in this disorder is typically due to retinal detachment, which occurs in 3% of patients.38 Similar to ROP, the retinal findings stem from vascular occlusion and include neovascularization and hemorrhages which lead to exudative and tractional detachments. Absence of a foveal pit with an enlarged foveal avascular zone is also often seen in this disorder. Non-retinal findings include microphthalmos, congenital cataract, and optic nerve atrophy.37
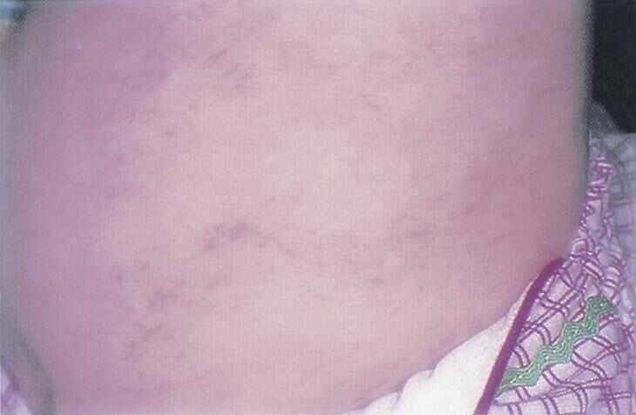
Figure 7. Pigmented skin lesions of incontinentia pigmenti (https://www.aao.org/image/pigmented-skin-lesions-of-incontinentia-pigmenti-2)
As with ROP, retinal detachments are typically tractional due to vitreous and preretinal fibrous proliferation causing traction on the retina. Laser photocoagulation to the avascular retina is the treatment of choice, ideally performed prior to retinal detachment. Unfortunately, the retinal findings are often not seen until detachment has occurred. For retinal detachment, vitrectomy is typically required to release the traction on the retina.39
Persistent Fetal Vasculature
Persistent fetal vasculature (PFV) (see also: https://eyewiki.aao.org/Persistent_hyperplastic_primary_vitreous) is characterized by the presence of intraocular fetal vasculature after birth. PFV can be present in the anterior segment of the eye, but this typically does not result in retinal detachment. However, posterior PFV, which can include vitreous stalks, retinal folds, posterior persistent hyperplastic primary vitreous (PHPV), or a hypoplastic optic nerve and macula, can result in retinal detachments in the pediatric population.40
PHPV (Figures 8-10) is a form of tractional retinal detachment that is present at birth and may progress. The detachment is due to traction from the persistent fetal vasculature, with possibly a component of traction from neuroectodermal portions of the primary vitreous.41 The primary vitreous is adhesive to the optic cup, resulting in an inability of the secondary vitreous to form. As a result, the adherent primary vasculature forms a tractional retinal detachment.40
Indications for surgery in these patients include progressive tractional detachment, a component of rhegmatogenous retinal detachment, to clear an amblyogenic opacity in the media, or to treat progressive angle-closure glaucoma. Early intervention has been advocated by some surgeons to prevent amblyopia.42 Vitrectomy, combined with lensectomy when indicated, is the ideal choice to treat this disease. The ciliary body and peripheral retina may be located more centrally and anteriorly in these eyes, prompting some surgeons to adopt an anterior translimbal approach for vitrectomy.43 Other surgeons prefer the posterior pars plicata approach, as this enables an earlier transection of the posterior hyaloid stalk. This also has the advantages of decreased anterior traction on the retina, more complete lensectomy, and decreased manipulation of the cornea.44,45
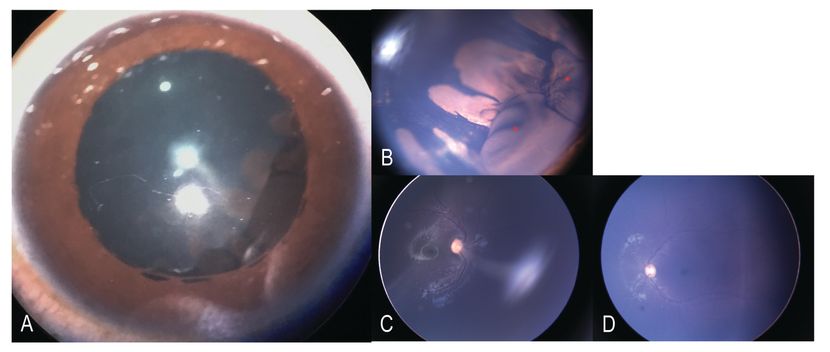
Figure 8. Pictures of a child with Persistent Hyperplastic Primary Vitreous: A, B Slit- lamp photo of the right eye showing elongated ciliary processes nasally with multiple large ciliary body cyst (red asterix). Localized cataract also called as Mittendorf cataract is seen. (C, D) Fundus photo of right eye showing the fibrovascular stalk from the optic disc to the posterior surface of the lens with normal macula and posterior pole. Fundus photos of the left eye with normal disc, macula and posterior pole. (Gonzalez A, Agarwal-Sinha S, Iyer SSR, Bolling JP. “Multiloculated Ciliary Body Cysts and Lenticular Coloboma: A Rare Phenotypic Variation Associated with Persistent Fetal Vasculature.” J Pediatr Ophthalmol Strabismus. 2018 Nov 2;55:e39-e41.)
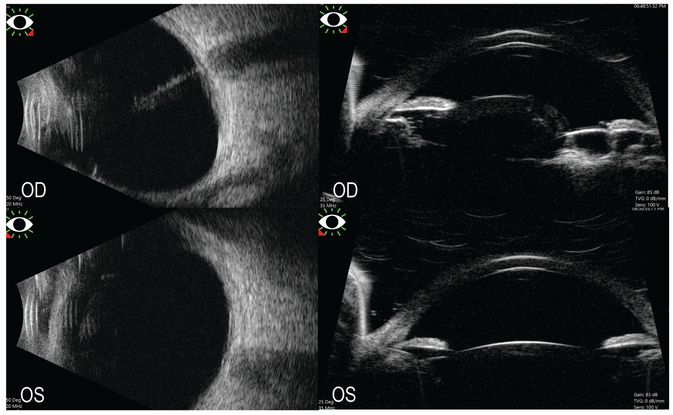
Figure 9. Left Top: Ultrasound B scan of the right showing hyperreflective stalk extending from the optic disc to the posterior surface of the lens in the vitreous cavity. Left Bottom: Ultrasound B scan of the left eye with acoustically clear vitreous cavity and attached retina. Right Top: Ultrasound biomicroscopy of the right eye showing multiple large ciliary body cysts nasally with subluxated lens temporally. Right Bottom: Ultrasound biomicroscopy of the left eye with normal anterior segment anatomy. (Gonzalez A, Agarwal-Sinha S, Iyer SSR, Bolling JP. Multiloculated Ciliary Body Cysts and Lenticular Coloboma: A Rare Phenotypic Variation Associated with Persistent Fetal Vasculature. J Pediatr Ophthalmol Strabismus. 2018 Nov 2;55:e39-e41.)
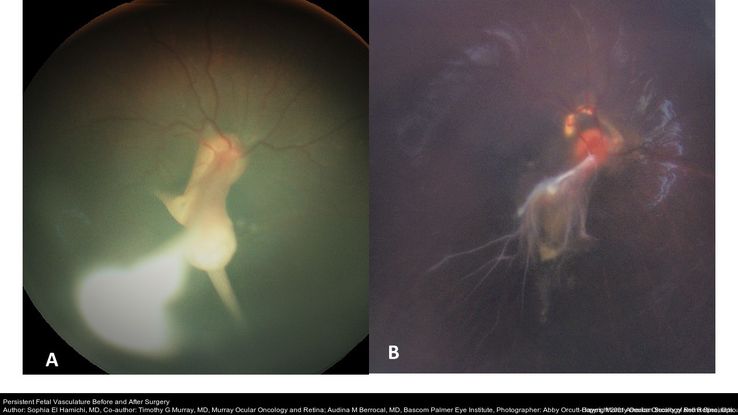
Figure 10. Fundus photograph A, showing a persistent fetal vasculature with secondary tractional detachment from PHPV. B: Fundus photograph of the patient eye after repair surgery with flat retina. (This image was originally published in the Retina Image Bank® website. Author: Sophia El Hamichi, MD; Photographer: Abby Orcutt-Hayes, Murray Ocular Oncology and Retina. Title: Persistent Fetal Vasculature Before and After Surgery. Retina Image Bank. Year 2020; Image Number 46637 © the American Society of Retina Specialists.)
X-Linked Retinoschisis
X-linked retinoschisis (see also: https://eyewiki.aao.org/X-linked_Retinoschisis) (Figures 11-14) predisposes young males with this disorder to retinal detachment. This disease is characterized by foveal schisis, and schisis often extends to the infero-temporal retina. Retinal breaks can form on the thinned inner layer of the schisis, resulting in rhegmatogenous retinal detachments. Retinal detachment occurs in 16% of patients with this disorder, with the causative retinal break ranging from small retinal holes to large retinal tears.46
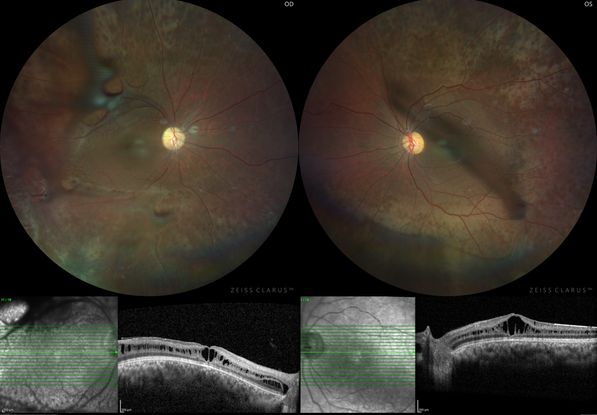
Figure 11. Color fundus photograph of the right and left eye of with juvenile x-linked retinoschisis showing with inner retinal defects along the superior and inferior arcades in both eyes. Corresponding OCT images at the bottom is showing retinal schisis involving fovea. (This image was originally published in the Retina Image Bank® website. Author: Shishir Verghese; Photographer: Shishir Verghese, Aravind Eye Hospital and Postgraduate institute of Ophthalmology, Coimbatore Title: Juvenile X-Linked Retinoschisis. Retina Image Bank. Year 2021; Image Number 73620 © the American Society of Retina Specialists.)
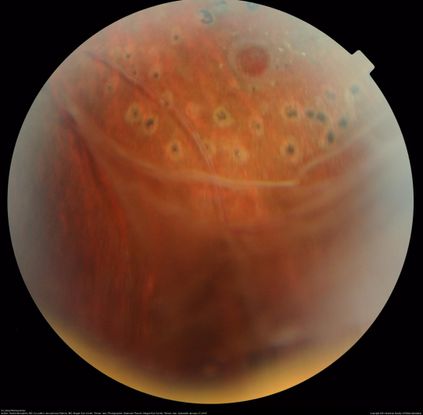
Figure 12. This image shows peripheral retinal schisis with elevated retinal vessels and localized retinal detachment in retinal periphery. Vitreous veil extends from the schisis cavity superiorly. Scattered laser scars are seen superiorly, surrounding the retinal hole with a cuff of subretinal fluid. (This image was originally published in the Retina Image Bank® website. Author: Hamid Ahmadieh, MD; Photographer: Shabnam Poureh, Negah Eye Center, Tehran, Iran. Title: X-Linked Retinoschisis. Retina Image Bank. Year 2015; Image Number 23947 © the American Society of Retina Specialists.)
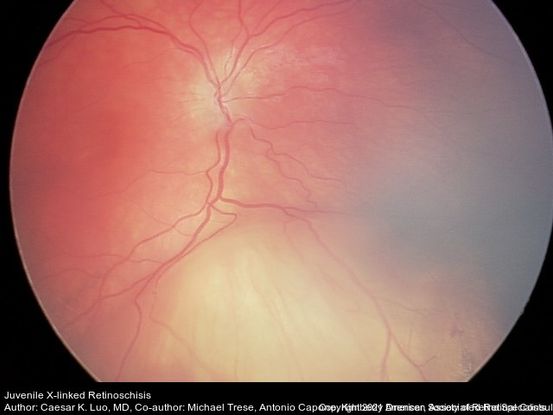
Figure 13. This image shows dome-shaped smooth retinal elevation in the inferonasal periphery with attached retina in a juvenile X-linked retinoschisis patient. (This image was originally published in the Retina Image Bank® website. Author: Caesar K Luo, MD; Photographer: Caesar Luo, Progressive Vision Institute, PA. Title: Juvenile X-Linked Retinoschisis. Retina Image Bank. Year 2014; Image Number 12912 © the American Society of Retina Specialists.)
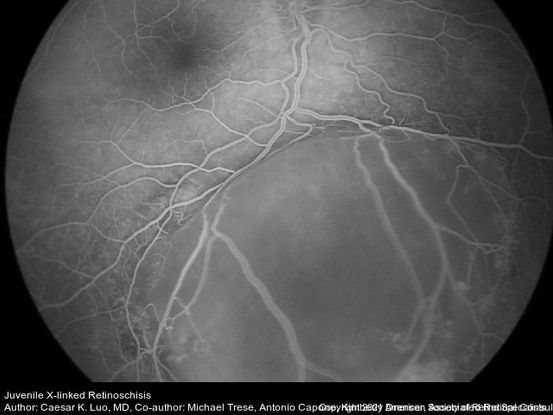
Figure 14. Mid-phase fluorescein angiography corresponds to the color picture, with smooth dome-shaped peripheral retinal elevation. Peripheral vascular changes are noted with an area of retinal nonperfusion and leakage from retinal capillaries in the periphery. (This image was originally published in the Retina Image Bank® website. Author: Caesar K Luo, MD; Photographer: Caesar Luo, Progressive Vision Institute, PA. Title: Juvenile X-Linked Retinoschisis. Retina Image Bank. Year 2014; Image Number 12913 © the American Society of Retina Specialists.)
Stickler Syndrome
Stickler syndrome (see also: https://eyewiki.aao.org/Stickler_syndrome) (Figure 15) was initially described in 1965 and includes ocular, skeletal, and facial abnormalities.47 Inheritance is typically via an autosomal dominant pattern, but sporadic cases have also been described. Non-ocular changes include cleft palate, flattened facies, diffuse and progressive arthropathy, neurosensory hearing loss, and spondyloepiphyseal dysplasia. The vitreous space is empty, a pathognomonic finding in this disease.48
Type I Stickler syndrome is the classic form of this disorder, and the vitreous has a membranous phenotype. A fibrillar retrolenticular membrane covers the pars plana and peripheral retina. Mutation in COL2A1, which encodes for synthesis of type II collagen, is the cause of this disorder. Type II Stickler syndrome presents with beaded vitreous condensations in the retrolenticular space. Lattice degeneration is also present, and there are perivascular pigmentary changes. Type II Stickler syndrome is due to mutation in the COL11A1 gene, which encodes for type XI collagen. Type III Stickler syndrome has also been described, but this disease does not include any ocular abnormalities and does not predispose to retinal detachment. Early-onset lamellar cataracts are common in Stickler syndrome. These cataracts are symmetric and bilateral. Myopia is present in 90% of affected patients.3,48
Peripheral retinal thinning, predisposing to retinal breaks, results in a high rate of retinal detachment. There is a tendency to form giant retinal tears, but any form of retinal break can be found. One-fifth of affected patients develop a retinal detachment prior to age 10 years.3,49 Prophylactic laser retinopexy in the fellow eye has been demonstrated to decrease risk of retinal detachment; rates of retinal detachment without prophylaxis are 65%, whereas prophylactic laser decreases the rate of detachment to 3%.50 In patients with this disorder, peripheral retinal examination is critical, and an examination under anesthesia to obtain a thorough depressed scleral examination may be indicated in young children unable to cooperate with depressed retinal exam in the outpatient setting. Given the autosomal dominant inheritance, screening of family members is indicated.
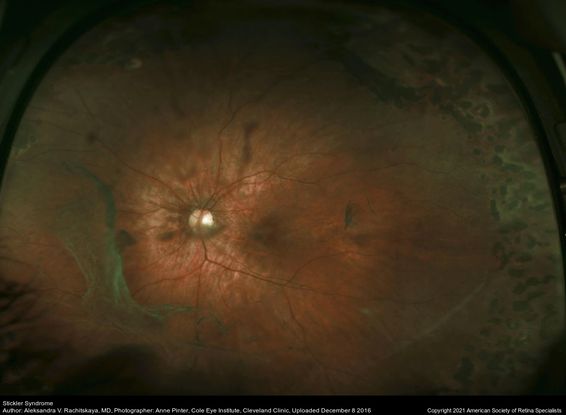
Figure 15. Optos wide-field fundus image of the left eye of a patient with Stickler Syndrome and COL2A1 gene mutation. The image shows laser scars in the periphery along with lattices and vitreous veils. (This image was originally published in the Retina Image Bank® website. Author: Aleksandra V. Rachitskaya, MD; Photographer: Anne Pinter, Cole Eye Institute, Cleveland Clinic. Title: Stickler Syndrome. Retina Image Bank. Year 2016; Image Number 26827© the American Society of Retina Specialists.)
Marfan Syndrome
Marfan syndrome (see also : https://eyewiki.org/Marfan_Syndrome and the disease review chapter Marfan Syndrome) (Figure 16) is inherited in an autosomal dominant fashion. The Ghent nosology is used to diagnose this disease, which encompasses ocular, cardiac, and skeletal abnormalities.51 The causative abnormality in Marfan syndrome is a mutation in the FBN1 gene on the chromosome 15, which encodes for fibrillin. Fibrillin is essential for the normal deposition of elastin and in microfibril assembly. Fibrillin is found in a multitude of ocular structures, including the lens, zonules, choroid, sclera, and Bruch membrane.52
Cardiac abnormalities in this syndrome can be life-threatening and include aortic dilation and dissections.53 Patients are typically above 95th percentile for height and have hyperextensible joints.54 Superotemporal dislocation of the lens is present in up to 80% of patients with Marfan syndrome, and patients can be highly myopic, with 21% of patients exhibiting 7 diopters of axial myopia or more. These patients also often exhibit poor pupillary dilation, limiting the view to the posterior segment.55
Retinal detachment is common in patients with Marfan syndrome, both due to inherent retinal abnormalities and increased risk conferred by early cataract surgery for ectopia lentis. The retinal detachment rate ranges from 5%-11% in surgically naïve patients, and can rise to 38% in those who have undergone cataract surgery.3,55 Early vitreous liquefaction, retinal thinning, and lattice degeneration predispose these patients to retinal detachment at a young age, with 70% of patients who develop a retinal detachment presenting before age 20 years.52 Most retinal breaks occur in the temporal half of the retina, and these patients are predisposed to giant retinal tears.56 Given poor pupillary dilation, iris retractors may be needed during vitreoretinal surgery. In patients with good pupillary dilation and without significant ectopia lentis, scleral buckling may be pursued. However, many patients require pars plana lensectomy, vitrectomy, and tamponade with intraocular gas or silicone oil. Scleral buckling can be considered in addition to vitrectomy in these patients. With these techniques, anatomic success rates are similar to those in patients without Marfan syndrome.56,57

Figure 16. Ectopia lentis in a patient with MFS with stretching of the zonular fibers (arrows). (EyeWiki) https://eyewiki.org/Marfan_Syndrome
Nontraumatic Inferotemporal Retinal Dialysis
Nontraumatic inferotemporal retinal dialysis (Figure 17) is a rare familial disorder with uncertain genetics. Affected patients present with inferotemporal retinal dialysis and subsequent retinal detachment without predisposing trauma. Young males in the second decade of life are more commonly affected, and they are typically emmetropic with normal, formed vitreous.58 These eyes have peripheral cystoid degeneration, thus predisposing them to retinal dialysis.59 The progression to retinal detachment after dialysis has formed is slow, as these patients do not have a posterior vitreous detachment. As a result, the retina often thins due to the chronicity of the detachment. Thus, this disease can be confused with retinoschisis; scleral depression is important to make the correct diagnosis. There is commonly pathology in the contralateral eye, and. detachments in this disease are typically asymptomatic until the macula is involved. The detachments are also typically shallow. PVR is rare; patients generally have good anatomic outcomes with scleral buckling. The fellow eye should be treated with cryotherapy if pathology is present.58

Figure 17. Ultra-wide field fundus photographs of the right and left eye showing bilateral idiopathic retinal dialysis in the temporal periphery with retinal detachment in the right eye. The black arrows point to the dialysis, the blue arrowheads to intraretinal macrocyst, and blue arrows vitreous opacities. (Published article: Vinod Kumar, Raghav Dinesh Ravani, Nikhil Kuthirummal, Kabiruddin Molla. “Ultra-wide field imaging of bilateral idiopathic retinal dialysis.” BMJ Case Rep. 2016 Jun 30;2016:bcr2016216212.)
Tumors and Inflammatory Causes of Childhood Retinal Detachment
Retinoblastoma
Retinoblastoma (see also: https://eyewiki.org/Retinoblastoma and Review of Retinoblastoma) (Figures 18-20) is the most common ocular malignant tumor of childhood, and it typically presents in young children. There can be a genetic predisposition in this disease due to mutations in the RB1 gene, which is a tumor-suppressing gene. Two active copies of this gene are typically carried in the cell; both of these must be mutated in order for retinoblastoma to develop (known as the “two-hit” hypothesis).60 Patients with a family history are more likely to present at a younger age and/or with bilateral disease. On presentation, 40% of retinoblastoma patients have bilateral disease. Presenting symptoms commonly include leukocoria and/or strabismus.
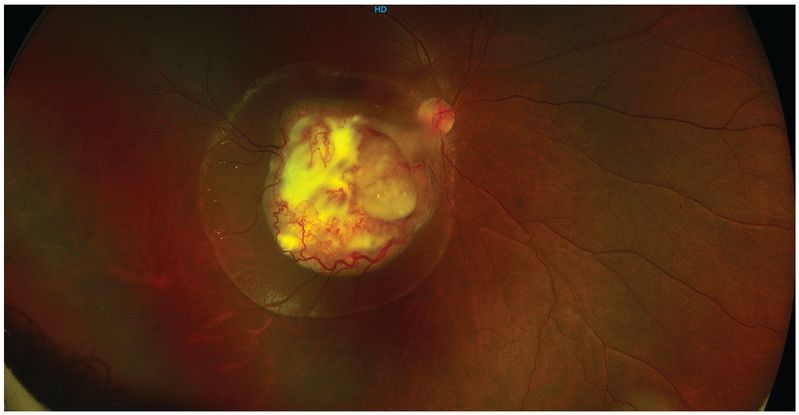
Figure 18. Color fundus picture of the right eye showing localized retinoblastoma lesion involving the macula and extending to the temporal optic margins with surrounding sub-retinal fluid. The overlying feeder vessels are dilated and torturous. The vitreous cavity is clear and rest of the retina is attached. ( “This image was provided by Vikas Khetan, MS, Senior Retinal Consultant, Sankara Nethralaya, Chennai, India. )
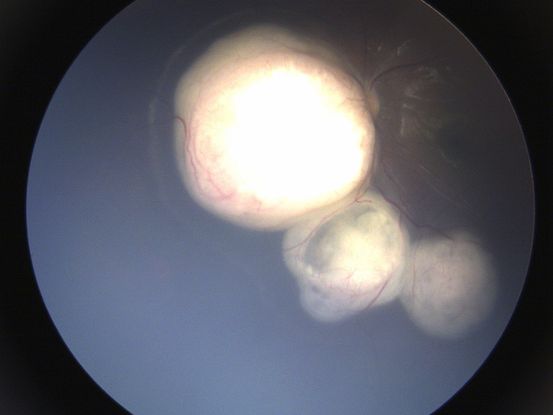
Figure 19. Color fundus picture of the left eye showing multiple retinoblastoma lesion involving the nasal and inferonasal retina and extending on to the optic discs with surrounding sub-retinal fluid. The vitreous cavity is clear and rest of the macula and retina is attached. ( “This image was provided by Vikas Khetan, MS, Senior Retinal Consultant, Sankara Nethralaya, Chennai, India. )
Figure 20. Color fundus photograph showing extensive exudative retinal detachment secondary to underlying retinoblastoma lesion with no view of the disc and macula. (This image was provided by Vikas Khetan, MS, Senior Retinal Consultant, Sankara Nethralaya, Chennai, India. )
Diagnostic workup should include a thorough dilated fundus examination of both eyes, as well as an extensive family history. Additional testing includes A- and B-scan ultrasound and magnetic resonance imaging of the brain and orbits, with the addition of lumbar puncture and/or bone marrow biopsy in patients with concern for extraocular extension.61
Retinoblastoma was initially graded based on the Reese-Ellsworth classification system, which helped predict probability of globe salvage after external beam radiation. More recently, chemotherapy has been favored to decrease the risk of secondary tumors due to radiation. As such, the International Classification of Retinoblastoma was developed in the 1990s to predict which patients can be cured without enucleation (Table 3).62
|
Table 3: International Classification of Retinoblastoma
|
|
Group
|
Examination Findings
|
|
A
|
Intraretinal tumors <3mm in size away from the fovea and optic disc
|
|
B
|
Tumors >3mm in size, located in the macula or near the optic disc, or with subretinal fluid
|
|
C
|
Tumor has local subretinal or vitreous seeding within 3 mm of the tumor
|
|
D
|
Tumor has distant subretinal or vitreous seeding, >3 mm from the tumor
|
|
E
|
Extensive retinoblastoma that occupies more than 50% of the globe. These may be associated with neovascular glaucoma, hemorrhage, or extension of the tumor into the anterior chamber or posteriorly along the optic nerve.
|
Management can include enucleation, radiation, or chemotherapy (administered systemically, into the ophthalmic artery, or via newer intravitreal techniques). As mentioned previously, radiation was previously a mainstay of treatment, but has largely been supplanted by chemotherapeutic treatments due to the risk of secondary tumors with radiation therapy. Systemic chemotherapy is often coupled with focal therapies, a concept called “chemoreduction.” Systemic chemotherapy aims to shrink the tumor, thus making focal therapies more efficacious. Enucleation may still be required for eyes with advanced disease, such as those with group E disease. More recently, intra-arterial chemotherapy has garnered interest. Typically, melphalan is utilized, but patients are placed at risk from multiple fluoroscopies. Further studies are needed in this field.63,64
Patients who pursue globe-conserving treatment in this disease are at risk for developing retinal detachments. Given the decrease in rates of enucleation as newer, more targeted treatments for retinoblastoma emerge, globe conservation is possible in increasing numbers patients; thus, a greater percentage of retinoblastoma survivors are now likely to develop associated retinal detachments. Retinal detachments can be present at the time of diagnosis, but often develop during treatment. Less commonly, they can also develop after treatment of retinoblastoma. Bilateral detachment is more likely to develop in patients with bilateral retinoblastoma. Approximately half of patients who develop retinal detachments present with exudative retinal detachments, while the remaining patients develop rhegmatogenous retinal detachments. Exudative detachments are managed with treatment of the retinoblastoma; rhegmatogenous detachments can develop due to retinal breaks or thinning and are typically managed with scleral buckling and cryotherapy. Scleral buckles can be combined with drainage of subretinal fluid when necessary.65
Toxoplasmosis
Ocular toxoplasmosis (see also: https://eyewiki.org/Toxoplasmosis) (Figures 21-24) is the most common cause of posterior uveitis in children. This disease can be congenitally transmitted or acquired. The offending organism is an intracellular protozoan, Toxoplasmosis gondii. This organism causes inflammation of the retina, choroid, retinal vasculature, and vitreous. Humans acquire T gondii typically through ingestion of undercooked meat, contact with infected cat excrement, or direct inoculation.66 This disease is managed medically with systemic antibiotics, including “triple therapy” with pyrimethamine, sulfadiazine, and corticosteroids. Alternative systemic antibiotic options include trimethoprim-sulfamethoxazole, clindamycin, or azithromycin. In some cases, systemic treatment can be augmented with intravitreal injections of clindamycin.67
Retinal detachments occur in 6% of patients with this disease, and an additional 5% of patients develop retinal breaks, although this cohort included both adults and children. Retinal detachments most often occur during active bouts of ocular toxoplasmosis and are more common in patients with concomitant myopia. The visual prognosis is poor, with more than half of patients progressing to visual acuities of less than 20/200. Exudative detachments are treated by managing the active toxoplasmosis; rhegmatogenous and tractional detachments can be managed with both scleral buckling and vitrectomy techniques.68
Figure 21. Pigmented retinochoroidal toxoplasmosis scar temporal to fovea. (This image was originally published in the Retina Image Bank® website. Author: Andrea Arriola-Lopez, MD MSc. Photographer: Innovacion Ocular. Title: Toxoplasmosis Scar. Image Bank. Year 2021; Image Number 82016© the American Society of Retina Specialists.)
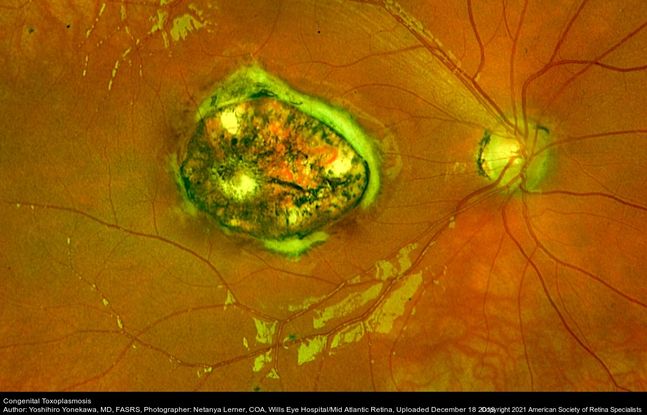
Figure 22. Widefield fundus image of the right eye with an inactive congenital toxoplasmosis macular lesion. (This image was originally published in the Retina Image Bank® website. Author: Yoshihiro Yonekawa, MD, FASRS. Photographer: Netanya Lerner, COA, Wills Eye Hospital/Mid Atlantic Retina. Title: Congenital Toxoplasmosis. Image Bank. Year 2019; Image Number 44596© the American Society of Retina Specialists. )
Figure 23. Color fundus photo of the right eye exhibiting extensive multifocal chorioretinal pigmented congenital toxoplasmosis scars involving the macula and peripheral retina with fibrous vitreous strands. Upper right: color fundus photo of left eye showing an extensive large macular scar extending between the arcades to the periphery. Bottom: fluorescein angiography photos of the right and left eyes depicting hyperfluorescent staining centrally with hypofluorescent borders with no indication of active disease. (This image was provided by Swati Agarwal-Sinha, MD, FASRS, United States .)
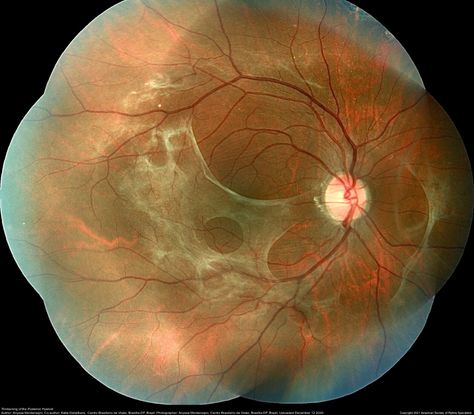
Figure 24. Color fundus photograph of the right eye showing thickening of the posterior hyaloid associated with an epiretinal membrane due to ocular toxoplasmosis. (This image was originally published in the Retina Image Bank® website. Author: Anyssa Montenegro. Photographer: Anyssa Montenegro, Centro Brasileiro da Visão, Brasília-DF, Brazil. Title: Thickening of the Posterior Hyaloid. Image Bank. Year 2020; Image Number 68404© the American Society of Retina Specialists.)
Toxocariasis
Toxocariasis (see also: https://eyewiki.org/Toxocariasis) (Figures 25-28) is due to infection of Toxocara canis or cati, which is a roundworm parasite that lives in the digestive tract of dogs and cats, respectively. Humans become infected via fecal-oral transmission or by eating contaminated foods. The median age of diagnosis is 8.5 years, but this disease can present as early as infancy and through late adulthood.66 Peripheral granulomas can form, and vitreous snowbanks indicate active uveitis.69 Medical management of toxocariasis includes steroid use (including topically, as a periocular injection, or systemically) to decrease inflammation and the subsequent formation of retinal tractional bands. Systemic antiparasitics, including albendazole and thiabendazole, are used by some clinicians but do not have proven efficacy.70
As a result of inflammation, fibrocellular bands form between the peripheral retina and macula or optic nerve. These bands can result in tractional retinal detachments; this traction can also result in retinal breaks, leading to rhegmatogenous retinal detachments. Detachments are typically managed with vitrectomy.69
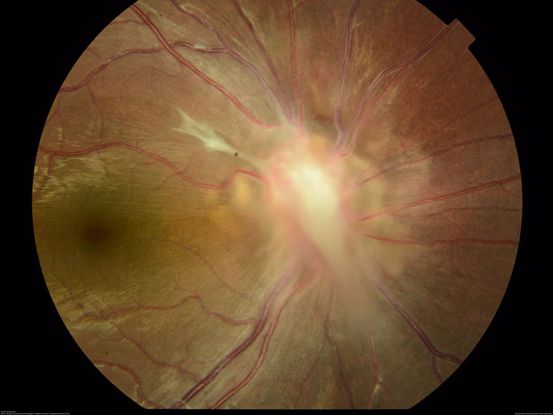
Figure 25. Fundus photo shows a classic localized Toxocara granuloma located over the optic nerve. (This image was originally published in the Retina Image Bank® website. Author: Bastian Schmidt Arias. Photographer: Bastian Schmidt. Title: Ocular Toxocariasis. Image Bank. Year 2018; Image Number 27812© the American Society of Retina Specialists.)
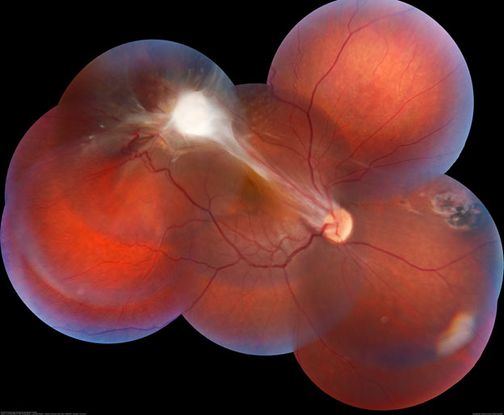
Figure 26. Fundus montage of a 15-year-old male patient with peripheral toxocariasis granuloma, and macular traction. (This image was originally published in the Retina Image Bank® website. Author: Lucas Zago Ribeiro, MD. Photographer: Lucas Zago Ribeiro, Federal University of São Paulo (UNIFESP). Title: Peripheral Toxocariasis Granuloma with Macular Traction. Image Bank. Year 2021; Image Number 78894© the American Society of Retina Specialists.)
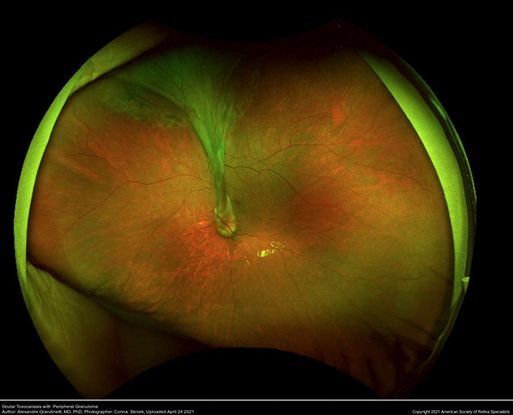
Figure 27. Optos fundus imaging of a 8-year-old boy with a retinal fold secondary to peripheral Toxocara canis granuloma localized on the superior retina. (This image was originally published in the Retina Image Bank® website. Author: Alexandre Grandinetti, MD, PhD. Photographer: Corina Skrzek. Title: Ocular Toxocariasis with Peripheral Granuloma. Image Bank. Year 2021; Image Number 74729© the American Society of Retina Specialists.)
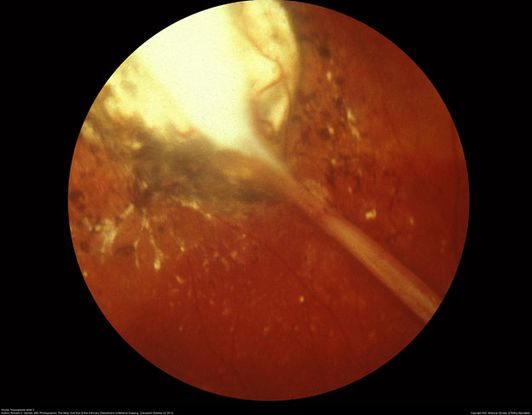
Figure 28. The subretinal scarred Toxocara granuloma in the periphery. It is white in color, elevated with pigment speckling around it. (This image was originally published in the Retina Image Bank® website. Author: Ronald C. Gentile, MD Photographer: The New York Eye & Ear Infirmary Department of Medical Imaging. Title: Ocular Toxocariasis slide 3. Image Bank. Year 2012; Image Number 1732© the American Society of Retina Specialists.)
Diffuse Unilateral Subretinal Neuroretinitis
Diffuse unilateral subretinal neuroretinitis (DUSN) (see also: https://eyewiki.org/Diffuse_Unilateral_Subacute_Neuroretinitis_(DUSN)) (Figures 29-30) is due to nematode-induced inflammation of the eye, resulting in degradation of the outer retina and retinal pigment epithelium (RPE). Patients present with vision loss, visual field defects, and/or photopsias. Later in the course of the disease, patients can develop vitritis, RPE pigmentary changes, and optic disc atrophy or swelling.71
Exudative detachments can form in this disorder due to inflammation from the causative nematode; these detachments can be worsened by the development of proliferative vitreoretinopathy.72 Anti-helminthic oral therapy can be used to kill the causative nematode, and are often prescribed along with corticosteroids. The nematode can also be walled off using later photocoagulation. These treatments decrease the inflammation causing exudation, thus ultimately resolving exudative retinal detachments.71 When surgical intervention is necessary, vitrectomy has been successful; silicone oil may be needed to facilitate retinal reattachment. The offending worm can be removed during vitrectomy if necessary.72
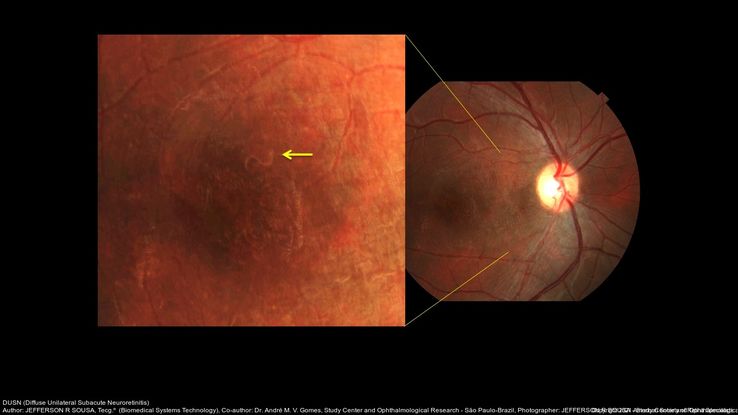
Figure 29. Fundus examination of the right eye shows pigment epithelium macular changes and a small larva juxtafoveal superiorly. (This image was originally published in the Retina Image Bank® website. Author: Jefferson R Sousa. Photographer: JEFFERSON R SOUSA - Study Center and Ophthalmological Research Dr. Andre M V Gomes, Institute Dr. Suel Abujamra São Paulo-Brazil.) Imaging Title: DUSN (Diffuse Unilateral Subacute Neuroretinitis). Year 2016; Image Number 26701© the American Society of Retina Specialists. Title: DUSN (Diffuse Unilateral Subacute Neuroretinitis). Year 2016; Image Number 26701© the American Society of Retina Specialists.
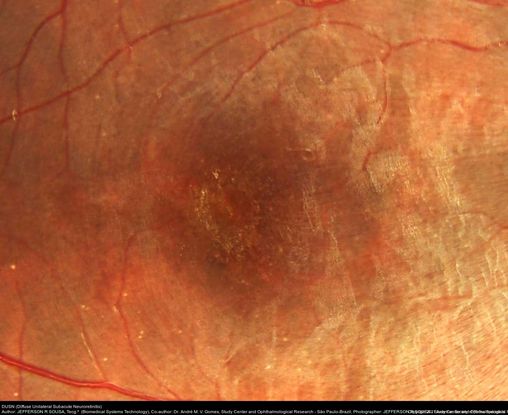
Figure 30. Fundus examination of the right eye shows pigment epithelium macular changes and a small larva juxtafoveal superiorly. (This image was originally published in the Retina Image Bank® website. Author: Jefferson R Sousa. Photographer: JEFFERSON R SOUSA - Study Center and Ophthalmological Research Dr. Andre M V Gomes, Institute Dr. Suel Abujamra São Paulo-Brazil. Imaging Title: DUSN (Diffuse Unilateral Subacute Neuroretinitis). Year 2016; Image Number 26702© the American Society of Retina Specialists.)
Juvenile Xanthogranuloma
Juvenile xanthogranuloma (see also: https://eyewiki.org/Juvenile_Xanthogranuloma) is an idiopathic non-Langerhans cell histiocytic disease that presents in early childhood. While the eye is not commonly affected, Ocular manifestations are numerous and include iris or ciliary body tumors, spontaneous hyphema, glaucoma, cataract, and uveitis.73 Medical management includes topical steroids; periocular or systemic steroids can be added in more recalcitrant cases.74 Rarely, this disease can result in tractional retinal detachments due to fibrosis. Vitrectomy with membrane delamination is necessary to release the tractional component to this disease; however, visual acuity outcomes even with aggressive surgical management can be poor.75
Other Neoplastic and Inflammatory Causes of Pediatric Retinal Detachments
There are other, less common, tumor and inflammation-induced causes of pediatric retinal detachments. These detachments are typically exudative and managed with control of the underlying disorder. These include choroidal hemangiomas (Figures 31-37), medulloepitheliomas (Figures 38-40), choroidal osteomas (Figures 41-45), Sturge-Weber syndrome (Figures 46-50), Wyburn-Mason syndrome (Figures 51-54), Vogt-Koyanagi-Harada disease (Figures 55-57), Bartonella neuroretinitis (Figures 58-62), and intraocular involvement of leukemia (Figures 64-64).
Choroidal Hemangioma
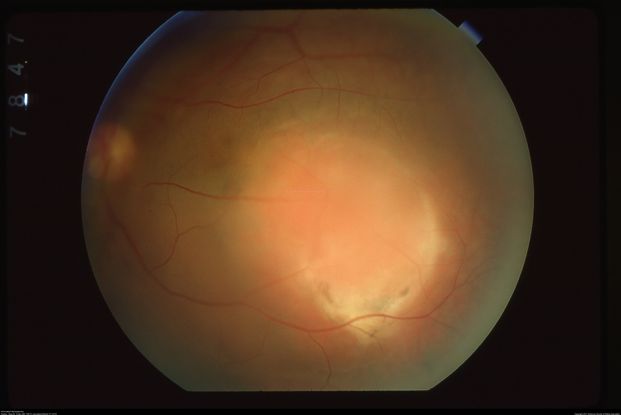
Figure 31. Color fundus of the left eye with choroidal hemangioma at the posterior pole including the macula with overlying subretinal fluid and neurosensory retinal detachment. (This image was originally published in the Retina Image Bank® website. Author: Gary R Cook, MD, FACS. Title: Choroidal Hemangioma Retina Image Bank. Year 2019; Image Number 29687© the American Society of Retina Specialists.)
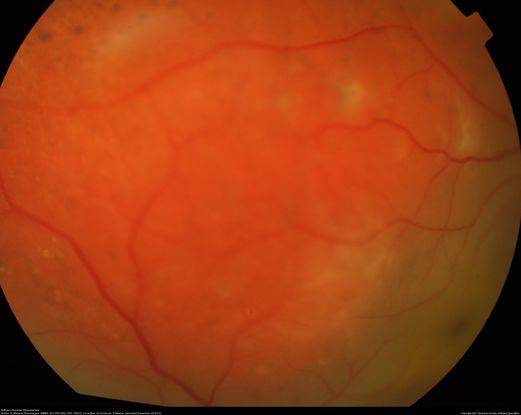
Figure 32. Color fundus of a 12-year-old patient with diffuse choroidal hemangioma and secondary retinal detachment. (This image was originally published in the Retina Image Bank® website. Author: P. Mahesh Shanmugam, MBBS, DO, FRCSEd, PhD, FAICO. Photographer: Sankara Eye Hospitals. Title: Diffuse Choroidal Hemangioma Retina Image Bank. Year 2015; Image Number 26243© the American Society of Retina Specialists.)
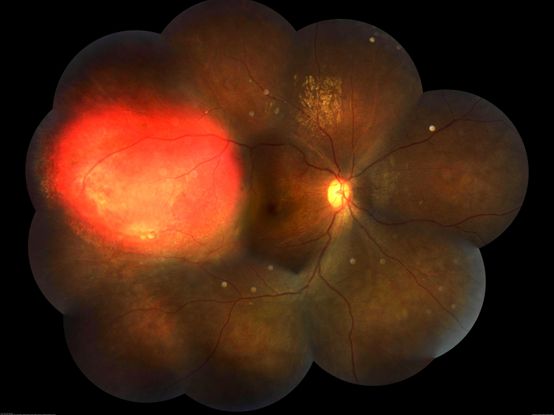
Figure 33. Right eye fundus photo montage of a 17-year-old boy showing a focal choroidal hemangioma temporally. (This image was originally published in the Retina Image Bank® website. Author: Somnath Chakraborty, MD. Photographer: Saptarshi Mehta, Retina Institute of Bengal. Title: Focal Choroidal Hemangioma Retina Image Bank. Year 2018; Image Number 28581© the American Society of Retina Specialists.)
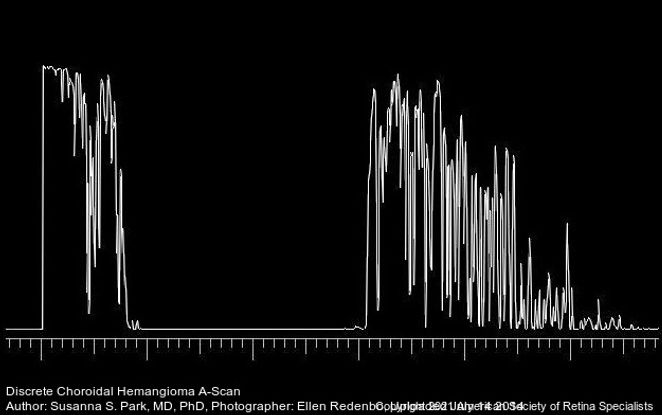
Figure 34. A-scan ultrasonography through a discrete choroidal hemangioma showing high internal reflectivity. (This image was originally published in the Retina Image Bank® website. Author: Susanna S Park, MD, PhD. Photographer: Ellen Redenbo, The University of California Davis Eye Center. Title: Discreet Choroidal Hemangioma A-Scan. Year 2014; Image Number 18800© the American Society of Retina Specialists.)
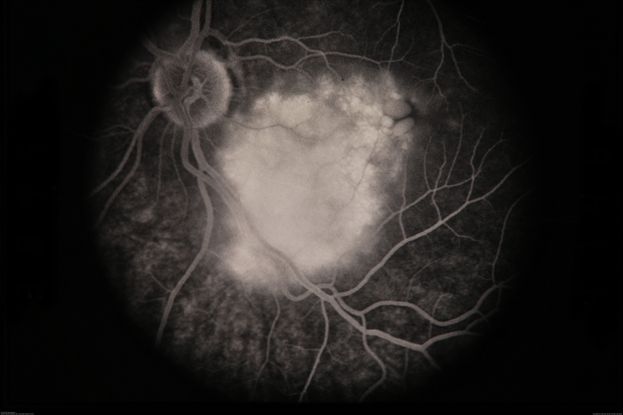
Figure 35. Fluorescein angiography of the left eye showing hyperfluorescent circumscribed choroidal lesion at the posterior pole. (This image was originally published in the Retina Image Bank® website. Author: David Callanan MD. Photographer: Texas Retina Associates Title: Choroidal Hemangioma. Year 2014; Image Number 20326 © the American Society of Retina Specialists.)
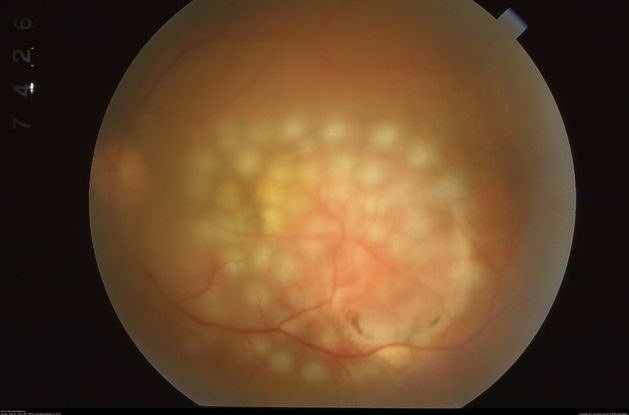
Figure 36. Color fundus photo of the left eye with circumscribed choroidal hemangioma treated with argon laser photocoagulation. (This image was originally published in the Retina Image Bank® website. Author: Gary R Cook, MD, FACS. Title: Choroidal Hemangioma. Year 2019; Image Number 29688© the American Society of Retina Specialists.)
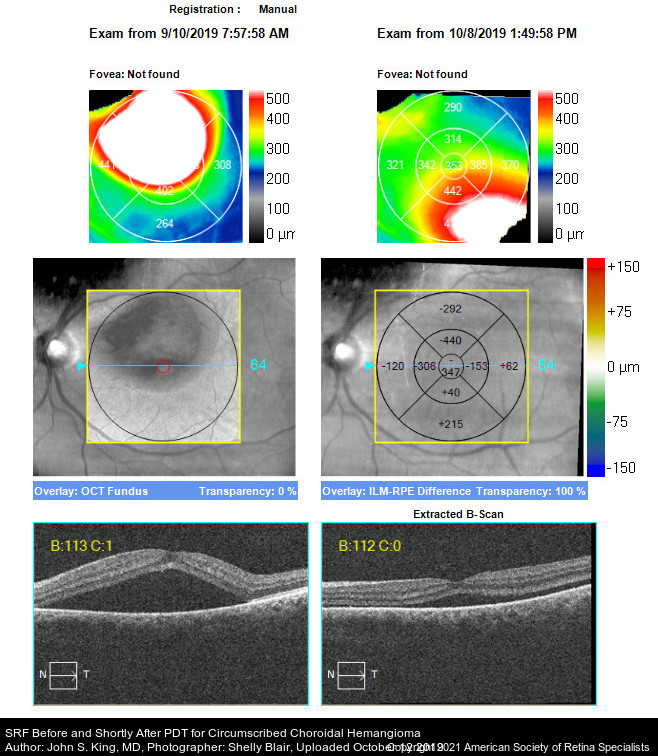
Figure 37. OCT images showing pre and post treatment of choroidal hemangioma with PDT. There is less subretinal fluid post treatment with PDT. (This image was originally published in the Retina Image Bank® website. Author: John S King, MD. Photographer: Shelly Blair, Retina Associates, PA. Title: SRF Before and Shortly After PDT for Circumscribed Choroidal Hemangioma. Year 2019; Image Number 38282© the American Society of Retina Specialists.)
Medulloepithelioma
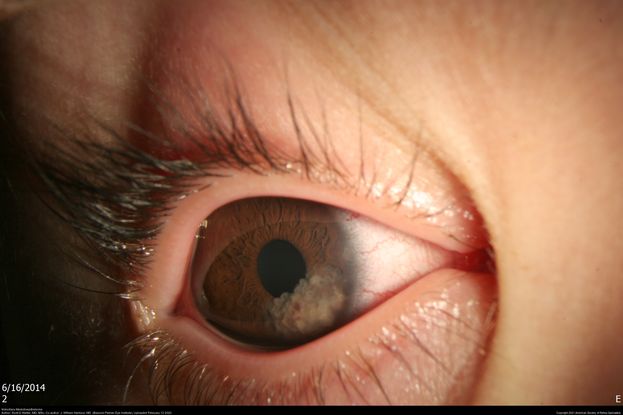
Figure 38. Anterior Segment picture of a 2-year-old male with a translucent, cystic, locally invasive iridociliary tumor consistent with medulloepithelioma prior to treatment. (This image was originally published in the Retina Image Bank® website. Author: Scott D Walter, MD, MSc. Photographer: Retina Consultants, P.C. Title: Iridociliary Medulloepithelioma. Year 2020; Image Number 50715© the American Society of Retina Specialists.)
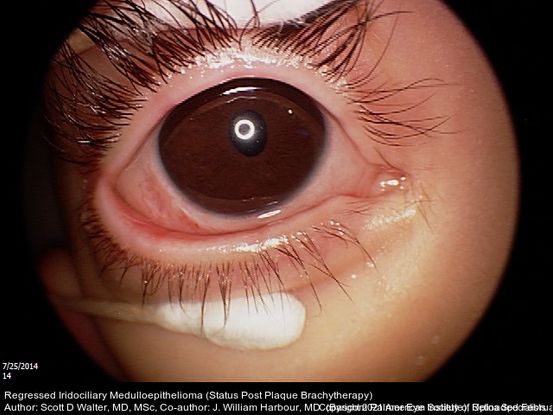
Figure 39. Anterior segment picture of a 2-year-old male with regressed iridociliary medulloepithelioma, one month status post plaque brachytherapy. (This image was originally published in the Retina Image Bank® website. Author: Scott D Walter, MD, MSc. Photographer: Retina Consultants, P.C. Title: Iridociliary Medulloepithelioma. Year 2020; Image Number 50719© the American Society of Retina Specialists.)
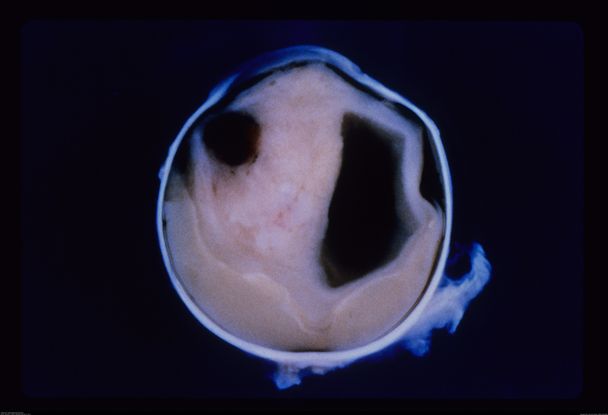
Figure 40. Enucleated eye of a 3-week-old infant with intraocular teratoid medulloepithelioma, filling the entire vitreous cavity. (This image was originally published in the Retina Image Bank® website. Author: Maurice F. Rabb, MD. Photographer: Maurice F. Rabb, MD Title: Intraocular Teratoid Medulloepithelioma. Year 2013; Image Number 11595© the American Society of Retina Specialists.)
Choroidal Osteoma
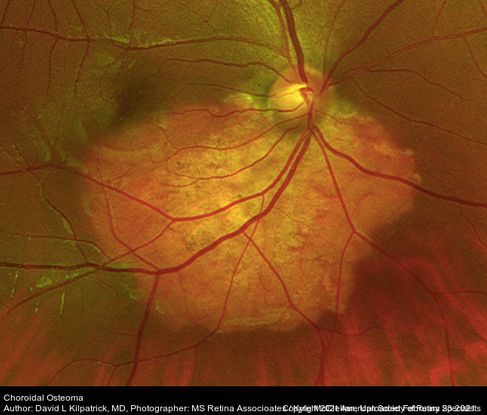
Figure 41. Color fundus photo of the right eye showing inferotemporal juxtapapillary choroidal osteoma. (This image was originally published in the Retina Image Bank® website. Author: David L Kilpatrick, MD. Photographer: Kyle McClellan, Mississippi Retina Associates Title: Choroidal Osteoma. Year 2021; Image Number 72587© the American Society of Retina Specialists.)
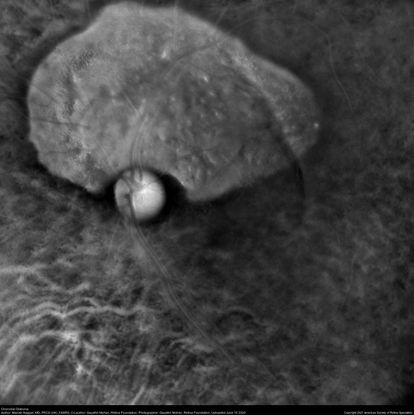
Figure 42. Retromode imaging (Mirante) of a patient with superior choroidal osteoma. (This image was originally published in the Retina Image Bank® website. Author: Manish Nagpal, MD, FRCS (UK), FASRS. Photographer: Gayathri Mohan, Retina Foundation .Title: Choroidal Osteoma. Year 2020; Image Number 57050© the American Society of Retina Specialists.)
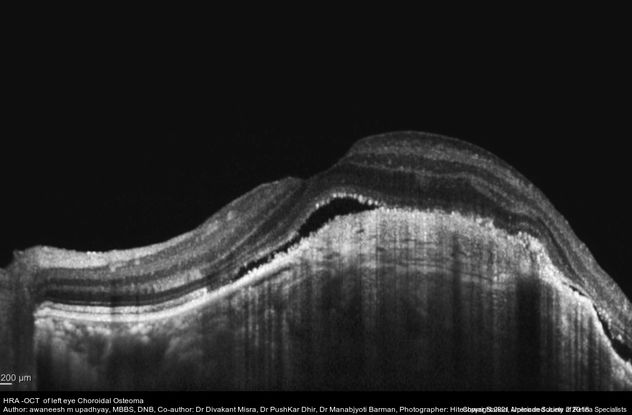
Figure 43. Optical coherence tomography image (Heidelberg Spectralis) showing undulating surface of choroidal osteoma with subretinal fluid and shaggy RPE. (This image was originally published in the Retina Image Bank® website. Author: Awaneesh M Upadhyay, MBBS, DNB. Photographer: Hiteshwar Saikia. Title: HRA -OCT of left eye Choroidal Osteoma. Year 2018; Image Number 28263© the American Society of Retina Specialists.)
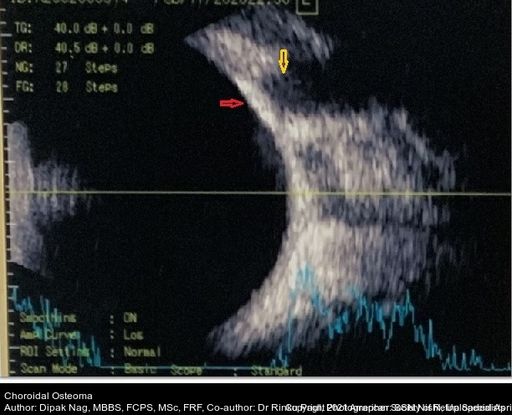
Figure 44. The B scan shows mildly elevated, high reflective choroidal mass (red arrow) with acoustic shadowing of “pseudo-optic nerve” (yellow arrow). The mass persists even in lower gain. A scan showed a high intensity spike. (This image was originally published in the Retina Image Bank® website. Author: Dipak Nag, MBBS, FCPS, MSc, FRF. Photographer: SSN Nishi. Title: Choroidal Osteoma. Year 2020; Image Number 52808© the American Society of Retina Specialists.)
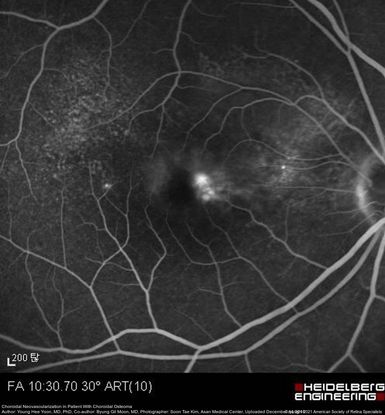
Figure 45. Fluorescein angiography showing choroidal neovascularization at the margin of the choroidal osteoma in the right eye. (This image was originally published in the Retina Image Bank® website. Author: Young Hee Yoon, MD, PhD. Photographer: Soon Tae Kim, Asan Medical Center. Title: Choroidal Neovascularization in Patient with Choroidal Osteoma. Year 2016; Image Number 26834© the American Society of Retina Specialists.)
Sturge-Weber Syndrome
Figure 46. Color fundus photo of a patient with Sturge-Weber syndrome with diffuse choroidal hemangioma with a “tomato catsup fundus.” (This image was originally published in the Retina Image Bank® website. Author: David Callanan MD. Photographer: Texas Retina Associates Title: Sturge-Weber/ Choroidal Hemangioma. Year 2015; Image Number 24735© the American Society of Retina Specialists.)
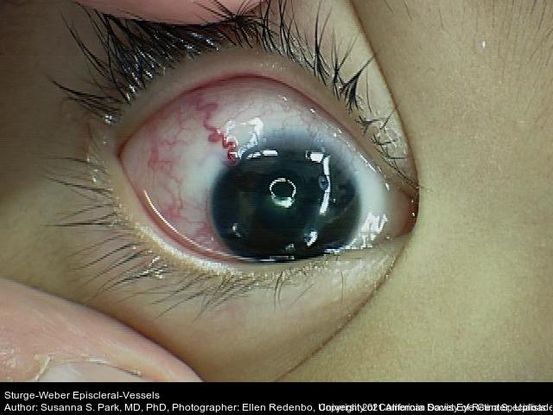
Figure 47. External photo of the right eye of the 8-year-old boy with Sturge-Weber Syndrome and diffuse choroidal hemangioma showing dilated episcleral vessels. (This image was originally published in the Retina Image Bank® website. Author: Susanna S Park, MD, PhD. Photographer: Ellen Redenbo, The University of California Davis Eye Center. Title: Discreet Choroidal Hemangioma A-Scan. Year 2014; Image Number 16767© the American Society of Retina Specialists.)

Figure 48. RetCam fundus photograph of the right eye of an 8-year-old boy with Sturge-Weber Syndrome showing total bullous exudative retinal detachment from a diffuse choroidal hemangioma. (This image was originally published in the Retina Image Bank® website. Author: Susanna S Park, MD, PhD. Photographer: Ellen Redenbo, The University of California Davis Eye Center. Title: Discreet Choroidal Hemangioma A-Scan. Year 2014; Image Number 16768© the American Society of Retina Specialists.)
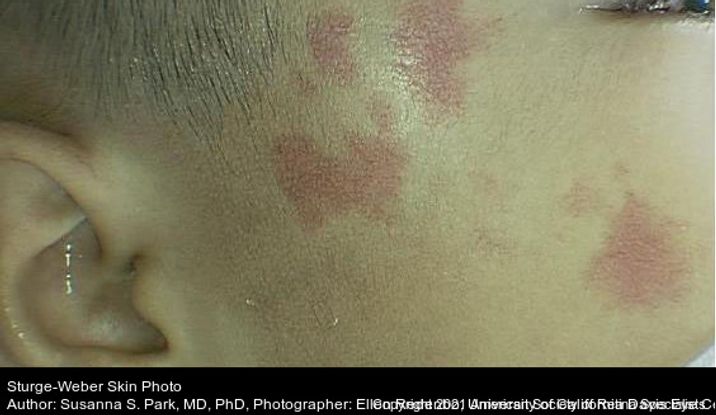
Figure 49. External photo of the face of this 8-year-old boy with Sturge-Weber syndrome and diffuse choroidal hemangioma showing the port wine stain on the ipsilateral side of the face. (This image was originally published in the Retina Image Bank® website. Author: Susanna S Park, MD, PhD. Photographer: Ellen Redenbo, The University of California Davis Eye Center. Title: Discreet Choroidal Hemangioma A-Scan. Year 2014; Image Number 16771© the American Society of Retina Specialists.)
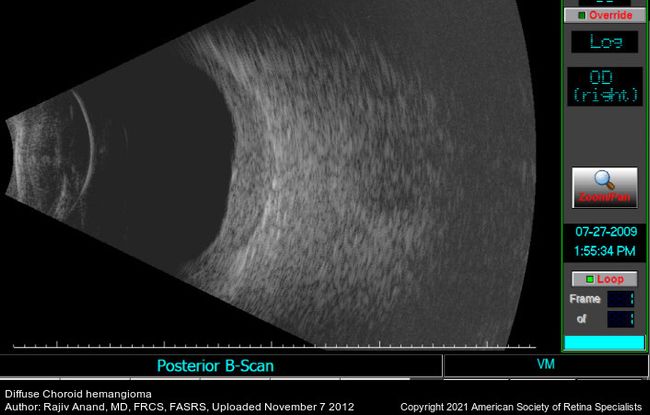
Figure 50. B-scan ultrasound shows the thickened choroid. (This image was originally published in the Retina Image Bank® website. Author: Rajiv Anand, MD, FRCS, FASRS. Photographer: Texas Retina Associates Title: Diffuse Choroidal Hemangioma. Year 2012; Image Number 1972© the American Society of Retina Specialists.)
Wyburn-Mason Syndrome
Figure 51. Color fundus picture of the right eye showing arteriovenous malformations in a patient with Wyburn-Mason Syndrome. (This image was originally published in the Retina Image Bank® website. Author: Gabriel Costa de Andrade, MD. Photographer: Gabriel Costa de Andrade, Retina Clinic/UNIFESP Title: Wyburn-Mason Syndrome. Image Bank. Year 2021; Image Number 72521© the American Society of Retina Specialists.)
Figure 52. Fundus photograph of a 16-year-old patient with poor vision in one eye demonstrates racemose angiomatosis as seen in Wyburn-Mason Syndrome with a sclerosed vessel. (This image was originally published in the Retina Image Bank® website. Author: Caesar K. Luo, MD, FASRS. Photographer: Joseph Trabucco, Progressive Vision Institute, Allentown, PA. Title: Wyburn-Mason Syndrome. Image Bank. Year 2018; Image Number 28305© the American Society of Retina Specialists.)
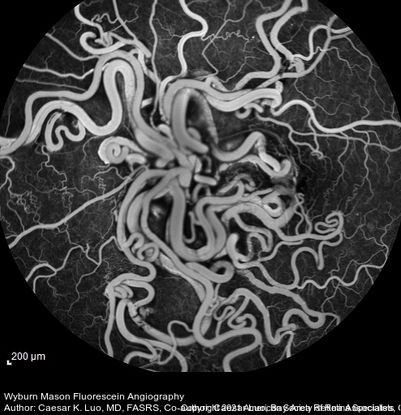
Figure 53. Fluorescein angiography of a 16-year-old patient with unilateral ocular Wyburn-Mason syndrome without fluorescein leak. (This image was originally published in the Retina Image Bank® website. Author: Caesar K. Luo, MD, FASRS. Photographer: Joseph Trabucco, Progressive Vision Institute, Allentown, PA. Title: Wyburn-Mason Syndrome. Image Bank. Year 2018; Image Number 28304© the American Society of Retina Specialists.)
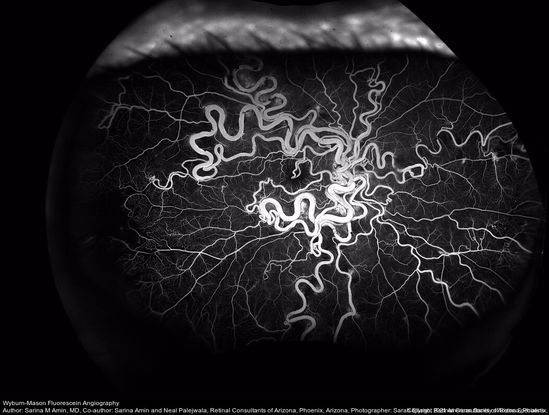
Figure 54. Wide-field fluorescein angiography of a 32-year-old woman with Wyburn-Mason syndrome showing temporal periphery capillary nonperfusion. (This image was originally published in the Retina Image Bank® website. Author: Sarina M Amin, MD. Photographer: Sarah Ellano, Retinal Consultants of Arizona, Phoenix, Arizona. Title: Wyburn-Mason Fluorescein Angiography. Image Bank. Year 2018; Image Number 28152© the American Society of Retina Specialists.)
Vogt-Koyanagi-Harada Disease
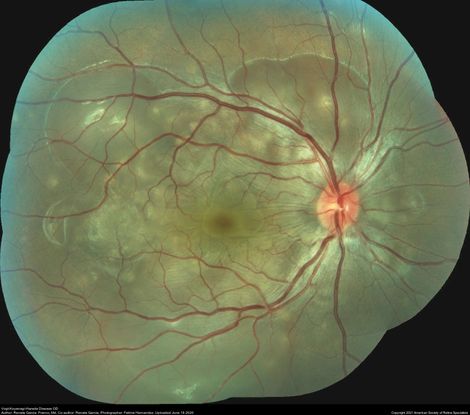
Figure 55. 25-year-old with VHK showing serous retinal detachment in both eyes. (This image was originally published in the Retina Image Bank® website. Author: Renata Garcia Franco, MD. Photographer: Fatima Hernandez, INDEREB. Title: Vogt-Koyanagi-Harada Disease OD. Image Bank. Year 2020; Image Number 58107© the American Society of Retina Specialists.)
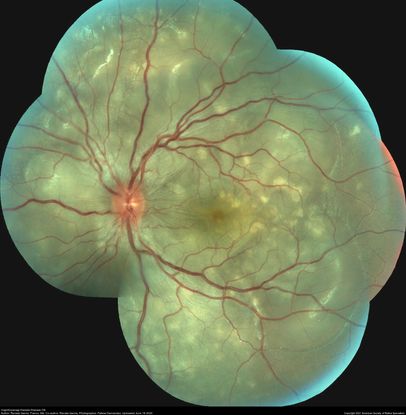
Figure 56. 25-year-old with VHK showing serous retinal detachment in both eyes. (This image was originally published in the Retina Image Bank® website. Author: Renata Garcia Franco, MD. Photographer: Fatima Hernandez, INDEREB. Title: Vogt-Koyanagi-Harada Disease OS. Image Bank. Year 2020; Image Number 58108© the American Society of Retina Specialists.)
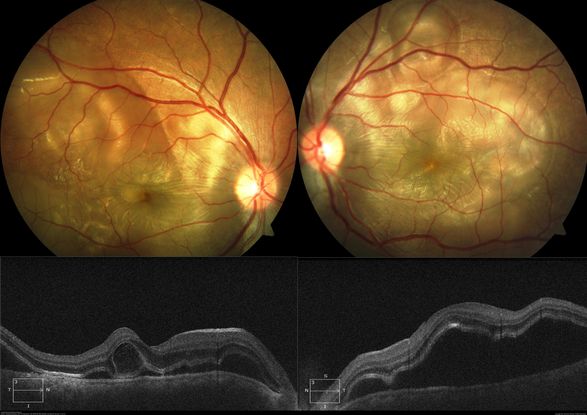
Figure 57. 18-year-old with fundus photographs showing classic picture of acute stage of VKH with multiple large serous RPE detachments and inflamed choroid. OCT shows hallmark features of VKF viz. multiple serous RPEDs with hyperreflective dots, subretinal septa and thick choroid. (This image was originally published in the Retina Image Bank® website. Author: Deepak Bhojwani, MS. Photographer: Deepak Bhojwani. Title: Vogt-Koyanagi-Harada Disease. Image Bank. Year 2019; Image Number 28851© the American Society of Retina Specialists.)
Bartonella
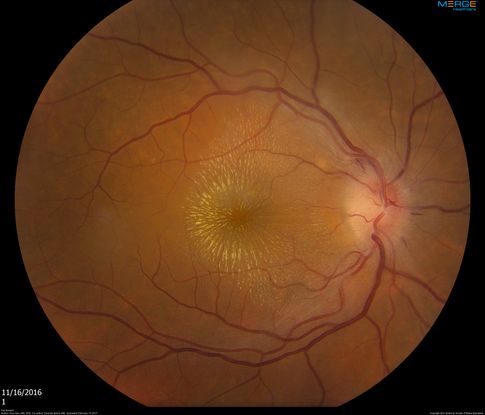
Figure 58. Classic neuroretinitis, exudates in a “macular star” pattern due to cat-scratch disease with positive Bartonella henselae antibodies. (This image was originally published in the Retina Image Bank® website. Author: Hua Gao, MD, PhD. Photographer: Retinal Diagnostic Center. Imaging Title: Cat Scratch. Year 2017; Image Number 26972© the American Society of Retina Specialists.)
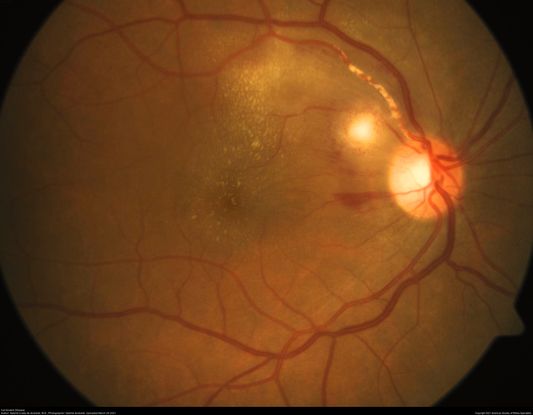
Figure 59. Fundus photograph showing macular vasculitis, pre retinal hemorrhage and exudation due to Bartonella henselae infection. (This image was originally published in the Retina Image Bank® website. Author: Gabriel Costa de Andrade, MD Photographer: Gabriel Andrade. Imaging. Title: Cat Scratch Disease. Image Bank. Year 2012; Image Number 74692© the American Society of Retina Specialists.)
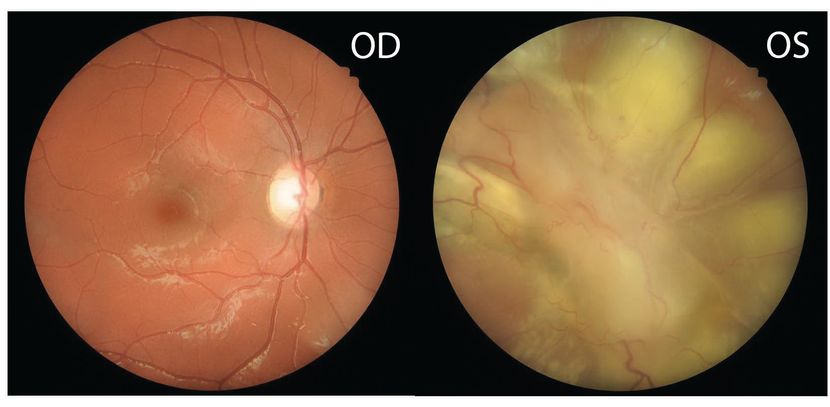
Figure 60. Color fundus photo of right eye showing normal optic disc, vessels, macula. Left eye 2 days after presentation with diffuse exudative retinal detachment with a large inflammatory retinal granuloma obscuring the optic disc. (This image was originally published in the Retina Image Bank® website. Author: Swati Agarwal-Sinha, MD, FASRS. Photographer: Harry Rosa, University of Florida. Imaging. Title: Bartonella Posterior Granulomatous Mass with an Exudative Retinal Detachment. Year 2020; Image Number 64263© the American Society of Retina Specialists.)
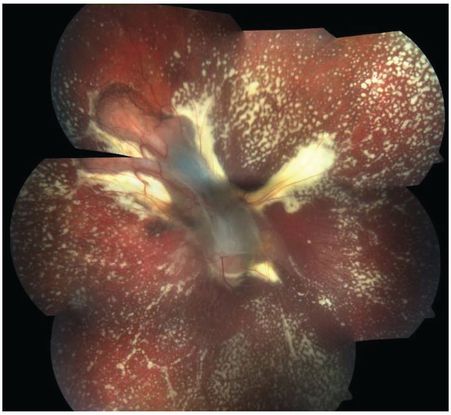
Figure 61. Color fundus photo of the left eye 2 months post treatment showing resolved exudative detachment, fibrosed granuloma at the optic disc, tractional detachment at the posterior pole and extensive diffuse subretinal exudates extending all around up to the retinal periphery. No retinal vascular changes are noted. (This image was originally published in the Retina Image Bank® website. Author: Swati Agarwal-Sinha, MD, FASRS. Photographer: Harry Rosa, University of Florida. Imaging. Title: Bartonella Posterior Granulomatous Mass with an Exudative Retinal Detachment Post 2 Months. Year 2020; Image Number 64265© the American Society of Retina Specialists.)
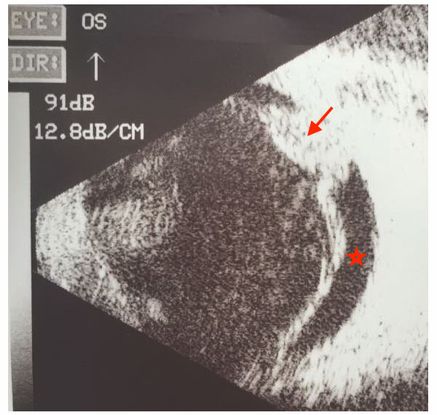
Figure 62. B-scan ultrasound of left eye showing localized inflammatory mass (arrow) at the optic disc with an exudative retinal detachment from Bartonella (*) (This image was provided by Swati Agarwal-Sinha, MD, FASRS, United States.)
Leukemia
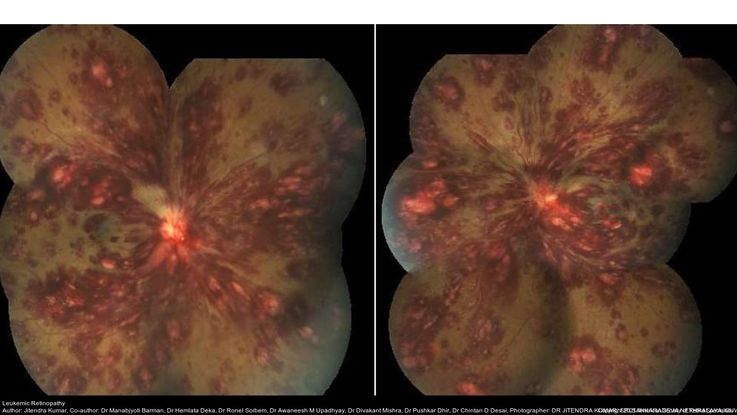
Figure 63. Fundus photograph of a patient with acute leukemia shows extensive retinal hemorrhages in all retinal layers with Roth spots in both eyes. (This image was originally published in the Retina Image Bank® website. Author: Jitendra Kumar. Photographer: Jitendra Kumar, SRI SANKARADEVA NETHRALAYA, GUWAHATI. Imaging Title: Leukemic Retinopathy. Year 2019; Image Number 29982© the American Society of Retina Specialists.)
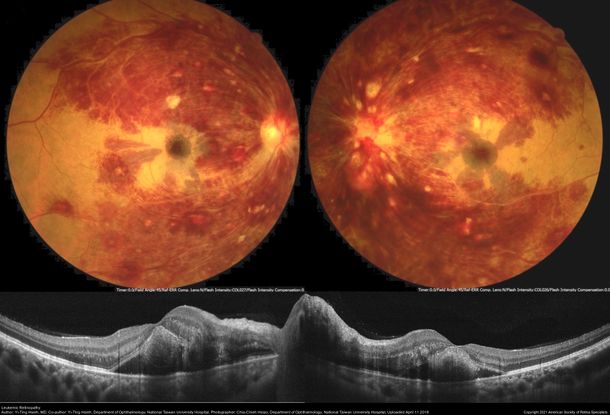
Figure 64. Fundus photograph of a patient with acute promyelocytic leukemia with low platelet count showing extensive retinal hemorrhages in all layers, roth spots extending to the midperiphery along the retinal arcades. The OCT shows macular thickening with edema in both eyes. (This image was originally published in the Retina Image Bank® website. Author: Yi-Ting Hsieh, MD. Photographer: Chia-Chieh Hsiao, Department of Ophthalmology, National Taiwan University Hospital. Imaging Title: Leukemic Retinopathy. Year 2018; Image Number 28076© the American Society of Retina Specialists.)
Combined Hamartoma
Combined hamartomas of the retina (Figure 65-67) and retinal pigment epithelium can be affiliated with epiretinal membranes and retinoschisis and are associated with tractional retinal detachments best managed with vitrectomy and membrane delamination.76 Von Hippel-Lindau disease (Figures 68-69) can result in retinal detachment due to the formation of retinal capillary hemangioblastomas. These tumors typically result in exudative detachments, but they can have a tractional component. Tractional detachments in this disease are best managed with vitrectomy, while exudative detachments are managed by treating the underlying hemangioblastoma.77 Behçet disease is an inflammatory disorder that can result in uveitis. Posterior uveitis can lead to exudative detachments from retinal vasculitis and can lead to rhegmatogenous detachments due to the formation of retinal tears at sites of active retinitis. Exudative detachments are treated with immunosuppression, while retinal tears are managed with barrier laser photocoagulation and/or cryotherapy.78,79
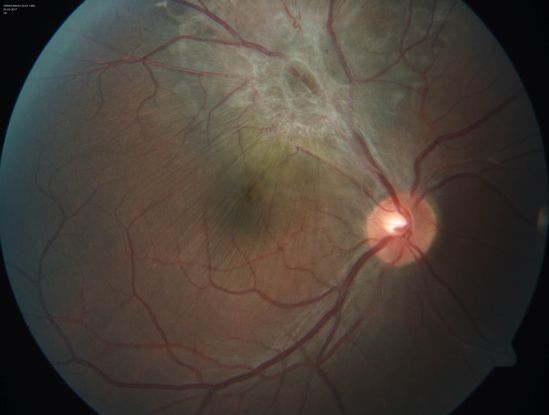
Figure 65. Color fundus photo of with CHRRPE, gliotic epiretinal membrane overlying deep greyish lesion. Retinal wrinkling and dragging of macula is seen. (This image was provided by Vikas Khetan, MS, Senior Retinal Consultant, Sankara Nethralaya, Chennai, India.)
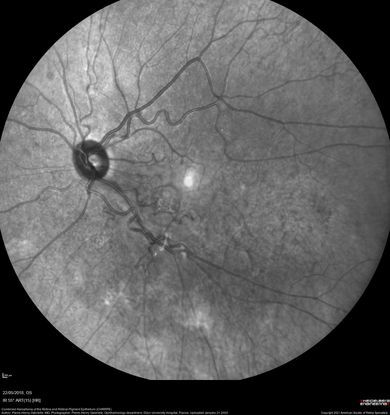
Figure 66. Scanning laser ophthalmoscope (Heidelberg Spectralis) infrared imaging of a 17-year-old with combined hamartomas of the retina and retinal pigment epithelium (CHRRPE) at the posterior pole of the left eye. (This image was originally published in the Retina Image Bank® website. Author: Pierre-Henry Gabrielle, MD. Photographer: Pierre-Henry Gabrielle, Ophthalmology department, Dijon University Hospital, France. Title: Combined Hamartoma of the Retina and Retinal Pigment Epithelium (CHRRPE). Image Bank. Year 2020; Image Number 46686© the American Society of Retina Specialists.)
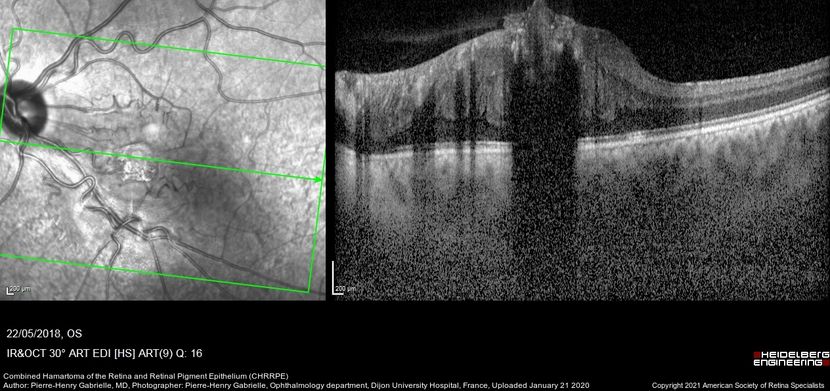
Figure 67. Coupled OCT B-scan and IR imaging of a 17-year-old with combined hamartomas of the retina and retinal pigment epithelium (CHRRPE) at the posterior pole of the left eye. A highly reflective elevated macular lesion with hyporeflective shadowing of the underlying tissue and obscuration of the normal retinal layers can be seen. (This image was originally published in the Retina Image Bank® website. Author: Pierre-Henry Gabrielle, MD. Photographer: Pierre-Henry Gabrielle, Ophthalmology department, Dijon University Hospital, France. Title: Combined Hamartoma of the Retina and Retinal Pigment Epithelium (CHRRPE). Image Bank. Year 2020; Image Number 46688© the American Society of Retina Specialists.)
Von Hippel-Lindau Syndrome
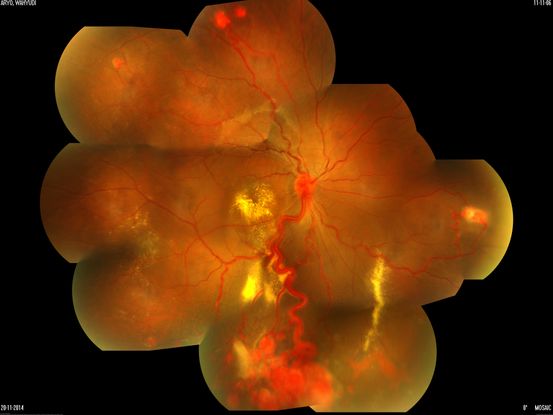
Figure 68. Fundus photograph of a patient with von Hippel-Lindau Disease and multiple retinal angiomas with exudates at the macula and in the retinal periphery. (This image was originally published in the Retina Image Bank® website. Author: Sjakon G Tahija, MD. Photographer: Avris Siahaan, Klinik Mata Nusantara. Title: Von Hippel-Lindau Syndrome. Image Bank. Year 2016; Image Number 26396© the American Society of Retina Specialists.)
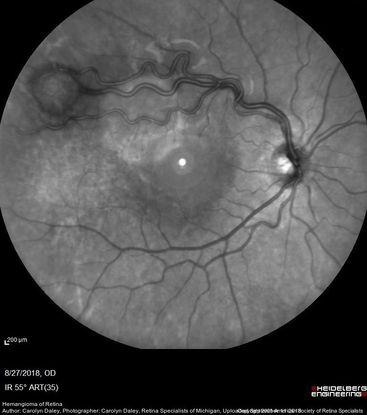
Figure 69. 50-degree OCT imaging (Heidelberg Spectralis) of a 20-year-old with multiple bilateral retinal hemangiomas diagnosed with von Hippel-Lindau Syndrome. (This image was originally published in the Retina Image Bank® website. Author: Carolyn Daley. Photographer: Carolyn Daley, Retina Specialists of Michigan. Title: Hemangioma of Retina. Image Bank. Year 2018; Image Number 28572© the American Society of Retina Specialists.)
Endophthalmitis
Endophthalmitis ((see also: https://eyewiki.aao.org/Endophthalmitis) (Figures 70-74) can also result in rhegmatogenous or exudative retinal detachments; in the pediatric population, endophthalmitis is more commonly due to trauma. It is presumed that the trauma that caused endophthalmitis also predisposed the child to a rhegmatogenous retinal detachment through a retinal break. This disorder is best managed with vitrectomy, combined with intravitreal antibiotics, as this treats the concomitant endophthalmitis.80 Systemic antibiotics are indicated when the endophthalmitis arises from an endogenous source.81 See Table 6 for further discussion of the above conditions.
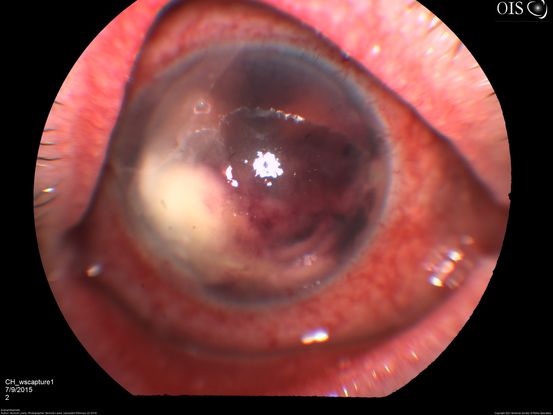
Figure 70. Anterior segment picture with hypopyon in AC and features of endophthalmitis. (This image was originally published in the Retina Image Bank® website. Author: Nichole Lewis. Photographer: Nichole Lewis, Retina Consultants of Sacramento. Imaging Title: Endophthalmitis. Year 2018; Image Number 27878© the American Society of Retina Specialists.)
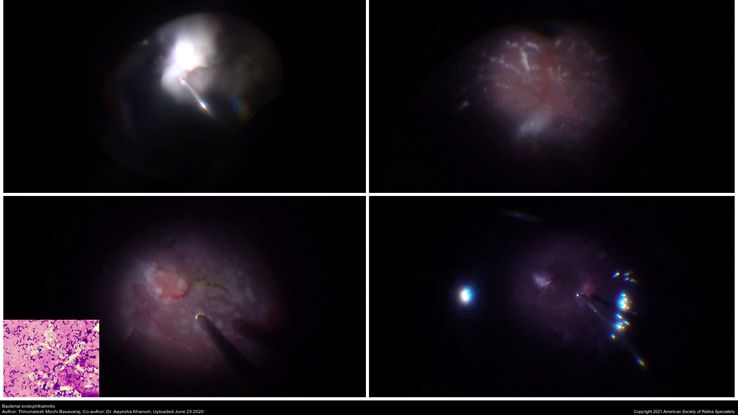
Figure 71. Intraoperative pictures showing bacterial colonies, lab-confirmed gram positive cocci (inset). (This image was originally published in the Retina Image Bank® website. Author: Thirumalesh Mochi Basavaraj. Photographer: Narayana Nethralaya. Imaging Title: Bacterial Endophthalmitis. Year 2020; Image Number 58118© the American Society of Retina Specialists.)
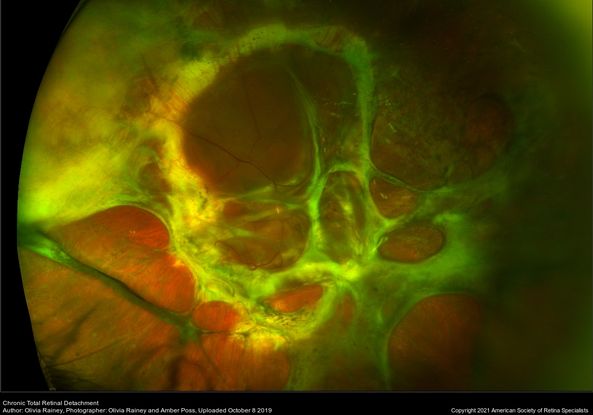
Figure 72. Ultra-wide field pseudocolor image of a patient with a chronic total retinal detachment affecting his right eye. Patient had Klebsiella endophthalmitis. (This image was originally published in the Retina Image Bank® website. Author: Olivia Rainey. Photographer: Olivia Rainey and Amber Poss, Retina Specialist of Michigan. Imaging Title: Chronic Total Retinal Detachment. Year 2019; Image Number 37264© the American Society of Retina Specialists.)
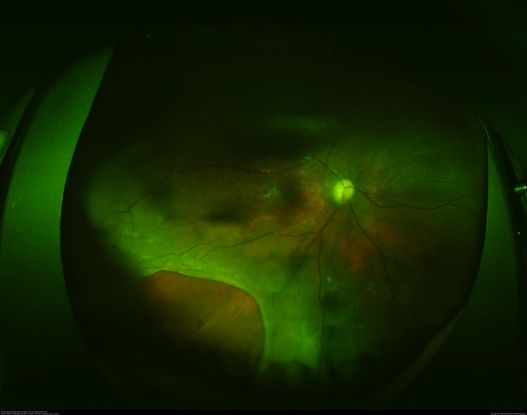
Figure 73. Fundus shows retinal detachment secondary to retinal necrosis following treatment of postoperative endophthalmitis. (This image was originally published in the Retina Image Bank® website. Author: Philip J. Polkinghorne, MD. Imaging Title: Post-Endophthalmitis Retinal Detachment and Necrosis. Year 2014; Image Number 18786© the American Society of Retina Specialists.)
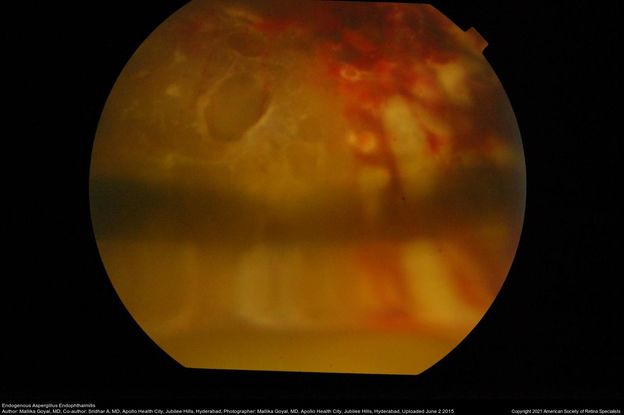
Figure 74. Fundus photograph of left eye of a 13-year-old boy at presentation with endogenous aspergillus endophthalmitis with retinal detachment and silicon oil in situ. Aspergillus was seen on smears and cultured from his vitreous. Retinal breaks, shallow retinal detachment and inferior meniscus of silicon oil are seen here. (This image was originally published in the Retina Image Bank® website. Author: Mallika Goyal, MD. Photographer: Mallika Goyal, MD, Apollo Health City, Jubilee Hills, Hyderabad. Imaging Title: Endogenous Aspergillus Endophthalmitis. Year 2015; Image Number 25044© the American Society of Retina Specialists.)
Other Common Causes of Pediatric Retinal Detachments
Trauma
Ocular trauma (Figures 75-85) is one of the most common causes of RD in males in the pediatric age group.82 Unlike other etiologies of rhegmatogenous retinal detachment in children, the contralateral eye frequently does not have any pathology predisposing it to retinal detachment, unless it was also involved in the trauma. Retinal detachment can occur both with open- and closed-globe injuries, although it is more common with open-globe injuries.83,84 PVR forms quickly in these patients and results in a worse anatomical and visual outcome. Thus, early surgical intervention is recommended. Retinal detachment repair surgery should be performed four to seven days after the initial trauma to allow vitreous detachment to occur, thus facilitating separation of vitreous from the retina.84
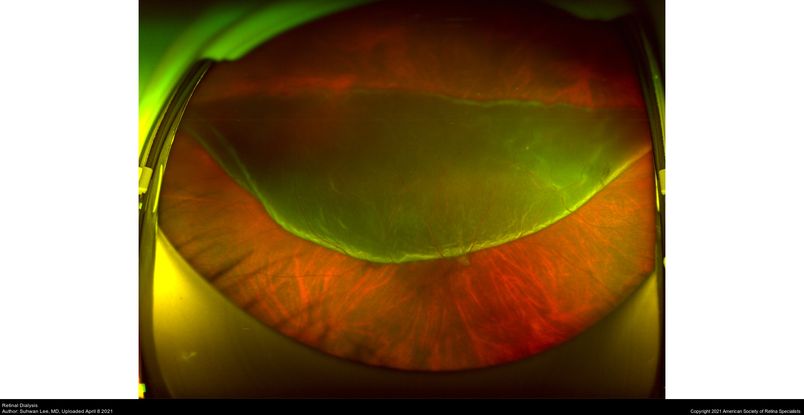
Figure 75. Ultra-wide fundus photograph of a patient with a retinal dialysis occurred after trauma. (This image was originally published in the Retina Image Bank® website. Author: Suhwan Lee, MD. Photographer: Hanvit Eye Center. Imaging Title: Retinal Dialysis. Year 2021; Image Number 74702© the American Society of Retina Specialists.)
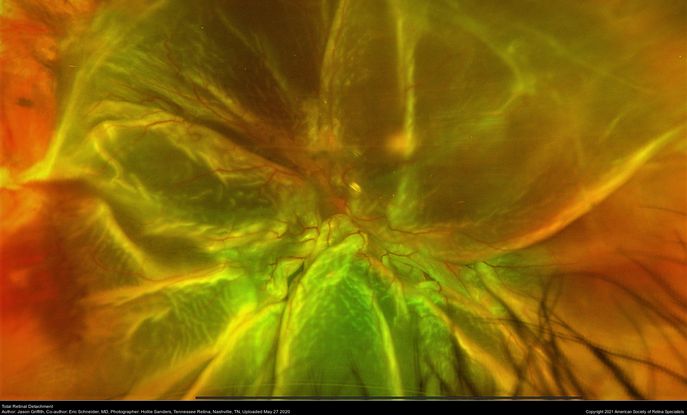
Figure 76. Fundus picture showing traumatic total retinal detachment following blunt trauma. (This image was originally published in the Retina Image Bank® website. Author: Jason Griffith. Photographer: Hollie Sanders, Tennessee Retina, Nashville, TN. Imaging Title: Total Retinal Detachment. Year 2020; Image Number 55981© the American Society of Retina Specialists.)
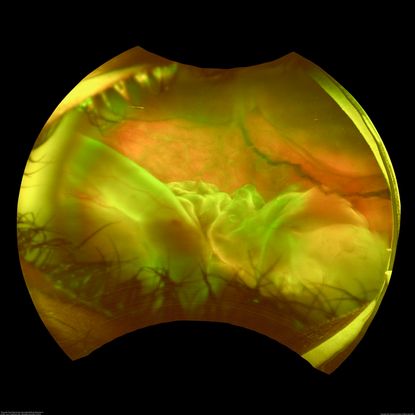
Figure 77. This wide field fundus photograph of the left eye shows a traumatic giant retinal tear associated with total retinal detachment. The image shows the torn superior retina folded over the macula with the underside of the retina visible. There is associated peripheral choroidal detachment due to hypotony from giant retinal tear. (This image was originally published in the Retina Image Bank® website. Author: Luis J Haddock, MD. Photographer: University of Miami. Imaging Title: Traumatic Giant Retinal Tear Associated Retinal Detachment. Year 2019; Image Number 40431© the American Society of Retina Specialists.)
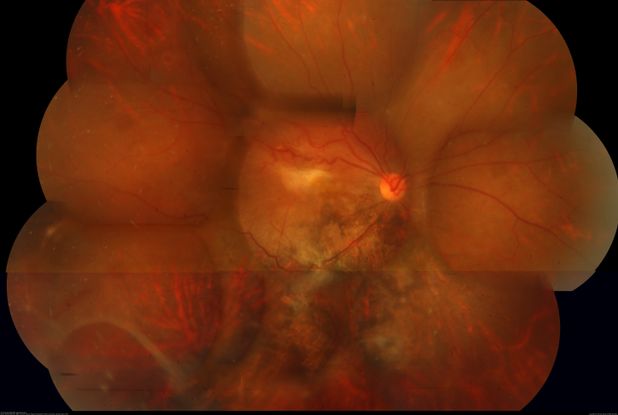
Figure 78. Fundus photo showing an epiretinal membrane with large retinal tear and pigmentary changes, 2 months following blunt trauma in a young male. (This image was originally published in the Retina Image Bank® website. Author: Navneet Mehrotra, DNB. Photographer: Mehul Choudhary, Retina Care and Retina Foundation. Imaging Title: Post Traumatic ERM With Large Retinal Tear. Year 2018; Image Number 28064© the American Society of Retina Specialists.)
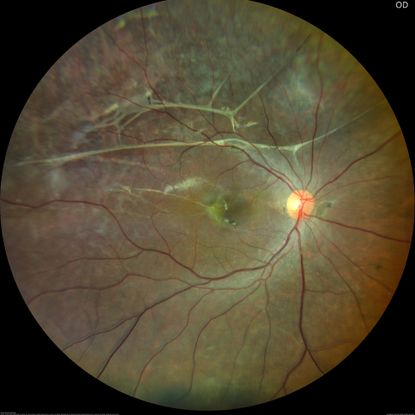
Figure 79. Fundus image of a 16-year-old patient with a history of trauma 1 year showing spontaneous regressed retinal detachment. (This image was originally published in the Retina Image Bank® website. Author: Vishal Gupta, MBBS, MS. Photographer: Dr Shobhit Chawla, Prakash Netra Kendr, Lucknow, UP, INDIA. Imaging Title: Spontaneously Settled RD. Year 2021; Image Number 80927© the American Society of Retina Specialists.)
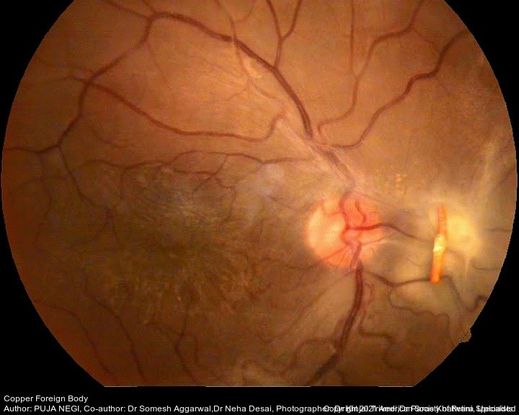
Figure 80. Fundus photo of young male patient following trauma with a copper wire. (This image was originally published in the Retina Image Bank® website. Author: Puja Negi. Photographer: Dr Kinjal Trivedi, Dr Paras Khatwani, M and J Institute of Ophthalmology, Ahmedabad. Imaging Title: Copper Foreign Body. Year 2021; Image Number 74796© the American Society of Retina Specialists.)
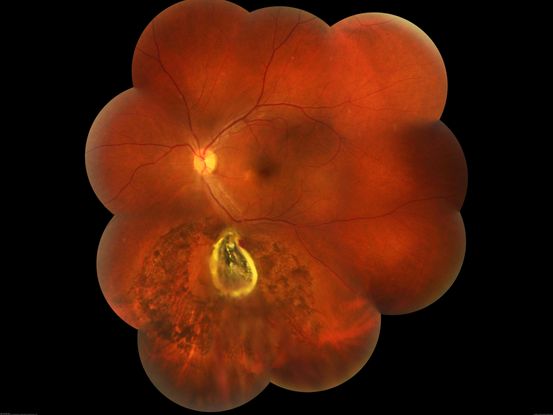
Figure 81. Fundus photo montage of left eye of a patient showing a large iron foreign body, impacted inferior to the inferotemporal branch vessels with a large patch of surrounding chorioretinal atrophy, secondary to resolving commotio retinae. (This image was originally published in the Retina Image Bank® website. Author: Somnath Chakraborty, MD. Photographer: Saptarshi Mehta, Retina institute of Bengal, Siliguri. Imaging Title: Intraocular Foreign Body. Year 2019; Image Number 28850© the American Society of Retina Specialists.)
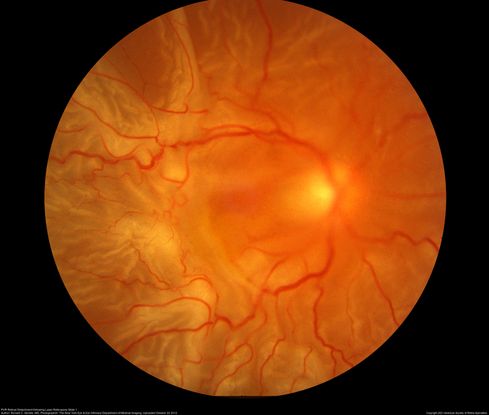
Figure 82. Fundus photos shows total retinal detachment with PVR post laser retinopexy. (This image was originally published in the Retina Image Bank® website. Author: Ronald C. Gentile, MD. Photographer: The New York Eye & Ear Infirmary Department of Medical Imaging. Imaging Title: PVR Retinal Detachment following Laser Retinopexy Slide 1. Year 2012; Image Number 1755© the American Society of Retina Specialists.)
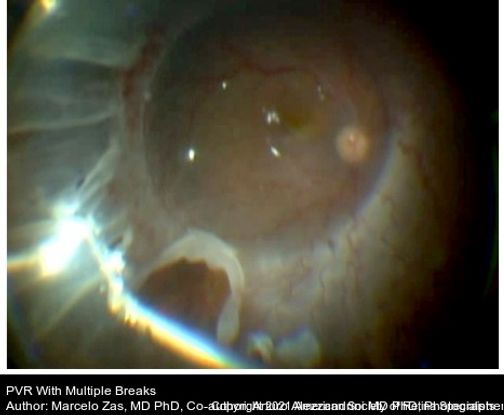
Figure 83. The image shows PVR with detached retina, PFCL in the posterior pole, diathermy done before the retinectomy. (This image was originally published in the Retina Image Bank® website. Author: Marcelo Zas MD PhD. Photographer: Marcelo Zas MD PhD. Imaging Title: PVR Retinal Detachment following Laser Retinopexy Slide 1. Year 2014; Image Number 15304© the American Society of Retina Specialists.)
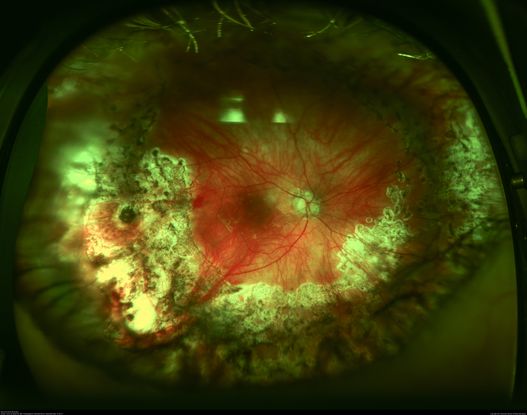
Figure 84. Optos wide field imaging shows recurrent PVR retinal detachment after repair. (This image was originally published in the Retina Image Bank® website. Author: John W. Kitchens, MD. Photographer: Michelle Buck, Retina Associates of Kentucky. Imaging Title: Recurrent RD (Post-Op). Year 2014; Image Number 18052© the American Society of Retina Specialists.)
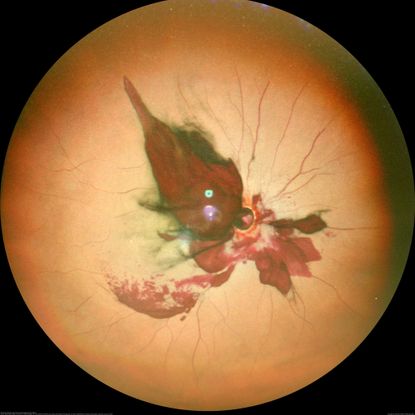
Figure 85. Fundus photo with history of trauma to RE showing optic nerve avulsion, vitreous hemorrhage and pale retina. (This image was originally published in the Retina Image Bank® website. Author: Sham Talati, DOMS. Photographer: Dr. Sham Talati, Retina Foundation, Ahmedabad. Imaging Title: Optic Nerve Avulsion with Vitreous Hemorrhage and Pale Retina. Year 2021; Image Number 71520© the American Society of Retina Specialists.)
Previous Intraocular Surgery
The rates of rhegmatogenous retinal detachment after prior intraocular surgery in children vary widely, so their true incidence is unknown. However, there is general significant lag time between the intraocular surgery and subsequent development of retinal detachment, with most detachments in this population presenting after age 10 years.9 Because many of these patients have had surgery in both eyes, such as for bilateral congenital cataracts, they are predisposed to presenting with bilateral retinal detachments.85 Pars plana vitrectomy with intraocular gas or silicone oil tamponade is the recommended treatment modality in these patients.58
Myopia
Retinal detachments secondary to myopia (Figures 86-92) in the pediatric population are rising due to the global epidemic of progressive myopia. The prevalence of myopia also rises with age, and this disease is especially common in East Asia, where up to 69% of fifteen-year-olds are affected.86 Multiple factors including genetics, exposure to outdoor light, prolonged near work, and intensity of near work are responsible for the dramatic increase in prevalence of myopia over the past 40 years.72,87–91 Myopia in younger children is frequently associated with congenital ocular or systemic anomalies. Like adults, children with myopia are predisposed to lattice degeneration and retinal tears, prompting formation of rhegmatogenous retinal detachments. Scleral buckling procedures are typically pursued as primary treatment in this population and have had success rates of 58% in patients with myopia; they can be successful even in eyes with >10 diopters of myopia.92
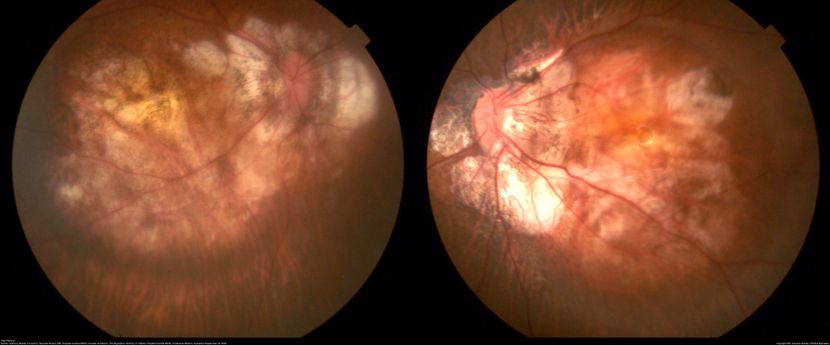
Figure 86. Fundus photos of both eyes with high myopia showing annular crescents with extensive chorioretinal atrophy at the posterior pole. (This image was originally published in the Retina Image Bank® website. Author: Anthony Maida. Photographer: Anthony C. Maida, Hospital Central Militar, Ciudad de México. Imaging Title: High Myopia. Year 2020; Image Number 64258© the American Society of Retina Specialists.)

Figure 87. Fundus image of a myopic fundus with temporal crescent and macular hole related retinal detachment at the posterior pole. (This image was originally published in the Retina Image Bank® website. Author: Deepak Bhojwani, MS. Photographer: DEEPAK BHOJWANI FOR OCCURA EYE CARE. Imaging Title: Retinal Detachment Associated with Myopic Macular Hole. Year 2021; Image Number 74695© the American Society of Retina Specialists.)
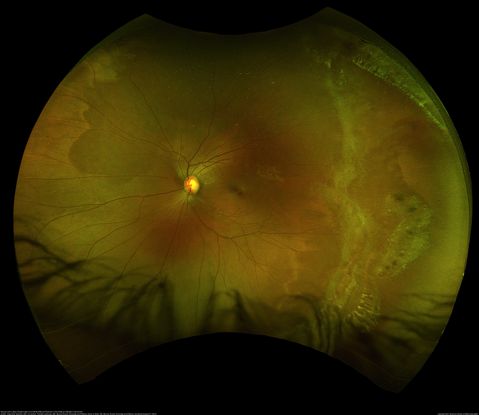
Figure 88. Fundus photo of a 1-year-old with Marfan syndrome, myopia, congenital nystagmus both eyes and exotropia right eye. Ultra-wide field imaging of her left eye shows lattice degeneration with atrophic retinal holes temporally, in addition to multiple sections of white without pressure. (This image was originally published in the Retina Image Bank® website. Author: Sophia El Hamichi, MD. Photographer: Murray Ocular Oncology and Retina. Imaging Title: Myopia with Lattice Degeneration and White Without Pressure in the Setting of Marfan's Syndrome. Year 2020; Image Number 62204© the American Society of Retina Specialists.)
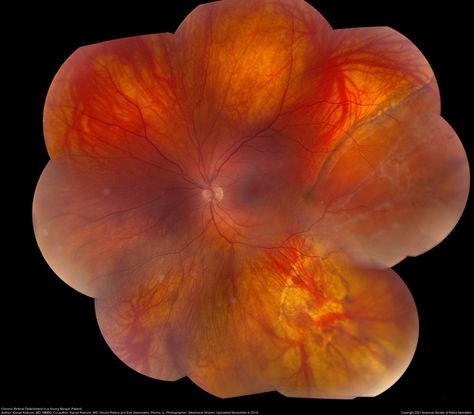
Figure 89. Chronic retinal detachment in a myopic patient showing spontaneous reattachment in inferotemporal quadrant, and demarcation line and subretinal gliosis in superotemporal quadrant. (This image was originally published in the Retina Image Bank® website. Author: Kamal Kishore, MD, MBBS. Photographer: Stephanie Shaver, Illinois Retina and Eye Associates. Imaging Title: Chronic Retinal Detachment in Young Myopic Patient. Year 2019; Image Number 40413© the American Society of Retina Specialists.)
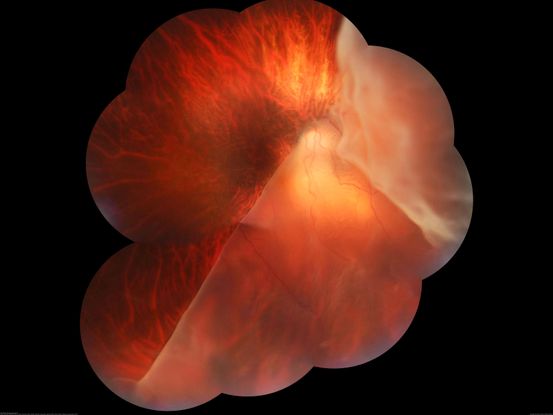
Figure 90. Fundus photograph of a patient with pathological myopia and retinal detachment secondary to giant retinal tear. (This image was originally published in the Retina Image Bank® website. Author: Somnath Chakraborty, MD. Photographer: Saptarshi Mehta, Retina Institute of Bengal. Imaging Title: Giant Retinal Tear-Retinal Detachment. Year 2017; Image Number 27206© the American Society of Retina Specialists.)
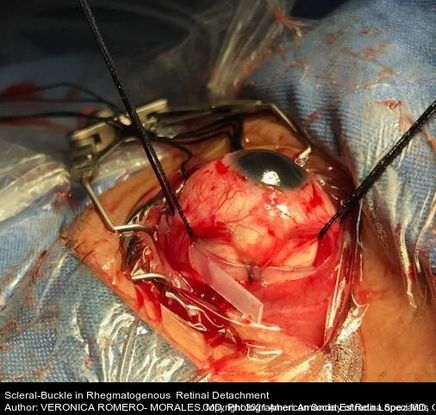
Figure 91. Trans-surgical photograph of a patient with phakic eye, high myopia and macula off rhegmatogenous retinal detachment. (This image was originally published in the Retina Image Bank® website. Author: Veronica Romero-Morales, MD. Photographer: Amanda Estrada López MD, GOVisión, Queretaro, Mexico. Imaging Title: Scleral-Buckle in Rhegmatogenous Retinal Detachment. Year 2019; Image Number 44599© the American Society of Retina Specialists.)
Coats Disease
Coats disease (see also: https://eyewiki.org/Coats_Disease) is a unilateral disorder of the retinal vasculature (Figure 92-97). Its etiology is unclear; at least 75% of patients are male. This disease is characterized by retinal aneurysms and telangiectasias, which can result in retinal exudates and subsequent exudative retinal detachment.93,94 Patients are diagnosed at an average age of 5 years. Shields and colleagues composed a classification scheme for Coats disease, described in Table 4.95
|
Table 4: Shields’ Classification of Coats Disease95
|
|
Stage
|
Examination Findings
|
|
1
|
Only retinal telangiectasias
|
|
2
|
Exudations and telangiectasias
A: exudation spares fovea
B: exudation involves fovea
|
|
3
|
Exudative retinal detachment
A: Subtotal detachment (further classified into 1/extrafoveal and 2/foveal)
B: Total detachment
|
|
4
|
Neovascular glaucoma due to total retinal detachment
|
|
5
|
End-stage disease
|
In this disorder, prevention of retinal detachment in the early stages is critical. Prophylactic laser and/or cryotherapy treatment is typically indicated for stage 2 disease. The goal in this stage is to ablate the telangiectasias via laser photocoagulation, or less commonly, cryopexy. Often, multiple treatment sessions are required.96 Treatment with anti-vascular endothelial growth factor (anti-VEGF) agents can be useful in the early stages of this disease.97
In more advanced-stage disease in which exudative retinal detachment exists, laser photocoagulation and/or cryotherapy can be considered when the retinal detachment is shallow. However, if cryotherapy is used, it should not be applied to more than 2 quadrants in a single session, as more widespread treatments can result in an increase in exudation.95 For more extensive detachments, surgical techniques vary widely. Some surgeons choose to pursue more intensive surgical repair, including vitrectomy, scleral buckling, tamponade with intraocular gas or silicone oil, and a posterior retinotomy.98,99
However, other surgeons advocate a less invasive approach, combining a posterior sclerotomy to drain subretinal fluid, cryotherapy, and infusion of balanced salt solution into the vitreous without vitrectomy. Some authors report that fairly equivalent outcomes have been achieved with either the more- or less-invasive approaches; success rates for each treatment vary by study.100 Rishi and colleagues found a 33% anatomic success rate with scleral buckling, 57% success with subretinal fluid drainage, and 88% success rate with vitrectomy; however, patients were not randomized to treatment, so there was no control for confounding variables which could skew the reported outcomes.101
Another, more novel, therapeutic technique for exudative retinal detachments in this population include external drainage of subretinal fluid and/or laser photocoagulation, coupled with a series of anti-VEGF injections.102,103 Early reports indicate good anatomical outcomes in these patients, although this approach may not be appropriate for patients who have already developed vitreoretinal fibrosis, as anti-VEGF agents in this population can cause further vitreoretinal traction.104
Figure 92. Widefield color fundus photo montage of a patient with RE Coats disease, showing diffuse exudates along with subfoveal nodule. (This image was originally published in the Retina Image Bank® website. Author: Gayathri Mohan. Photographer: Dr. Gayathri Mohan- Retina Foundation. Imaging Title: Coat's Disease - Widefield Montage. Year 2019; Image Number 41526© the American Society of Retina Specialists.)
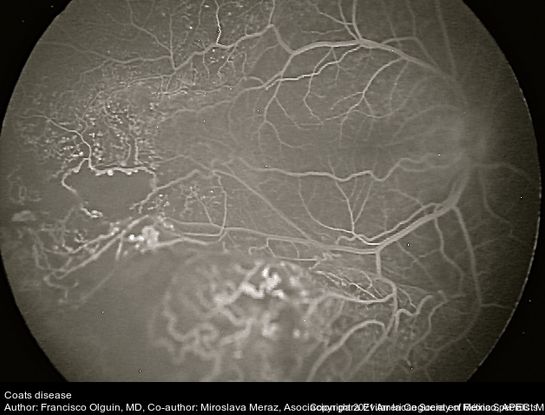
Figure 93. Fundus photograph of a 6-year-old child with leukocoria, FA showed peripheral areas of non-perfusion, telangiectasias and aneurysms. (This image was originally published in the Retina Image Bank® website. Author: Francisco Olguin, MD. Photographer: Francisco Olguin, Asociacion para Evitar la Ceguera en México, APEC. Mexico City. Imaging Title: Coat's Disease. Year 2018; Image Number 27846© the American Society of Retina Specialists.)
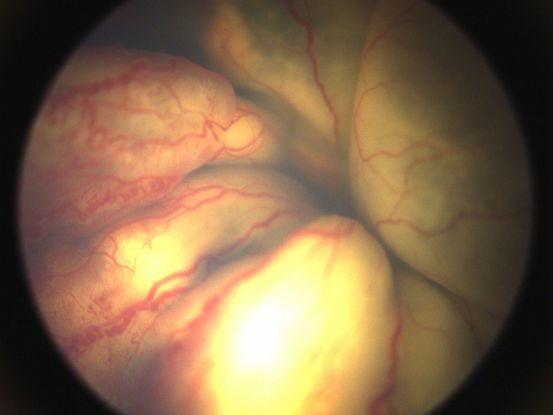
Figure 94. Fundus photos of the right eye showing total exudative retinal detachment with telangiectasias and saccular aneurysms of the overlying retinal vessels. (This image was provided by Vikas Khetan, MS, Senior Retinal Consultant, Sankara Nethralaya, Chennai, India.)
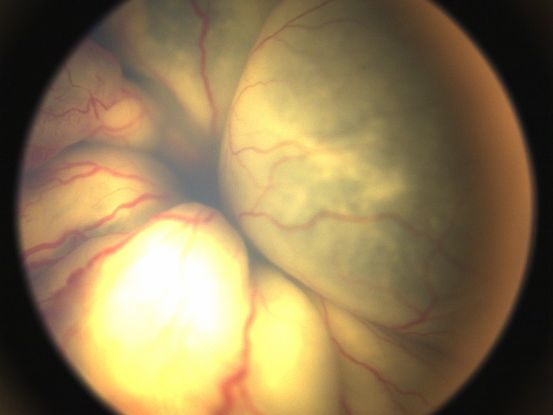
Figure 95. Fundus photos of the left eye showing total exudative retinal detachment with telangiectasias and saccular aneurysms of the overlying retinal vessels. (This image was provided by Vikas Khetan, MS, Senior Retinal Consultant, Sankara Nethralaya, Chennai, India.)
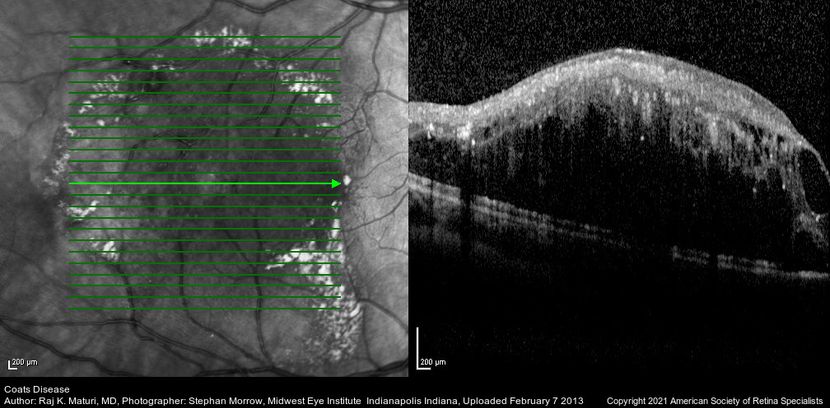
Figure 96. Scanning laser ophthalmoscope (Heidelberg Spectralis) imaging in a 8-year-old with Coats disease. (This image was originally published in the Retina Image Bank® website. Author: Raj K. Maturi, MD. Photographer: Stephan Morrow, Midwest Eye Institute Indianapolis Indiana. Imaging Title: Coat's Disease. Year 2013; Image Number 3420© the American Society of Retina Specialists.)
Table 5: Genetic/Vascular Causes of Pediatric Retinal Detachments
|
Disease
|
Age of Onset
|
Inheritance
|
Gene/Locus
|
Clinical Features
|
Management
|
|
Familial Exudative Vitreoretinopathy16,115–117
|
Often presents within the first year of life. However, misdiagnosis is common.
|
X-linked (NDP mutation)
Autosomal dominant (FZD4, LPR5 mutations)
Autosomal recessive (LPR5 mutation)
|
NDP (X chromosome)
FZD4 (chromosome 11q)
LPR5 (chromosome 11q)
|
Bilateral, macular dragging, retinal neovascularization, subretinal exudation, tractional retinal detachment, radial retinal folds.
Retinal detachments can be exudative, tractional, or rhegmatogenous.
|
Laser photocoagulation, cryotherapy, and anti-VEGF agents can help manage exudation and neovascularization.
Retinal detachment can be managed with vitrectomy or scleral buckling.
|
|
Retinopathy of Prematurity25
|
Birth
|
None
|
None
|
Bilateral, associated with premature birth, fibrovascular proliferation, and avascular retina. Retinal detachments are tractional.
|
Laser photocoagulation and/or anti-VEGF agents for patients without detachments.
Retinal detachments are managed with scleral buckling or vitrectomy; combining these 2 procedures is not of benefit.
|
|
Norrie Disease36,117
|
Early infancy
|
X-linked recessive
|
NDP (Xp11.4-p11.3)
|
Bilateral, leukocoria, glaucoma, cataract, microphthalmos, total retinal detachment, gray/yellow glistening mass that replaces the retina and is made of immature retinal cells, sensorineural deafness. Retinal detachments are tractional.
|
Laser photocoagulation can have moderate success at birth.
Early vitrectomy can help maintain light perception.
Visual prognosis is very poor even with the above treatments.
|
|
Incontinentia Pigmenti39,118
|
Retinal detachment typically present by age 6
|
X-linked dominant; lethal in males
|
IKBKG (Xq2)
|
Bilateral, peripheral retinal avascularity neovascularization, and hemorrhages; optic nerve atrophy, cherry red fovea; seizures. Systemic manifestations include defects of the hair, skin, and nails. Retinal detachments are tractional and or exudative.
|
Laser photocoagulation or cryotherapy to avascular retina.
Retinal detachments are typically treated with vitrectomy, sometimes with the addition of a scleral buckle.
|
|
Persistent Hyperplastic Primary Vitreous41,43
|
Birth
|
Not applicable
|
Not applicable
|
Unilateral, leukocoria, microphthalmos, cataract, shallow anterior chamber, retrolental fibrovascular membrane. Retinal detachments are tractional.
|
Vitrectomy combined with lensectomy (when cataract is present)
|
|
X-linked Retinoschisis46,119
|
Age of onset of retinal detachment varies
|
X-linked
|
RS1 gene (Xp22)
|
Blunted foveal reflex with cystic fovea present 100% with 50% peripheral retinoschisis involving the nerve fiber layer, pigmentary atrophy at the fovea in older patients, vitreous hemorrhage. Retinal detachments are rhegmatogenous.
|
Retinal detachment can be managed either with scleral buckling or vitrectomy.
|
|
Stickler Syndrome3,49,50
|
20% of patients have a retinal detachment before age 10
|
Autosomal dominant
|
COL2A1/ 12q13.11
(type 1); COL11A1/ 1p21.1
(Type 2)
|
Cleft palate, flattened facies, diffuse and progressive arthropathy, neurosensory hearing loss, and spondyloepiphyseal dysplasia,
empty vitreous, vitreous veils/strands, radial lattice degeneration. Retinal detachments are rhegmatogenous.
|
Prophylactic barrier laser photocoagulation can prevent retinal detachments.
Vitrectomy is recommended over scleral buckling.
|
|
Marfan Syndrome52,56
|
Mean age of onset of retinal detachment is 26 years, but can present in childhood.
|
Autosomal dominant
|
FBN1 (15q21.1)
|
Tall, skeletal anomalies, aortic dissection, ectopia lentis, myopia, poor pupillary dilation. Retinal detachments are rhegmatogenous.
|
Retinal detachments can be managed with either vitrectomy or scleral buckling.
|
|
Nontraumatic Inferotemporal Retinal Dialysis58,59
|
Young males in the second decade of life
|
Unknown
|
Unknown
|
Patients are typically emmetropic with normal vitreous. They have peripheral cystoid degeneration, resulting in retinal dialysis. Detachments are often shallow, thin, and chronic; patients generally do not present until the macula is involved.
|
Scleral buckling with cryotherapy in the contralateral eye if pathology is present.
|
Table 6: Tumors and Uveitis as Causes of Pediatric Retinal Detachments
|
Disease
|
Age of Onset
|
Clinical Features
|
Management
|
|
Retinoblastoma64,65
|
Can present with exudative detachment, or during treatment of retinoblastoma, but can also occur years later.
|
Patients often present with leukocoria or strabismus. Retinal detachments are exudative or rhegmatogenous. The retina can develop thinning or atrophic holes, predisposing to detachment.
|
Exudative detachments typically resolve spontaneously, at an average of fifteen weeks with treatment. Rhegmatogenous detachments can be successfully managed with scleral buckling and cryotherapy or laser.
|
|
Choroidal hemangioma120
|
Typically, presents in the third to sixth decade of life, but can present as early as age six.
|
Presents as a circular, orange or red elevated mass or as diffuse choroidal hemangioma in patients with Sturge Weber syndrome. Patients can develop floaters, blurred vision, visual field deficits, or metamorphopsia. Retinal detachments are exudative.
|
Retinal detachments are typically managed by treating the tumor. Tumor can be treated with photodynamic therapy, external beam radiation, transpupillary thermotherapy, plaque brachytherapy, anti-VEGF agents, or laser photocoagulation.
|
|
Medulloepithelioma121
|
Typically presents between ages 2-10 years.
|
Patients can present with strabismus. Cataracts and glaucoma are common. Retinal detachments are exudative.
|
Management of the medulloepithelioma is typically with enucleation, cryotherapy, plaque brachytherapy, external beam radiotherapy, or resection. Specific management of the detachment is not indicated.
|
|
Choroidal Osteoma122
|
Varies
|
Presents as a yellow/orange mass with well-defined borders and branching vessels. Choroidal neovascularization is often present.
Retinal detachments are exudative.
|
Subretinal fluid typically resolves spontaneously. Anti-VEGF agents can be used to decreased choroidal neovascularization and associated subretinal fluid and exudative detachment.
|
|
Sturge-Weber Syndrome123
|
Varies
|
Port-wine stain, glaucoma, and increased conjunctival vascularization, circumscribed or diffuse choroidal hemangioma. Retinal detachments are exudative.
|
Retinal detachments are treated by tumor destruction or decreasing its leakage. Laser photocoagulation, photodynamic therapy, anti-VEGF injections, brachytherapy, and external beam radiotherapy have all been used.
|
|
Combined Hamartoma of the Retina and Retinal Pigment Epithelium76
|
Typically diagnosed in school-age children
|
Patients may have concomitant neurofibromatosis. Epiretinal membranes and retinoschisis are common. Retinal detachments are tractional.
|
Retinal detachments are typically managed with vitrectomy with membrane delineation to release epiretinal traction.
|
|
Von Hippel-Lindau Syndrome77
|
Varies widely—can present from school-age children to the elderly
|
Patients are predisposed to systemic tumors, both benign and malignant. Retinal detachments are due to concomitant retinal capillary hemangioblastoma. Retinal detachments are typically exudative, but may have tractional components.
|
Vitrectomy cam be employed for tractional retinal detachments. Exudative detachments are treated by managing the underlying hemangioblastoma, which can be done with photocoagulation or cryotherapy. Using anti-VEGF agents is increasingly under consideration.
|
|
Wyburn-Mason Syndrome124,125
|
Varies
|
Manifestations are due to location of arteriovenous malformations. Neurologic abnormalities, including seizures, visual field deficits, and cranial neuropathies, can be found. Ocular findings include retinal or vitreous hemorrhages, retinal vein occlusions, optic disc edema, and optic atrophy. Retinal detachments are exudative.
|
Exudative detachments in this disorder are treated by managing the arteriovenous malformation, typically via anti-VEGF injections, sub-tenon triamcinolone injection, or laser photocoagulation.
|
|
Behçet Disease78,79
|
Varies
|
Other ocular manifestations include anterior uveitis and retinal vasculitis. Retinal detachments can be exudative or rhegmatogenous.
|
Exudative detachments are typically due to retinal vasculitis and are treated with immunosuppression. Retinal tears can be found at the site of active lesions and are managed with laser photocoagulation.
|
|
Vogt-Koyanagi-Harada Disease126
|
Typically, late adolescence or early adulthood, but can present later
|
Presents with uveitis, neurologic symptoms, yellow-orange fundus, alopecia, and vitiligo. Retinal detachments are exudative.
|
Exudative detachments typically resolve with management of the underlying uveitis.
|
|
Toxoplasmosis68
|
Varies; can present as early as five years of age
|
Chorioretinal scarring is typically seen.
Retinal detachments and breaks form during active attacks of toxoplasmosis. Both rhegmatogenous and tractional retinal detachments can occur.
|
Either vitrectomy or scleral buckling can be performed.
|
|
Toxocariasis69
|
Varies; can present as young as age six years
|
Retinal granuloma, which can involve the posterior pole. Retinal tears can form from localized retinal traction. Retinal detachments are rhegmatogenous or tractional.
|
Pars plana vitrectomy is typically performed in both tractional and rhegmatogenous detachments.
|
|
Bartonella127
|
Varies
|
Retinal vasculitis and uveitis are common. Associated optic disc edema can cause peripapillary serous retinal detachments. Retinal detachments are exudative.
|
Retinal detachments typically resolve with steroids over a period of weeks.
|
|
Diffuse Unilateral Subretinal Neuroretinitis71,72
|
Varies, but typically presents before twenty years of age
|
Patients can present with optic disc edema or atrophy, vitritis, and retinal arteriolar narrowing. Retinal detachments are exudative.
|
Vitrectomy can be performed, during which the causative worm can be extracted.
|
|
Leukemia128
|
Varies; typically, before ten years of age
|
Leukemic masses can be present subretinally. Intraretinal hemorrhages and orbital granulomatous sarcomas can also be found. Retinal detachments are exudative.
|
Treatment of the underlying leukemia treats the retinal detachment.
|
|
Juvenile Xanthogranuloma73,75
|
Early childhood
|
Can present with hyphema, heterochromia, and anisocoria. Retinal detachments are tractional.
|
Retinal detachments are treated with vitrectomy and membrane delamination.
|
|
Endophthalmitis80
|
Varies
|
Endophthalmitis is more commonly due to trauma in children than in adults, and thus some retinal detachments are presumed due to the inciting trauma. Retinal detachments are rhegmatogenous or exudative.
|
Retinal detachments are typically managed with vitrectomy with concomitant endophthalmitis, as this also treats the causative endophthalmitis.
|
Table 7. Other Common Causes of Pediatric Retinal Detachment
|
Disease
|
Age of Onset
|
Clinical Features
|
Management
|
|
Myopia129
|
Varies; the earlier the onset of myopia, the more likely the patient is to reach high myopia and subsequent complications
|
Poor uncorrected visual acuity that typically worsens with time. Retinal detachments are rhegmatogenous.
|
Prophylaxis of retinal breaks with laser photocoagulation.
Management of retinal detachment in children is typically with scleral buckling procedures. Scleral buckling can be combined with vitrectomy when needed.
|
|
Coats Disease93–104
|
Elementary school age
|
Most commonly males; exudation, telangiectasias. Retinal detachments are exudative.
|
Treat telangiectasias with photocoagulation or cryopexy as prophylaxis against retinal detachment. Anti-VEGF agents are growing in popularity.
Retinal detachments can be treated by draining subretinal fluid via sclerotomies. More invasive approaches with vitrectomy and scleral buckling have also been used. Silicone oil is often used as tamponade in vitrectomy cases.
|
|
Trauma84
|
Typically, older children
|
History of trauma; often have other sequelae of trauma, such as globe rupture. Retinal detachments are rhegmatogenous.
|
Removal of intraocular foreign body and repair of ruptured globe, if present.
Traumatic retinal breaks can be treated with laser photocoagulation.
Vitrectomy is often delayed until seven days post trauma to allow time for the vitreous to separate from the retina.
|
|
Prior Intraocular Surgery9,58,85
|
Varies with the age at which intraocular surgery was performed, but often presents long after surgery (after age 10)
|
History of previous intraocular surgery.
Often with a history of other ocular anomalies (such as congenital cataract, glaucoma, etc.). Retinal detachments are rhegmatogenous.
|
Pars plana vitrectomy with intraocular gas or silicone oil tamponade.
Patients have often had bilateral surgery (i.e., bilateral congenital cataracts), so careful examination of the fellow eye is warranted.
|
|
Choroidal Coloboma109–111
|
Birth
|
Unilateral or bilateral, microphthalmos, microcornea, myopia, iris colobomas. Retinal detachments are rhegmatogenous.
|
Vitrectomy with laser photocoagulation at the edge of the coloboma. Intraocular gas is preferred for tamponade.
|
Tables 5-7: Causes of pediatric retinal detachments. VEGF—vascular endothelial growth factor
Chorioretinal Coloboma
Choroidal colobomas (see also: https://eyewiki.org/Coloboma) (Figures 97-99) occur in approximately 0.5-2.2 cases in 10,000 live births.105,106 They are caused by incomplete closure of the embryonic fissure, which occurs during the seventh week of gestation. The retina divides into two layers at the edge of the coloboma; the coloboma itself does not have normal retina, retinal pigment epithelium, or choroid. The retina thins at the edge of the coloboma, predisposing the retina to breaks in this area.107 Estimates of retinal detachment rates range from 8% to 40% of patients with this disorder.108
If the area of detachment does not involve the coloboma, surgical repair of the detachment is the same as for other pediatric retinal detachments. However, frequently, the coloboma and/or optic nerve is involved, complicating surgical repair. Scleral buckling was the preferred method in the 1980s,108,109 but with the advent of smaller-gauge pars plana vitrectomy, most surgeons now pursue vitrectomy as the initial approach. The coloboma must be carefully separated from the remainder of the retina, and laser retinopexy is recommended around the edge of the coloboma to create a rim of chorioretinal adhesion. Tamponade with intraocular gas is preferred over silicone oil to prevent silicone oil extruding into the subretinal space.110,111 If the optic nerve is involved in the coloboma, peripapillary laser can result in significant visual field deficits; however, these eyes often have limited vision due to concomitant amblyopia. Some surgeons recommend postoperative laser in the papillomacular bundle, but this can be challenging to perform without general anesthesia in the pediatric population.112
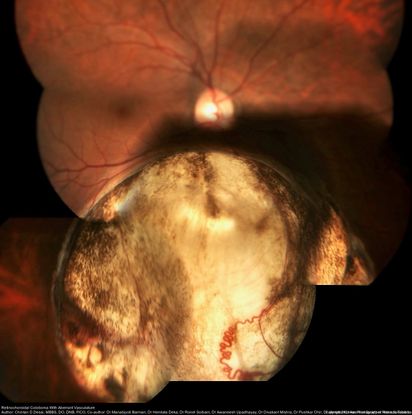
Figure 97. Fundus photo montage of a patient with a retinochoroidal coloboma Ida Mann classification type 3 with a spring coil shaped aberrant vessel. (This image was originally published in the Retina Image Bank® website. Author: Chintan D Desai, MBBS, DO, DNB, FICO. Photographer: Kankan Talukdar, Sankaradeva Nethralaya, Guwahati. Imaging Title: Retinochoroidal Coloboma with Aberrant Vasculature. Year 2018; Image Number 28681© the American Society of Retina Specialists.)
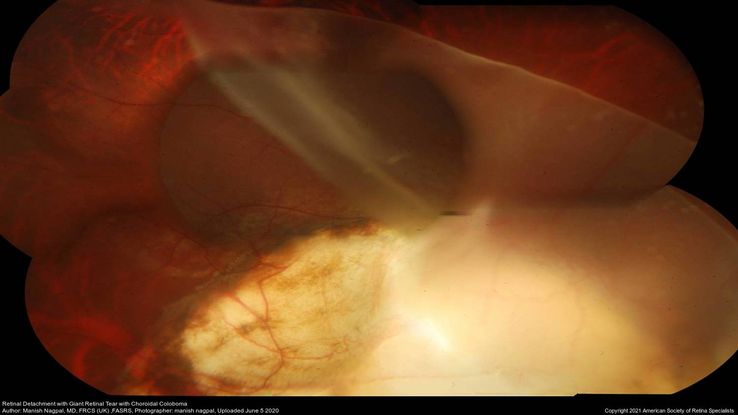
Figure 98. Fundus photo showing with retinal detachment, giant retinal tear in an eye with choroidal coloboma. (This image was originally published in the Retina Image Bank® website. Author: Manish Nagpal, MD, FRCS (UK), FASRS. Photographer: Manish Nagpal, Retina Foundation, Ahmedabad. Imaging Title: Retinal Detachment with Giant Retinal Tear with Choroidal Coloboma. Year 2020; Image Number 56022© the American Society of Retina Specialists.)
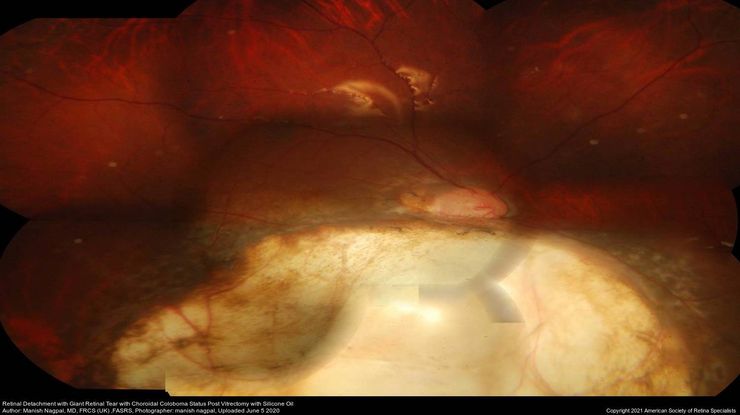
Figure 99. Fundus photo post surgery filled with silicone oil for giant retinal tear and retinal detachment in an eye with choroidal coloboma. (This image was originally published in the Retina Image Bank® website. Author: Manish Nagpal, MD, FRCS (UK), FASRS. Photographer: Manish Nagpal, Retina Foundation, Ahmedabad. Imaging Title: Retinal Detachment with Giant Retinal Tear with Choroidal Coloboma Status Post Vitrectomy with Silicone Oil. Year 2020; Image Number 56023© the American Society of Retina Specialists.)
Surgical Management
The surgical approaches to pediatric retinal detachment are similar to those performed in adults, but scleral buckling is pursued as the primary surgery more frequently. Treatment recommended for specific disorders is described above.
Scleral Buckling
Scleral buckling provides a significant advantage in pediatric patients, who typically have formed vitreous. In these patients, it can be challenging to surgically induce a posterior vitreous detachment during vitrectomy surgery. Additionally, there is lower risk of cataract formation, and there is no need for a second surgery, as is required when silicone oil tamponade is placed during vitrectomy surgery, unless the scleral buckle needs to later be removed. Either encircling or segmental buckling can be used, based on the specific pathology seen. However, in complicated retinal detachments, scleral buckling is usually not sufficient, and vitrectomy must be employed. Additionally, scleral buckling can cause axial myopia and induce strabismus. Sometimes these changes are permanent, even if the scleral buckle is removed.4 Strabismus often needs to be addressed surgically, increasing morbidity for these patients.
Vitrectomy
Vitrectomy is more commonly employed when PVR grade C is encountered. It is also more commonly used when the etiology of the detachment is trauma, or when posterior tears or media opacities are present.4,113 Vitrectomy is also the recommended second-line procedure if a retinal re-detachment occurs after primary scleral buckling.4 However, with vitrectomy, internal tamponade with gas or silicone oil is often needed. Because intraocular gas requires specific positioning, which is typically challenging for children, silicone oil is the preferred tamponade in this age group. However, silicone oil placement can be complicated by emulsification, rise in intraocular pressure/glaucoma, cataract formation, and corneal damage.7 Additionally, silicone oil requires a second surgery to remove, requiring an additional general anesthesia for these children. Thus, it is important that patients and their families be appropriately counseled prior to the placement of silicone oil.
Success Rates
Anatomical success rates vary based on the underling etiology. Surgical success rates are highest after trauma and lowest with congenital or developmental retinal anomalies.4 Children have a greater rate of postoperative complications and a lower rate of anatomical success rates as compared to adults with retinal detachments.114 As such, the average number of surgeries each patient requires is 2.2.6 Over 50% of patients have improved visual acuity after retinal detachment repair while 20% have stable visual acuity.4 Worse outcomes are found with younger age, worse initial visual acuity, presence of PVR grade C, giant retinal tears, total retinal detachment, involvement of the macula, prior intraocular surgery, and when a vitrectomy is required.58
Summary
Retinal detachments in the pediatric population are uncommon. They can be caused by a wide range of pathology, including trauma, myopia, previous intraocular surgery, and congenital/developmental anomalies. The surgical treatment in these patients needs to be individualized to the patient. Anatomic and visual success rates depend on the underlying etiology and severity of the detachment at presentation. In the pediatric age range, there are the additional complications of strabismus and amblyopia which need to be actively managed along with the retinal disease. Collaboration between the retinal specialist and the pediatric ophthalmologist is essential in providing the best management for these patients.
References
-
Meier P. [Retinal detachment in children: differential diagnosis and current therapy. ] Klin Monatsbl Augenheilkd. 2008;225(9):779-790. doi:10.1055/s-2008-1027515.
-
Winslow RL, Tasman W. Juvenile rhegmatogenous retinal detachment. Ophthalmology. 1978;85(6):607-618. doi:10.1016/s0161-6420(78)35641-4.
-
Gan NY, Lam W-C. Retinal detachments in the pediatric population. Taiwan J Ophthalmol. 2018;8(4):222-236. doi:10.4103/tjo.tjo_104_18.
-
Nuzzi R, Lavia C, Spinetta R. Paediatric retinal detachment: a review. Int J Ophthalmol. 2017;10(10):1592-1603. doi:10.18240/ijo.2017.10.18.
-
Gurler B, Coskun E, Öner V, Comez A, Erbagci I. Clinical characteristics and surgical outcomes of pediatric rhegmatogenous retinal detachment. Int Ophthalmol. 2016;36(4):521-525. doi:10.1007/s10792-015-0158-3.
-
Fivgas GD, Capone A. Pediatric rhegmatogenous retinal detachment. Retina Phila Pa. 2001;21(2):101-106. doi:10.1097/00006982-200104000-00001.
-
Wadhwa N, Venkatesh P, Sampangi R, Garg S. Rhegmatogenous retinal detachments in children in India: Clinical characteristics, risk factors, and surgical outcomes. J Am Assoc Pediatr Ophthalmol Strabismus. 2008;12(6):551-554. doi:10.1016/j.jaapos.2008.05.002.
-
Wang N-K, Tsai C-H, Chen Y-P, et al. Pediatric rhegmatogenous retinal detachment in East Asians. Ophthalmology. 2005;112(11):1890-1895. doi:10.1016/j.ophtha.2005.06.019.
-
Soheilian M, Ramezani A, Malihi M, et al. Clinical features and surgical outcomesof pediatric rhegmatogenous retinal detachment. Retina. 2009;29(4):545-551. doi:10.1097/IAE.0b013e318194fd1a.
-
Weinberg DV, Lyon AT, Greenwald MJ, Mets MB. Rhegmatogenous retinal detachments in children. Ophthalmology. 2003;110(9):1708-1713. doi:10.1016/S0161-6420(03)00569-4.
-
Gonzales CR, Singh S, Yu F, Kreiger AE, Gupta A, Schwartz SD. Pediatric rhegmatogenous retinal detachment: Clinical Features and Surgical Outcomes. Retina. 2008;28(6):847-852. doi:10.1097/IAE.0b013e3181679f79.
-
Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991;112(2):159-165. doi:10.1016/s0002-9394(14)76695-4.
-
Chen S-N, Jiunn-Feng H, Te-Cheng Y. Pediatric rhegmatogenous retinal detachment in taiwan. Retina Phila Pa. 2006;26(4):410-414. doi:10.1097/01.iae.0000238546.51756.cd.
-
Gow J, Oliver GL. Familial exudative vitreoretinopathy. An expanded view. Arch Ophthalmol Chic Ill 1960. 1971;86(2):150-155. doi:10.1001/archopht.1971.01000010152007.
-
Canny CL, Oliver GL. Fluorescein angiographic findings in familial exudative vitreoretinopathy. Arch Ophthalmol Chic Ill 1960. 1976;94(7):1114-1120. doi:10.1001/archopht.1976.03910040034006.
-
Ranchod TM, Ho LY, Drenser KA, Capone A, Trese MT. Clinical Presentation of Familial Exudative Vitreoretinopathy. Ophthalmology. 2011;118(10):2070-2075. doi:10.1016/j.ophtha.2011.06.020.
-
Chen C, Huang S, Sun L, et al. Analysis of etiologic factors in pediatric rhegmatogenous retinal detachment with genetic testing. Am J Ophthalmol. February 2020:S0002939420300684. doi:10.1016/j.ajo.2020.02.015.
-
Shukla D, Singh J, Sudheer G, et al. Familial exudative vitreoretinopathy (FEVR). Clinical profile and management. Indian J Ophthalmol. 2003;51(4):323-328.
-
Quiram PA, Drenser KA, Lai MM, Capone A, Trese MT. Treatment of vascularly active familial exudative vitreoretinopathy with pegaptanib sodium (Macugen). Retina. 2008;28(Supplement):S8-S12. doi:10.1097/IAE.0b013e3181679bf6.
-
Pendergast SD, Trese MT. Familial exudative vitreoretinopathy. Results of surgical management. Ophthalmology. 1998;105(6):1015-1023. doi:10.1016/S0161-6420(98)96002-X.
-
Rahi JS, Cable N. Severe visual impairment and blindness in children in the UK. The Lancet. 2003;362(9393):1359-1365. doi:10.1016/S0140-6736(03)14631-4.
-
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol Chic Ill 1960. 2005;123(7):991-999. doi:10.1001/archopht.123.7.991.
-
An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol Chic Ill 1960. 1984;102(8):1130-1134. doi:10.1001/archopht.1984.01040030908011.
-
An international classification of retinopathy of prematurity. II. The classification of retinal detachment. The International Committee for the Classification of the Late Stages of Retinopathy of Prematurity. Arch Ophthalmol Chic Ill 1960. 1987;105(7):906-912.
-
Shulman J, Hartnett M. Retinopathy of Prematurity. In: Pediatric Retina. Philadelphia, PA: Wolters Kluwer; 2014:491-508.
-
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol Chic Ill 1960. 2003;121(12):1684-1694. doi:10.1001/archopht.121.12.1684.
-
VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB, Lambert SR. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity. Ophthalmology. 2017;124(5):619-633. doi:10.1016/j.ophtha.2016.12.025.
-
Shah PK, Narendran V, Kalpana N. Safety and efficacy of simultaneous bilateral 25-gauge lens-sparing vitrectomy for vascularly active stage 4 retinopathy of prematurity. Eye. 2015;29(8):1046-1050. doi:10.1038/eye.2015.78.
-
Chow DR, Ferrone PJ, Trese MT. Refractive changes associated with scleral buckling and division in retinopathy of prematurity. Arch Ophthalmol Chic Ill 1960. 1998;116(11):1446-1448. doi:10.1001/archopht.116.11.1446.
-
Sears JE, Sonnie C. Anatomic success of lens-sparing vitrectomy with and without scleral buckle for stage 4 retinopathy of prematurity. Am J Ophthalmol. 2007;143(5):810-813. doi:10.1016/j.ajo.2007.01.017.
-
Jang SY, Choi KS, Lee SJ. Delayed-onset retinal detachment after an intravitreal injection of ranibizumab for zone 1 plus retinopathy of prematurity. J Am Assoc Pediatr Ophthalmol Strabismus. 2010;14(5):457-459. doi:10.1016/j.jaapos.2010.05.011.
-
Shah RJ, Garcia-Gonzalez JM, Blair MP, Galasso J, Shapiro MJ. Concurrent scleral buckle and intravitreal bevacizumab for advanced retinopathy of prematurity-related retinal detachment. Retin Cases Brief Rep. 2016;10(2):183-186. doi:10.1097/ICB.0000000000000221.
-
Shah PK, Narendran V, Kalpana N, Tawansy KA. Anatomical and visual outcome of stages 4 and 5 retinopathy of prematurity. Eye Lond Engl. 2009;23(1):176-180. doi:10.1038/sj.eye.6702939.
-
Black G, Redmond RM. The molecular biology of Norrie’s disease. Eye Lond Engl. 1994;8 ( Pt 5):491-496. doi:10.1038/eye.1994.124.
-
Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883-895. doi:10.1016/s0092-8674(04)00216-8.
-
Walsh MK. Early vitrectomy effective for Norrie Disease. Arch Ophthalmol. 2010;128(4):456. doi:10.1001/archophthalmol.2009.403.
-
Baranano D, Goldberg M. Incontinentia Pigmenti. In: Pediatric Retina. 2nd ed. Philadelphia, PA: Wolters Kluwer; 2014:354-359.
-
Carney RG. Incontinentia pigmenti: a world statistical analysis. Arch Dermatol. 1976;112(4):535. doi:10.1001/archderm.1976.01630280059017.
-
Wald KJ, Mehta MC, Katsumi O, Sabates NR, Hirose T. Retinal detachments in incontinentia pigmenti. Arch Ophthalmol Chic Ill 1960. 1993;111(5):614-617. doi:10.1001/archopht.1993.01090050048026.
-
Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1997;124(5):587-626. doi:10.1016/s0002-9394(14)70899-2.
-
Manschot WA. Persistent hyperplastic primary vitreous; special reference to preretinal glial tissue as a pathological characteristic and to the development of the primary vitreous. AMA Arch Ophthalmol. 1958;59(2):188-203.
-
Bosjolie A, Ferrone P. Visual outcome in early vitrectomy for posterior persistent fetal vasculature associated with traction retinal detachment. Retina. 2015;35(3):570-576. doi:10.1097/IAE.0000000000000353.
-
Federman JL, Shields JA, Altman B, Koller H. The surgical and nonsurgical management of persistent hyperplastic primary vitreous. Ophthalmology. 1982;89(1):20-24. doi:10.1016/s0161-6420(82)34854-x.
-
Goldberg MF, Peyman GA. Pars plicata surgery in the child for pupillary membranes, persistent hyperplastic primary vitreous, and infantile cataract. Trans New Orleans Acad Ophthalmol. 1983;31:228-262.
-
Soheilian M, Vistamehr S, Rahmani B, et al. Outcomes of surgical (pars plicata and limbal lensectomy, vitrectomy) and non-surgical management of persistent fetal vasculature (PFV): an analysis of 54 eyes. Eur J Ophthalmol. 2002;12(6):523-533. doi:10.1177/112067210201200613.
-
George ND, Yates JR, Moore AT. Clinical features in affected males with X-linked retinoschisis. Arch Ophthalmol Chic Ill 1960. 1996;114(3):274-280. doi:10.1001/archopht.1996.01100130270007.
-
Stickler GB, Belau PG, Farrell FJ, et al. Hereditary progressive arthro-ophthalmopathy. Mayo Clin Proc. 1965;40:433-455.
-
Snead MP, Yates JR. Clinical and molecular genetics of Stickler syndrome. J Med Genet. 1999;36(5):353-359.
-
Stickler GB, Hughes W, Houchin P. Clinical features of hereditary progressive arthro-ophthalmopathy (Stickler syndrome): a survey. Genet Med Off J Am Coll Med Genet. 2001;3(3):192-196. doi:10.1097/00125817-200105000-00008.
-
Ang A, Poulson AV, Goodburn SF, Richards AJ, Scott JD, Snead MP. Retinal detachment and prophylaxis in type 1 Stickler syndrome. Ophthalmology. 2008;115(1):164-168. doi:10.1016/j.ophtha.2007.03.059.
-
Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476-485. doi:10.1136/jmg.2009.072785.
-
Remulla JF, Tolentino FI. Retinal detachment in Marfan’s syndrome. Int Ophthalmol Clin. 2001;41(4):235-240. doi:10.1097/00004397-200110000-00021.
-
Pyeritz RE, McKusick VA. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979;300(14):772-777. doi:10.1056/NEJM197904053001406.
-
Magid D, Pyeritz RE, Fishman EK. Musculoskeletal manifestations of the Marfan syndrome: radiologic features. AJR Am J Roentgenol. 1990;155(1):99-104. doi:10.2214/ajr.155.1.2112876.
-
Maumenee IH. The eye in the Marfan syndrome. Trans Am Ophthalmol Soc. 1981;79:684-733.
-
Sharma T, Gopal L, Shanmugam MP, et al. Retinal detachment in Marfan syndrome: clinical characteristics and surgical outcome. Retina Phila Pa. 2002;22(4):423-428. doi:10.1097/00006982-200208000-00005.
-
Abboud EB. Retinal detachment surgery in Marfan’s syndrome. Retina Phila Pa. 1998;18(5):405-409. doi:10.1097/00006982-199805000-00003.
-
Soliman MM, Macky TA. Pediatric rhegmatogenous retinal detachment. Int Ophthalmol Clin. 2011;51(1):147-171. doi:10.1097/IIO.0b013e31820099c5.
-
Kinyoun JL, Knobloch WH. Idiopathic retinal dialysis. Retina Phila Pa. 1984;4(1):9-14. doi:10.1097/00006982-198400410-00003.
-
Abramson DH, Schefler AC. Update on retinoblastoma. Retina Phila Pa. 2004;24(6):828-848. doi:10.1097/00006982-200412000-00002.
-
Lin P, O’Brien JM. Frontiers in the management of retinoblastoma. Am J Ophthalmol. 2009;148(2):192-198. doi:10.1016/j.ajo.2009.04.004.
-
Shields CL, Mashayekhi A, Au AK, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276-2280. doi:10.1016/j.ophtha.2006.06.018.
-
Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A Phase I/II Study of Direct Intraarterial (Ophthalmic Artery) Chemotherapy with Melphalan for Intraocular Retinoblastoma. Ophthalmology. 2008;115(8):1398-1404.e1. doi:10.1016/j.ophtha.2007.12.014.
-
O’Brien J, Gombos D. Retinoblastoma. In: Pediatric Retina. 2nd ed. Philadelphia, PA: Wolters Kluwer; 2014:313-329.
-
Elaraoud I, Ch’ng S, Karl D, Kalogeropoulos D, Chavan R, Sharma A. Management of retinal detachment in retinoblastoma with globe conserving treatment. J Curr Ophthalmol. 2019;31(1):43-48. doi:10.1016/j.joco.2018.09.002.
-
Vitale A, Kump L, Foster C. Uveitis Affecting Infants and Children. In: Pediatric Retina. 2nd ed. Philadelphia, PA: Wolters Kluwer; 2014:386-431.
-
Kim SJ, Scott IU, Brown GC, et al. Interventions for toxoplasma retinochoroiditis. Ophthalmology. 2013;120(2):371-378. doi:10.1016/j.ophtha.2012.07.061.
-
Bosch-Driessen LH, Karimi S, Stilma JS, Rothova A. Retinal detachment in ocular toxoplasmosis. Ophthalmology. 2000;107(1):36-40. doi:10.1016/s0161-6420(99)00013-5.
-
Ahn SJ, Ryoo N-K, Woo SJ. Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia Pac Allergy. 2014;4(3):134. doi:10.5415/apallergy.2014.4.3.134.
-
Schneier AJ, Durand ML. Ocular toxocariasis: Advances in diagnosis and treatment. Int Ophthalmol Clin. 2011;51(4):135-144. doi:10.1097/IIO.0b013e31822d6a5a.
-
Relhan N, Pathengay A, Raval V, Nayak S, Choudhury H, Harry Flynn, Jr. H. Clinical experience in treatment of diffuse unilateral subretinal neuroretinitis. Clin Ophthalmol. September 2015:1799. doi:10.2147/OPTH.S86989.
-
Poppert S, Heideking M, Agostini H, et al. Diffuse unilateral subacute neuroretinitis caused by Ancylostoma hookworm. Emerg Infect Dis. 2017;23(2):343-344. doi:10.3201/eid2302.142064.
-
Zamir E, Wang RC, Krishnakumar S, Aiello Leverant A, Dugel PU, Rao NA. Juvenile xanthogranuloma masquerading as pediatric chronic uveitis: a clinicopathologic study. Surv Ophthalmol. 2001;46(2):164-171. doi:10.1016/s0039-6257(01)00253-3.
-
Karcioglu ZA, Mullaney PB. Diagnosis and management of iris juvenile xanthogranuloma. J Pediatr Ophthalmol Strabismus. 1997;34(1):44-51.
-
Hildebrand GD. Juvenile xanthogranuloma with presumed involvement of the optic disc and retina. Arch Ophthalmol. 2004;122(10):1551. doi:10.1001/archopht.122.10.1551.
-
Dedania VS, Ozgonul C, Zacks DN, Besirli CG. Novel classification system for combined hamartona of the retina and retinal pigment epithelium. Retina. 2018;38(1):12-19. doi:10.1097/IAE.0000000000001499.
-
Chew EY. Ocular manifestations of von Hippel-Lindau disease: clinical and genetic investigations. Trans Am Ophthalmol Soc. 2005;103:495-511.
-
Vrabec TR. Exudative retinal detachment in Behçet disease. Arch Ophthalmol Chic Ill 1960. 2001;119(9):1383-1386.
-
Akova YA, Yilmaz G, Aydin P. Retinal tears associated with panuveitis and Behçet’s disease. Ophthalmic Surg Lasers. 1999;30(9):762-765.
-
Thordsen JE, Harris L, Hubbard GB. Pediatric endophthalmitis: A 10-year consecutive series. Retina. 2008;28(Supplement):S3-S7. doi:10.1097/IAE.0b013e318159ec7f.
-
Murugan G, Shah PK, Narendran V. Clinical profile and outcomes of pediatric endogenous endophthalmitis: A report of 11 cases from South India. World J Clin Pediatr. 2016;5(4):370. doi:10.5409/wjcp.v5.i4.370.
-
Nelson LB, Wilson TW, Jeffers JB. Eye injuries in childhood: demography, etiology, and prevention. Pediatrics. 1989;84(3):438-441.
-
Sarrazin L, Averbukh E, Halpert M, Hemo I, Rumelt S. Traumatic pediatric retinal detachment: a comparison between open and closed globe injuries. Am J Ophthalmol. 2004;137(6):1042-1049. doi:10.1016/j.ajo.2004.01.011.
-
Wang N-K, Chen Y-P, Yeung L, et al. Traumatic pediatric retinal detachment following open globe injury. Ophthalmol J Int Ophtalmol Int J Ophthalmol Z Augenheilkd. 2007;221(4):255-263. doi:10.1159/000101928.
-
Soliman M, Macky TA. Pediatric rhegmatogenous retinal detachment: clinical features, management and visual outcome. EVRS Educ Electr J. 2005;2:5-12.
-
Rudnicka AR, Kapetanakis VV, Wathern AK, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2016;100(7):882-890. doi:10.1136/bjophthalmol-2015-307724.
-
Cao K, Wan Y, Yusufu M, Wang N. Significance of outdoor time for myopia prevention: A systematic review and meta-analysis based on randomized controlled trials. Ophthalmic Res. 2020;63(2):97-105. doi:10.1159/000501937.
-
Cheng H-C, Chang K, Shen E, Luo K-S, Ying Y-H. Risk factors and behaviours of schoolchildren with myopia in Taiwan. Int J Environ Res Public Health. 2020;17(6):1967. doi:10.3390/ijerph17061967.
-
Grzybowski A, Kanclerz P, Tsubota K, Lanca C, Saw S-M. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020;20(1):27. doi:10.1186/s12886-019-1220-0.
-
Wen L, Cao Y, Cheng Q, et al. Objectively measured near work, outdoor exposure and myopia in children. Br J Ophthalmol. February 2020:bjophthalmol-2019-315258. doi:10.1136/bjophthalmol-2019-315258.
-
Wu P-C, Chen C-T, Lin K-K, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125(8):1239-1250. doi:10.1016/j.ophtha.2017.12.011.
-
Errera M-H, Liyanage SE, Moya R, Wong SC, Ezra E. Primary scleral buckling for pediatric rhegmatogenous retinal detachment. Retina. 2015;35(7):1441-1449. doi:10.1097/IAE.0000000000000480.
-
Coats G. Forms of retinal disease with massive exudation. R Lond Ophthal Hosp Rev. 1908;17(3):440–525.
-
Sigler EJ, Randolph JC, Calzada JI, Wilson MW, Haik BG. Current management of Coats disease. Surv Ophthalmol. 2014;59(1):30-46. doi:10.1016/j.survophthal.2013.03.007.
-
Shields JA, Shields CL, Honavar SG, Demirci H, Cater J. Classification and management of Coats disease: the 2000 Proctor Lecture. Am J Ophthalmol. 2001;131(5):572-583. doi:10.1016/s0002-9394(01)00896-0.
-
Mulvihill A, Morris B. A population-based study of Coats disease in the United Kingdom II: investigation, treatment, and outcomes. Eye. 2010;24(12):1802-1807. doi:10.1038/eye.2010.127.
-
Ghorbanian S, Jaulim A, Chatziralli IP. Diagnosis and treatment of coats’ disease: a review of the literature. Ophthalmol J Int Ophtalmol Int J Ophthalmol Z Augenheilkd. 2012;227(4):175-182. doi:10.1159/000336906.
-
Yoshizumi MO, Kreiger AE, Lewis H, Foxman B, Hakakha BA. Vitrectomy techniques in late-stage Coats’-like exudative retinal detachment.Doc Ophthalmol Adv Ophthalmol. 1995;90(4):387-394. doi:10.1007/bf01268124
-
Egerer I, Tasman W, Tomer TT. Coats disease. Arch Ophthalmol Chic Ill 1960. 1974;92(2):109-112. doi:10.1001/archopht.1974.01010010115006.
-
Adam RS, Kertes PJ, Lam W-C. Observations on the management of Coats’ disease: less is more. Br J Ophthalmol. 2007;91(3):303-306. doi:10.1136/bjo.2006.103382.
-
Rishi P, Rishi E, Uparkar M, et al. Coats′ disease: An Indian perspective. Indian J Ophthalmol. 2010;58(2):119. doi:10.4103/0301-4738.60081.
-
Lin C-J, Hwang J-F, Chen Y-T, Chen S-N. The effect of intravitreal bevacizumab in the treatment of Coat’s disease in children. Retina. 2010;30(4):617-622. doi:10.1097/IAE.0b013e3181c2e0b7.
-
Stanga PE, Jaberansari H, Bindra MS, Gil-Martinez M, Biswas S. Transcleral drainage of subretinal fluid, anti-vascular endothelial growth factor, and wide-field imaging-guided laser in Coats exudative retinal detachment. Retina. 2016;36(1):156-162. doi:10.1097/IAE.0000000000000669.
-
Ramasubramanian A, Shields CL. Bevacizumab for Coats’ disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol. 2012;96(3):356-359. doi:10.1136/bjophthalmol-2011-300141.
-
Warburg M. Classification of microphthalmos and coloboma. J Med Genet. 1993;30(8):664-669. doi:10.1136/jmg.30.8.664.
-
Stoll C, Alembik Y, Dott B, Roth MP. Epidemiology of congenital eye malformations in 131,760 consecutive births. Ophthalmic Paediatr Genet. 1992;13(3):179-186. doi:10.3109/13816819209046487.
-
Gopal L. A clinical and optical coherence tomography study of choroidal colobomas. Curr Opin Ophthalmol. 2008;19(3):248-254. doi:10.1097/ICU.0b013e3282fc2604.
-
Daufenbach DR, Ruttum MS, Pulido JS, Keech RV. Chorioretinal colobomas in a pediatric population. Ophthalmology. 1998;105(8):1455-1458. doi:10.1016/S0161-6420(98)98028-9.
-
Patnaik B, Kalsi R. Retinal detachment with coloboma of the choroid. Indian J Ophthalmol. 1981;29(4):345-349.
-
Gopal L, Kini MM, Badrinath SS, Sharma T. Management of retinal detachment with choroidal coloboma. Ophthalmology. 1991;98(11):1622-1627. doi:10.1016/s0161-6420(91)32075-x.
-
Jalali S, Das T. Selection of surgical technique for retinal detachment with coloboma of the choroid. Indian J Ophthalmol. 1994;42(1):27-30.
-
McDonald HR, Lewis H, Brown G, Sipperley JO. Vitreous surgery for retinal detachment associated with choroidal coloboma. Arch Ophthalmol Chic Ill 1960. 1991;109(10):1399-1402. doi:10.1001/archopht.1991.01080100079046.
-
Oono Y, Uehara K, Haruta M, Yamakawa R. Characteristics and surgical outcomes of pediatric rhegmatogenous retinal detachment. Clin Ophthalmol Auckl NZ. 2012;6:939-943. doi:10.2147/OPTH.S31765.
-
Rumelt S, Sarrazin L, Averbukh E, Halpert M, Hemo I. Paediatric vs adult retinal detachment. Eye Lond Engl. 2007;21(12):1473-1478. doi:10.1038/sj.eye.6702511.
-
Dickinson JL, Sale MM, Passmore A, et al. Mutations in the NDP gene: contribution to Norrie disease, familial exudative vitreoretinopathy and retinopathy of prematurity. Clin Experiment Ophthalmol. 2006;34(7):682-688. doi:10.1111/j.1442-9071.2006.01314.x.
-
Jiao X, Ventruto V, Trese MT, Shastry BS, Hejtmancik JF. Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in LRP5. Am J Hum Genet. 2004;75(5):878-884. doi:10.1086/425080.
-
Toomes C, Bottomley HM, Jackson RM, et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet. 2004;74(4):721-730. doi:10.1086/383202.
-
Greene-Roethke C. Incontinentia pigmenti: A summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31(6):e45-e52. doi:10.1016/j.pedhc.2017.07.003.
-
Sauer CG, Gehrig A, Warneke-Wittstock R, et al. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet. 1997;17(2):164-170. doi:10.1038/ng1097-164.
-
Karimi S, Nourinia R, Mashayekhi A. Circumscribed choroidal hemangioma. J Ophthalmic Vis Res. 2015;10(3):320. doi:10.4103/2008-322X.170353.
-
Tadepalli S, Shields C, Shields J, Honavar S. Intraocular medulloepithelioma – A review of clinical features, DICER 1 mutation, and management. Indian J Ophthalmol. 2019;67(6):755. doi:10.4103/ijo.IJO_845_19.
-
Mansour AhmadM, Alameddine RamziM, Kahtani E. Review of choroidal osteomas. Middle East Afr J Ophthalmol. 2014;21(3):244. doi:10.4103/0974-9233.134686.
-
Lambiase A, Mantelli F, Bruscolini A, La Cava M, Abdolrahimzadeh S. Ocular manifestations of Sturge-Weber syndrome: pathogenesis, diagnosis, and management. Clin Ophthalmol. May 2016:871. doi:10.2147/OPTH.S101963.
-
So JM, Holman RE. Wyburn-Mason Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. http://www.ncbi.nlm.nih.gov/books/NBK493218/. Accessed April 8, 2020.
-
Pangtey BPS, Kohli P, Ramasamy K. Wyburn-Mason syndrome presenting with bilateral retinal racemose hemangioma with unilateral serous retinal detachment. Indian J Ophthalmol. 2018;66(12):1869-1871. doi:10.4103/ijo.IJO_455_18.
-
Lavezzo MM, Sakata VM, Morita C, et al. Vogt-Koyanagi-Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis. 2016;11:29. doi:10.1186/s13023-016-0412-4.
-
Kalogeropoulos CD, Koumpoulis, Mentis, Pappa, Zafeiropoulos, Aspiotis. Bartonella and intraocular inflammation: a series of cases and review of literature. Clin Ophthalmol. June 2011:817. doi:10.2147/OPTH.S20157.
-
Orhan B, Malbora B, Akça Bayar S, Avcı Z, Alioğlu B, Özbek N. Ophthalmologic findings in children with leukemia: A single-center study. Türk Oftalmol Derg. 2016;46(2):62-67. doi:10.4274/tjo.03880.
-
Wang N-K, Chen Y-P, Lai C-C, et al. Paediatric retinal detachment: comparison of high myopia and extreme myopia. Br J Ophthalmol. 2009;93(5):650-655. doi:10.1136/bjo.2008.145920.