Many acquired conditions lead to glaucoma, which is by definition secondary glaucoma because it occurs as a result of the acquired condition. These conditions can primarily involve the eye, or be part of a wider systemic condition. Glaucoma may develop many years after the onset of the acquired condition, so it is vital to be aware of the relationship of these conditions with glaucoma. The mechanisms leading to glaucoma are broadly similar to those occurring in nonacquired conditions, and more than one mechanism can contribute to the development of glaucoma or to its progression.
Trauma
Definition and incidence
Trauma is usually classified as blunt or penetrating – both types can lead to secondary glaucoma. The presentation may be immediate at the time of the injury (acute or delayed), or develop over a period of time after the injury (chronic). Most chronic presentations are painless and slow in onset so patients at high risk of glaucoma should have regular lifelong monitoring of their intraocular pressure (IOP). Occasionally a later-onset form may present with sudden onset of pain and photophobia from raised IOP (classically seen after surgical closure of a cyclodialysis cleft, or in some neovascular glaucoma patients).
Mechanism of glaucoma
A variety of mechanisms may contribute to secondarily raised IOP; they are determined by the nature and severity of the injury, as well as its management. Predictors for the development of chronic glaucoma after injury are increased angle pigmentation, higher baseline IOP, absence of cyclodialysis cleft, hyphema, angle recession greater than 180 degrees, and lens injury.1 The various mechanisms for glaucoma and approaches to their management are discussed below.
Hyphema
Hyphema can be caused by either blunt or penetrating injury, but when it is a result of penetrating trauma, its management and role in glaucoma are dictated by the overall globe injury. In blunt trauma situations hyphema is caused by tears of the iris, iris root, and ciliary body. There may also be recurrent hemorrhage (“re-bleed”) from the original site, which can exacerbate existing ocular hypertension (OHT) or cause acutely raised IOP a few days (typically 3-5 days) after the injury.
Mechanism of glaucoma
This is an open-angle glaucoma mechanism with pre-trabecular meshwork (TM) obstruction by red blood cells (RBCs), accompanying inflammatory mediators, and/or clot. The likelihood and severity of IOP elevation is proportional to the degree of hyphema2 and can be extremely high, requiring urgent attention. Concomitant damage to the angle structures, if present, may compound the elevated IOP, or mitigate it if there is a cyclodialysis cleft or ciliary body damage. Be aware that in cases of sickle cell disease and trait, significantly elevated IOP can accompany a relatively benign-appearing hyphema. (See Special considerations in sickle cell disease (SCD) and sickle cell trait (SCT).) . More rarely, an acute angle-closure situation can develop from secondary pupillary block by a large blood clot. Posterior synechiae (PS) and peripheral anterior synechiae (PAS) may develop from prolonged or more severe hyphema, which can also predispose to angle closure.
Management
Management in the acute situation is directed at globe integrity and controlling underlying risks of hemorrhage or re-bleed. Close monitoring and medical treatment of raised IOP will be sufficient in most cases. Cycloplegia, topical steroids, and limitation of physical activity are also advised. If, despite medications, IOP is considered too high for the safety of the optic nerve or it risks blood staining of the cornea, surgery may be required. The World Glaucoma Association (WGA) Childhood Glaucoma consensus group found general agreement that situations requiring prompt surgery are total hyphema, partial hyphema with IOP sustained at greater than 30 mmHg on maximally tolerated medical therapy, and development of corneal blood staining.3 There was, however, less consensus on the surgical procedure of choice. Although anterior chamber (AC) washout of the hemorrhage is agreed by many, the role of trabeculectomy at the time of washout is less clearly defined. Angle surgery in the form of gonioaspiration has also been reported for small hypertensive hyphemas.4 One disadvantage of early surgery, however, is that deflating the globe and introducing irrigation or instruments into the eye may disturb the clot and precipitate fresh bleeding. These risks must be weighed against each other when determining the most suitable approach for each case.
Special considerations in sickle cell disease (SCD) and sickle cell trait (SCT)
Children with SCD are at greater risk of optic nerve damage for a given IOP than the general population. Raised IOP stresses perfusion of susceptible ocular tissues. The RBCs in SCD cases are more vulnerable to lower oxygenation and adopt a sickled shape, which hinders their passage through vasculature, causing vessel occlusion and tissue hypoxia (analogous to the crises experienced in other parts of the body). The central retinal artery can thus be more under stress at lower IOPs in SCD patients than would otherwise be the case, and loss of vision due to optic atrophy from acute IOP elevation may occur. Similarly, affected RBCs in the AC adopt sickled shapes and do not clear through the trabecular meshwork (TM) as quickly as normal RBCs might, which prolongs hyphema and contributes to elevated IOP.5 Topical carbonic anhydrase inhibitors (CAIs) may aggravate this situation with further acidotic stress on the AC RBCs, and are therefore contraindicated.
SCD patients are also more likely to experience re-bleeds than the general population. Persistent or recurrent hemorrhage should alert the clinician to this possible underlying risk factor.
Glaucoma (and other) medications, such as the CAIs, that induce acidosis may also aggravate sickling. This may be limited to the AC in the case of topical CAIs, but systemic administration may have more general systemic adverse effects. Using mannitol or glycerol to lower IOP may also compound the situation by causing dehydration.
Hyphema in SCD children should be managed more aggressively than in other children, with closer follow-up and a lower threshold for surgery if IOP control by medical therapy is proving insufficient. The CAI class of medications should be avoided.
Although SCT children would not be expected to demonstrate the same vulnerabilities to hyphema as SCD children, there is still a likelihood of higher IOPs, re-bleeds, and poorer visual outcomes for SCT children than for non–sickle-cell children. Similar precautions should be exercised in children with SCT as in those with SCD.6,7
Pearl: In any child with a hyphema, consider the possibility of SCD/SCT and test for it if there is any ethnicity or ancestry suspicious for SCD, or atypical presentation in terms of disproportionate hypertension for size of hyphema, persistence of hyphema, or re-bleed.
Traumatic iritis
In the immediate aftermath of a significant injury, inflammation and ciliary body shutdown may lessen IOP, but as ciliary body function recovers, and as inflammatory debris accumulates in the angle, the IOP increases and may become abnormally elevated.8
Mechanism of glaucoma
Acutely raised IOP may result from inflammatory cells and inflammatory products clogging the TM (pre-trabecular open-angle mechanism), which may alter TM function at a cellular level (trabecular open-angle mechanism) and lead to PS and PAS in the long term, which may be progressive (angle-closure mechanism). Inadequately treated inflammation predisposes to PS and PAS, which both lead to forms of secondary angle-closure glaucoma.
Management
Some degree of inflammation accompanies most injuries and its treatment is important in the prevention of secondary glaucoma. Treating inflammation with topical steroids also helps with pain relief. Cycloplegia is helpful to minimize the risk of posterior synechiae developing and to reduce photophobia.
Steroid treatment in itself can lead to open-angle glaucoma, which in children develops more quickly (See Corticosteroids,) and can be more profound than in adults.
Lens damage – subluxation or dislocation
Partial zonular rupture results in lens subluxation and complete rupture in lens dislocation, either anteriorly into the AC or posteriorly into the vitreous. These ruptures result from blunt or penetrating injuries and suggest a significant injury. However, in children with underlying zonular fragility (such as Marfan syndrome and homocystinuria, or in buphthalmic eyes) trivial trauma can dislocate the lens.
Mechanism of glaucoma
A subluxed lens leads to angle-closure glaucoma by forward displacement of the lens-iris diaphragm narrowing the angle, and/or by a secondary pupillary block mechanism. Anterior dislocation can result in the lens becoming trapped in the pupil, causing acute angle closure from secondary pupillary block or, if completely dislocated into the AC, may cause raised IOP by reverse pupil block. Posterior dislocation is less likely to lead to glaucoma. However, there is still a possibility that vitreous can occlude the pupil and cause acute angle closure, or that if the lens capsule has been breached, leached proteins can cause an inflammatory response leading to a secondary uveitic glaucoma (phacoanaphylactic glaucoma). A posteriorly dislocated lens that becomes cataractous can also lead to phacolytic glaucoma if it leaks lens proteins.
Repeated episodes of raised IOP result in angle damage. So even in the presence of normal IOP, finding PAS on gonioscopy and other signs of glaucoma is highly suggestive of intermittent raised IOP from angle closure secondary to lens subluxation/dislocation. However, it may be difficult to distinguish this from previous angle damage in an eye that has had significant previous trauma. Children are often not able to describe the symptoms of intermittent angle closure with raised IOP, and a careful history from parents probing for symptoms suggestive of episodic eye pain, headaches, and photophobia may help.
Management
Topical and systemic glaucoma medications are used in the acute situation. Following anterior lens dislocation, pilocarpine may be helpful to keep a mobile lens in the posterior segment as a temporizing measure. However, treating the underlying cause of the secondary glaucoma is required, so lens extraction might be necessary. If elevated IOP remains an issue, further glaucoma surgery, such as implantation of a glaucoma drainage device (GDD) may be required. For a more detailed approached to management of lens subluxation /dislocation, refer to connective tissue disorders in section 2 (See Lens damage – subluxation or dislocation.) .
Pearl: Temporizing measures for a child presenting with a dislocated crystalline lens in the AC: dilate the pupil with a short-acting mydriatic eye drop, keep the child supine and face up until the lens has dropped back into the vitreous cavity, then instill pilocarpine to constrict the pupil.
Angle injury – angle recession and cyclodialysis cleft
Angle recession is typically caused by blunt trauma, which forces aqueous humor into the angle to shear the iris root posteriorly from its insertion, tearing apart the longitudinal and circular muscles of the ciliary body. There is also tearing of structures as anterior-posterior compression distends the globe in its equatorial circumference. Angle recession in the immediate post-injury period may, but does not necessarily, cause raised IOP (although accompanying hyphema and iritis may do so); however, glaucoma can develop months to many years after the injury. This may be a result of chronic scarring of TM and SC or perhaps altered TM and SC dynamics following loss of ciliary muscle tension on the scleral spur.
Its reported incidence varies, but in general 5-20% of patients with angle recession develop glaucoma.9 The risk is associated with the circumferential extent of the recession, being most likely when more than 180 degrees of angle is involved.
A cyclodialysis cleft usually results from significant blunt globe injury in which the iris root and ciliary body are torn away from their insertions to create a gap that is visible on gonioscopy as a bright white line (internal sclera previously covered by ciliary body). Such an injury is more likely to produce hypotony than raised IOP because it allows aqueous to pass from the AC into the suprachoroidal space. However, at first a cleft can be blocked by blood or other tissues, and hypotony may only become apparent later. Previously undiagnosed clefts can present after spontaneous closure years later, with acutely elevated IOP, which can be extremely painful. This is similar to the situation following surgical repair of clefts in which there is often a period of markedly elevated IOP.
Management
The onset of glaucoma may be delayed many years after the original injury and is asymptomatic, so lifelong IOP monitoring is necessary in children with angle recession. First-line treatment is with medical therapy, and if that is insufficient, surgery is indicated. Surgery bypassing the drainage mechanism is required, such as trabeculectomy or GDD. The choice of surgery will be influenced by other factors relating to the extent of trauma (such as cataract) and the visual prognosis.
Posterior segment injury
Mechanism of glaucoma
Trauma that results in vitreous hemorrhage and posterior segment injury is often severe enough to contribute multiple glaucoma mechanisms in the acute setting. Persistent vitreous hemorrhage can lead to late-onset chronic open-angle glaucoma from altered RBCs that become “ghost cells” in the AC. These cells are not as pliable as fresh RBCs and are not cleared as effectively by TM cells, which leads to TM obstruction and raised IOP. Ghost cell glaucoma is rare in children.10 In adults it typically presents approximately 3 weeks after the vitreous hemorrhage11 and may be asymptomatic until there is advanced damage, especially if vision has already been compromised.
Injuries that lead to chronic retinal detachment may be complicated by angle neovascularization, leading to secondary closed-angle glaucoma. Retinal detachment repair using silicone oil can also produce a secondary glaucoma (either open-angle glaucoma with TM obstruction and damage by silicone oil particles, or acute pupil-block glaucoma from oil in the vitreous in the absence of a functioning peripheral iridectomy.12
Pearl: An inferior iridectomy should always be performed at the time of silicone oil placement to avoid pupil-block glaucoma by the oil.
Pearl: Do not be fooled by a deep AC and clear cornea in an oil-filled eye with high IOP – when the oil fill is such that the AC is totally occupied by oil, the cornea remains clear.13
Management
This usually requires collaboration between a retinal specialist and the other relevant specialists. Depending on the severity of the injury, the initial goal may be to achieve globe integrity, or to save vision from retinal detachment. High IOP in this setting is best treated medically. Once the eye is as stable as possible, glaucoma surgery may be indicated.
In the case of ghost cell glaucoma, removing the source of RBCs is important, which may require vitrectomy. If IOP does not settle with medications or vitrectomy and washout, glaucoma surgery may be required.
Silicone oil removal is often not possible in these cases and is unlikely to reverse secondary angle damage once established. GDD surgery and trabeculectomy do not perform well long-term,14,15 so transscleral cyclodiode becomes the final option. If there is angle closure with a pupillary block element, a surgical peripheral iridotomy (SPI) is more likely to remain patent than a laser PI, because of ocular inflammation.
Considerations in children with existing glaucoma who experience globe trauma
Children with buphthalmos are more susceptible to globe trauma, partly because a large eye is less well protected by the orbit, and partly because thinned sclera is more likely than normal sclera to rupture. The ocular integrity is further weakened if there has been previous glaucoma filtering surgery. Injuries in these eyes can be difficult to manage and can disturb glaucoma control, or be the final event that leads to loss of the eye. These children should be encouraged to live as full a life as possible but also to take sensible precautions such as wearing sports eye protection and avoiding excessively risky contact sports.
Consideration for eyes with very poor visual prognosis
In an eye with a dismal visual prognosis, refraining from treatment of asymptomatic elevated IOP is an option that should seriously be considered. This is especially so in children, as the impact of medications and surgeries may significantly affect quality of life and development. However, in a child experiencing pain, or in the very young when there is concern for the potentially negative effects of increasing buphthalmos or phthisis, the balance tips in favor of treatment.
Surgery
Secondary glaucoma may develop following a variety of other ocular surgeries in children. Furthermore, pre-existing glaucoma can also be exacerbated by surgery for other related or unrelated ocular conditions. The elevation of IOP may be acute, which is most often in the immediate postoperative stage, from a number of reasons such as inflammation, hyphema, uveitis, or retained viscoelastic. This tends to be painful if the IOP is high enough. Slower, more insidious elevations in IOP can occur over the longer term following ocular surgeries. Hypotony and flat anterior chamber following any surgery can predispose to PAS and later elevated eye pressures. Sutured closure of all paracenteses and wounds in any intraocular surgery is required in children to prevent hypotony from leaks. Because of the slower onset, the child is less likely to experience pain, and the development of OHT/glaucoma may be missed if there is inadequate postoperative follow-up of the original surgery. Steroid-related glaucoma falls into this category, although it can occur within a week or two of its commencement (earlier than in adults) and can cause pain.
Pearl: Beware the child in the postoperative period in whom there is a 1-2 line drop in visual acuity. Check for microcystic edema and obtain a reliable IOP.
Corneal surgery
Secondary glaucoma after corneal surgery is common and reported to complicate penetrating keratoplasty in approximately 25% of children, and to a lesser extent, lamellar keratoplasty in 5%.16
Mechanism of glaucoma
Glaucoma may be due to multiple mechanisms such as PAS, TM dysfunction from inflammation and steroid-induced OHT (graft survival requires intensive and sustained steroid therapy). Uncontrolled glaucoma adversely affects graft survival in turn.
A critical component of successful Descemet stripping endothelial keratoplasty (DSEK) is instilling an air bubble into the AC and keeping the patient supine in the early postoperative period, to maintain the donor endothelial button against the exposed host stroma. This requires highly motivated care-givers, and patient selection is critical. There is a risk of acute pupil-block glaucoma if the air bubble becomes trapped behind the iris, and of graft failure if the button becomes dislodged.
Retinal surgery
Early postoperative IOP “spikes” often occur following vitrectomy and scleral buckle procedures. Some specialists prefer silicone oil to gas tamponades because of a lower incidence of early postoperative pressure elevations.17
Mechanism of glaucoma
Glaucoma can occur by both open- and closed-angle mechanisms. Causes of early postoperative IOP elevation are retained viscoelastic, oil or gas overfill, expansion of the gas (especially if not titrated correctly), hyphema, and pupil block from a gas bubble or oil in aphakic children. Postoperative inflammation, persistent vitreous hemorrhage, and steroid administration may also contribute to later elevated IOP.
Lens surgery
This is covered in the Secondary Glaucoma chapter specifically devoted to glaucoma following cataract surgery (GFCS).
Management of glaucoma secondary to other surgeries
Careful follow-up with examinations under anesthesia and co-management by a team of ophthalmologists (including corneal, pediatric, glaucoma, and retina specialists, as required) is crucial to best care for children with ocular comorbidities.18 Medical treatment is usually first line, followed by surgery. Many factors will influence choice of treatment, and close collaboration between specialists is required. Parents' expectations should be managed and the general well-being and long-term future of the child considered as much as possible when making these difficult management decisions.
Retinopathy of Prematurity
Secondary glaucoma in formerly premature infants with retinopathy of prematurity (ROP), a disease of the retinal vasculature, is a well-recognized complication. The incidence in children with severe retinopathy of prematurity and retinal detachments has been reported as high as 30%.19 Both the Cryotherapy for Retinopathy of Prematurity Cooperative Group (CRYO-ROP) and Early Treatment for Retinopathy of Prematurity (ETROP) studies reported secondary glaucoma occurring with increasing frequency with age. In the CRYO-ROP study (infants with bilateral threshold ROP), 5% of the treated eyes and 11% of the control eyes developed glaucoma by 10 years of age.20 In the ETROP study (infants with high-risk pre-threshold ROP), 2% of the eyes developed glaucoma within the first 6 years of life.
Mechanism of glaucoma
The predominant mechanism of glaucoma in ROP is due to shallowing of the anterior chamber and narrowing of the angle, leading to angle closure. The diagnosis should be suspected with severe shallowing of the AC, corneal edema, increased corneal diameter, and raised IOP. Angle-closure glaucoma is usually due to anterior displacement of the lens-iris diaphragm,21-23 for example from a cicatricial retrolental mass. Secondary glaucoma can also result from neovascularization (Figure 1).
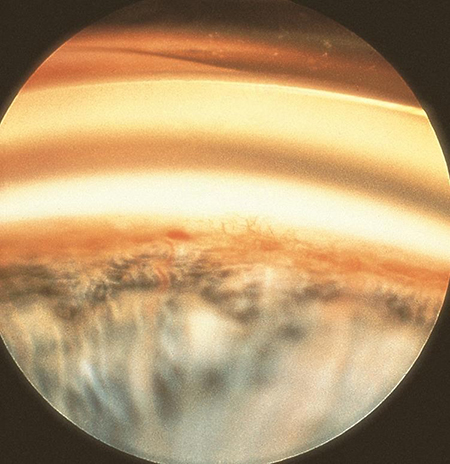
Figure 1. Iris neovascularization resulting in secondary glaucoma
Glaucoma can occur early after laser retinal photocoagulation for retinopathy of prematurity24-26 by a number of mechanisms, such as choroidal detachment after excessive photocoagulation, ciliary body edema, and relative changes in lens size. Hyphema leading to posterior synechiae formation and acute pupillary block glaucoma has been described.27 Furthermore, intraocular inflammation following laser treatment can exacerbate angle closure by encouraging the formation of PAS and PS.
Glaucoma is common complication after surgical treatment for stage 4/5 ROP after vitrectomy and scleral buckling procedures, although onset can be delayed.
Even in the absence of treatment for ROP, the anterior segments of infants with retinopathy of prematurity are often abnormal. Hartnett observed immature angle structures in 70% of premature infants with stages 4 and 5 ROP.28
Management
Topical medications are first-line treatment. Further management is guided by the mechanism of glaucoma. Surgical peripheral iridectomy (SPI) for pupillary block is recommended. Lensectomy may be considered if lens displacement is suspected to be contributing significantly to progressive angle closure. Following failed medical treatment for chronic angle closure, GDD or diode laser surgery should be considered, depending on the visual prognosis.
Given the increased risk of glaucoma in children with a history of treated or untreated Stage 4 or 5 ROP, and the fact it can develop years later, lifelong monitoring for glaucoma is crucial.
Elevated episcleral venous pressure
Elevated episcleral venous pressure (EEVP) is a cause of secondary glaucoma in children. The characteristic finding of EEVP is blood in Schlemm's canal, best observed by gonioscopy. Patients may also have prominent episcleral and conjunctival vessels. EEVP is most commonly associated with Sturge-Weber syndrome and other nonacquired conditions that are discussed in that chapter. Acquired causes of EEVP include carotid-cavernous-sinus fistula,29,30 cavernous sinus thrombosis, dural arteriolar venous shunts, superior vena cava syndrome, and orbital obstructive lesions. In cases of open-angle glaucoma and EEVP without a known cause, the condition is known as idiopathic elevated episcleral venous pressure (IEEVP). In a review by Rhee et al of IEEVP, most cases presented in early adulthood or late adolescence.31 If patients required filtration surgery, there were frequently good outcomes. However, as with other causes of elevated episcleral venous pressure, caution must be taken to avoid choroidal effusions in the postoperative period following filtration surgery.
Exudative retinal detachment
Exudative retinal detachments may cause secondary angle-closure glaucoma by anterior displacement of the lens-iris diaphragm in a number of conditions affecting children. The most common underlying diseases fall in the nonacquired category (eg, nanophthalmos, optic disc pits, morning glory syndrome,32 familial exudative vitreoretinopathy [FEVR] and cutis marmorata).33 (See the Secondary Glaucoma chapter on Glaucoma Associated With Non-Acquired Ocular Anomalies). Acquired causes include posterior uveitis, following PRP, posterior pole malignancy, Coats disease, and posterior scleritis.
Malignancy
Tumors in the eye are rare but can lead to secondary glaucoma and may masquerade as uveitis or be missed because of the difficulty examining young children. A high index of suspicion is required in any atypical presentation of glaucoma and examination under anesthesia should be performed if necessary. Tumors may be primary ocular tumors (retinoblastoma, medulloepithelioma, and melanoma) or secondary to systemic disease elsewhere (leukemia). Tumors may be benign (iris cysts) or malignant (retinoblastoma). The mechanisms producing glaucoma are multifactorial and include direct invasion of the angle, aqueous-borne tumor cells collecting in the angle and obstructing the TM, inflammation, hemorrhage, neovascularization, effusion, and mass effect pushing iris and/or lens forward to narrow the angle. The prompt and correct diagnosis of these conditions is important, as inadvertent glaucoma surgery in those with malignant characteristics may worsen the patient’s systemic prognosis by seeding malignant tumor cells outside of the eye.
Ocular malignancy
Iris cysts
Types and causes of iris cysts are covered elsewhere. They are rare in childhood and their management depends on the underlying etiology but is usually conservative if primary and uncomplicated.34 If cysts are associated with secondary glaucoma, however, regardless of underlying cause, treatment is required. Angle closure is the usual mechanism of raised IOP, with or without pupil block. Laser PI is required if there is any pupil block component, and its impact on the conformation of the angle should be assessed and monitored, as angle closure may not resolve or may recur at a later point. The iris cysts themselves should also be treated, by laser or surgery as indicated, to remove the primary cause of elevated IOP. Argon laser iridoplasty may be helpful in areas of persistent appositional angle closure. Medications (or surgery) to control IOP may also be required.
Pearl: Primary angle closure is exceedingly rare in children. It is necessary to always look for secondary causes, which may be acquired (such as tumors of iris and/or ciliary body) or nonacquired (such as nanophthalmos).35, 36
Medulloepithelioma (Diktyoma)
This ciliary body tumor usually presents in young childhood. It can be benign or malignant. Although rare, it should be considered in any case of unexplained unilateral glaucoma, especially if accompanied by uveitis, unilateral cataract, and/or neovascularization. Glaucoma is found in approximately 50% of cases, and is most commonly due to angle neovascularization, though direct tumor invasion, anterior displacement of the iris, inflammation, and other conditions can also contribute to secondary glaucoma.37 Management of glaucoma is dictated by factors related to the tumor and the overall prognosis for the eye and the child. This requires multidisciplinary input, and incisional surgery for glaucoma is contraindicated in the presence of malignancy.
Pearl: It is necessary to have a high index of suspicion of intraocular malignancy in any child with unexplained neovascularization of the iris, and to look especially carefully at the ciliary body for medulloepithelioma.
Retinoblastoma
Glaucoma may be the presenting sign of retinoblastoma and is found in 17% of a large cohort of retinoblastoma cases.38 Glaucoma (presenting as buphthalmos in younger patients) is associated with more invasive disease,39 and iris neovascularization is predictive of choroidal invasion.40 The mechanism is most commonly neovascular glaucoma (70%).38 Other mechanisms include angle closure from anterior lens-iris displacement by tumor or an associated retinal detachment, or, less commonly, open-angle glaucoma (obstruction by tumor cells). Management of the tumor takes priority, with IOP usually managed by medications, or if painful in a poor-prognosis eye, by enucleation. Filtering surgery for glaucoma is contraindicated.
Uveal melanoma
Uveal melanomas are less common in children than adults but are still an important cause of secondary glaucoma. Secondary glaucoma is more likely in anterior uveal melanomas (ie, iris and ciliary body melanomas), than choroidal melanomas, because of their relatively close relationship with the angle. In a large study of iris melanoma by Shields et al, secondary glaucoma developed in approximately 17% of patients younger than 20 years, compared to 38% of adults over 60 years in age.41 The mechanisms are similar to those mentioned above for other tumors, with direct-angle infiltration being the most common for iris melanomas,42 pigment dispersion, and tumor invasion of the angle in ciliary body melanomas, and neovascularization in choroidal melanomas.38 Additionally, radiotherapy causes TM inflammation and scarring, and there may be acute IOP rises immediately after treatment, as well as over time.
Although topical medications may be used for raised IOP, the presence of secondary glaucoma is a poor prognostic sign, and primary enucleation may be recommended in such circumstances. There is still hesitation about performing glaucoma surgery in these eyes, for fear of systemic spread, though a recent study has questioned this.43
Systemic malignancies
Juvenile xanthogranuloma (JXG)
Juvenile xanthogranuloma is a rare systemic condition peculiar to children, especially the very young. It is predominantly an anterior segment disease in which abnormal histiocytes infiltrate the iris and other structures of the anterior segment and excite an inflammatory response. Although it predominantly involves skin, the eye is involved in 0.4-10% of cases.44, 45
Mechanism of glaucoma: Secondary glaucoma develops from mechanisms that reflect recurrent bouts of inflammation, hyphema, and histiocytic cells obstructing the TM. Neovascular glaucoma can also occur. Although it is a benign tumor in terms of its nonmetastatic behavior, it can be sight threatening, especially when complicated by glaucoma. It is important to have a high index of suspicion for this disease in children with unexplained uveitis and spontaneous hyphema, or in infants with unilateral glaucoma.44
Pearl: Have a high index of suspicion of JXG in any child with unexplained uveitis and spontaneous hyphema.
Management
Management is aimed at the underlying condition (topical and/systemic steroids, immunosuppression, irradiation) and controlling IOP. If glaucoma medications are insufficient, surgery is required, and the choice is based on the surgeon’s preference and the prognosis for the eye.
Other systemic malignancies
The eye may be secondarily involved in some malignancies (by metastases or tumors that affect multiple organs). Systemic malignancies such as leukemia can involve both the anterior and posterior segments of the eye, leading to secondary open-angle glaucoma (eg, tumor cells obstructing TM in acute lymphocytic leukemia46) or secondary closed-angle glaucoma (eg, large choroidal hemorrhage complicating choroidal infiltration in chronic myeloid leukemia47). Additionally, steroids are often a component of chemotherapy and may induce raised IOP and glaucoma. Treatment of the glaucoma is generally conservative, prioritizing management of the underlying disorder.48
Infections
This section refers to glaucoma resulting from infections of the eye acquired after birth (which distinguishes it from the TORCH group). There are many scenarios in which a severe ocular or periorbital infection could temporarily lead to raised IOP, including pre-septal cellulitis, orbital cellulitis, severe corneal infection with endophthalmitis, infection following trauma, or foreign bodies. Previous surgical sites (trabeculectomy bleb or GDD erosion) can be portals for endophthalmitis, which can complicate the management of underlying glaucoma.
Herpes simplex
Ocular herpes simplex infections (keratitis, uveitis) may be accompanied by a particularly high IOP or an elevation in IOP that seems disproportionate to the degree of ocular inflammation.
Mechanism of glaucoma
The herpes viruses can specifically target the TM cells, causing trabeculitis. Inflammatory debris from coexisting keratouveitis also obstructs aqueous access to the angle, creating secondary open-angle OHT/glaucoma. Corticosteroid therapy may compound this. Over time, and with repeated episodes of inflammation, PAS develop and progressively narrow and close the angle. Severe corneal disease may ultimately require PKP and prolonged steroid usage, both of which also contribute to the risk of glaucoma.
Management
Topical glaucoma medications are first-line treatment for raised IOP, but glaucoma surgery may be required. This becomes more likely after recurrent attacks, prolonged steroid dependency, or after penetrating keratoplasty (PKP). Arguments were made against using prostaglandin analogues (PGAs) because of the risk of provoking recurrent herpetic disease, but currently many will offer a trial of PGAs in the hope of avoiding surgery. Angle surgery is not advocated, and trabeculectomy or GDD surgery are recommended, the latter especially if PKP has been/is likely to be performed. Treating the underlying infection with antivirals such as acyclovir is also important. Some advocate topical steroids only if inflammation is not controlled with antivirals; others recommend steroid therapy routinely.
Pearl: Check corneal sensation in any child presenting with unilateral mild iritis and high IOP – reduced or absent corneal sensation suggests herpetic etiology.
Uveitis
Uveitis in children is rare and the majority of cases are idiopathic. Uveitis may be isolated to the eye, or part of a larger systemic inflammatory condition.
Mechanism
Glaucoma secondary to pediatric uveitis is most commonly open angle,49 but angle-closure glaucoma is also not infrequent. There are many underlying mechanisms that contribute; these can be found in Table 1. Presentation and management will depend on the underlying mechanisms and associated disease.
|
Table 1. Mechanisms of Glaucoma in Pediatric Uveitis3
- Open-angle glaucoma
- Trabecular meshwork obstruction from inflammatory cells and proteins
- Trabecular meshwork damage from chemical mediators
- Trabecular cell dysfunction/inflammation
- Steroid response
- Angle-closure glaucoma
- Posterior synechiae
- Peripheral anterior synechiae
- Pupil closure from fibrin membrane
- Anterior rotation of ciliary body
|
Management
Treatment of inflammation begins with corticosteroids (topical, intraocular and/or systemic) as well as nonsteroidal anti-inflammatory drugs. Inadequate disease control, steroid-related side effects, or elevated IOP response to corticosteroids are reasons to add or change to steroid-sparing medications, known as disease modifying anti-rheumatic drugs (DMARDs). Systemic steroid and DMARD therapies are best managed in conjunction with a pediatric rheumatologist experienced in monitoring these medications in children.
Treatment of raised IOP is challenging due the variety of factors that may be affecting it (detailed in Table 1). Treating the underlying inflammatory condition or conversely, reducing steroid dosage in those already on treatment (with the caveat that controlling inflammation is the priority), and undertreating uveitis in an effort to avoid steroid-responsive IOP elevation is, in the long-run, counterproductive. In acute angle closure, consider intensive cycloplegia to break existing posterior synechiae and resolve iris bombé. If unsuccessful, SPI is required. Early prophylaxis using short-acting cycloplegic drops may prevent posterior synechiae formation in the first place. Removal of an intumescent lens, or one leaking protein, will remove a source of inflammation.
Pearl: Performing a laser peripheral iridotomy (LPI) on a child is difficult. Furthermore, LPIs performed in inflamed eyes are more likely to close, require higher energy to penetrate an inflamed swollen iris, and are more likely to hemorrhage because of engorged iris vessels. A surgical approach (SPI) provides a more definitive treatment in this situation.
In terms of reducing IOP itself, treatment begins with topical medications, usually a topical beta blocker and/or topical carbonic anhydrase inhibitor. Use of prostaglandin analogues (PGA) and topical alpha-2 agonists (in older children) is usually introduced as a second-line treatment. Although there are hypothetical concerns for increased risks of inflammation with PGAs, Chang et al suggest that there was no increased risk of uveitis or macular edema,50 and when faced with the choice between adding a PGA versus surgery, most would opt for the former. Pilocarpine reduces the blood-aqueous barrier and may promote inflammation, as well as miosis, both of which predispose to PS formation; pilocarpine is thus best avoided in inflamed eyes. Systemic carbonic anhydrase inhibitors may also be used as a bridging medication while waiting for surgery, or when there is hope that the period of IOP elevation is transitory; because of their side effects, they are not usually considered a long-term option,
Surgical preferences vary based on surgeon experience and practice patterns. Adequate control of inflammation prior to surgery is highly desirable, and temporizing IOP control using agents such as oral carbonic anhydrase inhibitors, or compromising by allowing the patient to continue with a slightly higher IOP than desired, to allow safer glaucoma surgery at a later date, may be prudent. Ideally the eye should be “quiet” for a minimum period of three months before performing intraocular surgery. Most surgeons opt for GDD surgery or trabeculectomy. If the angle is open, some surgeons advocate beginning with goniosurgery51 and, if unsuccessful, moving on to GDD surgery. Hypotony complicates surgery in pediatric uveitic glaucoma more commonly than in the general population, and adjustments to surgical technique are required to minimize the risks of hypotony52 both in the immediate postoperative period, and later, in case there is a uveitic flare in the presence of a filter or GDD.
Juvenile idiopathic arthritis
Juvenile idiopathic arthritis (JIA) is a heterogeneous group of diseases with many systemic manifestations. JIA-associated iridocyclitis is one of the leading causes of visual morbidity in children53 and a common cause of uveitic glaucoma in children.54 Managing glaucoma in children with uveitis is challenging, and control of intraocular inflammation is crucial. Glaucoma in JIA is largely associated with long-standing poorly controlled inflammation. In a series by Foster et al, 42% of the patients with JIA-associated iridocyclitis developed glaucoma, and only 36% were successfully treated medically.
Other acquired systemic inflammatory conditions
The most common inflammatory diseases other than JIA that cause glaucoma in children are idiopathic (in which no underlying cause is found), and much more rarely, diseases such as sarcoidosis, rubella,55 herpes,56 and syphilis. Although sarcoidosis typically presents in adults between 20 and 40 years of age, it can present in children. Ocular involvement is most commonly anterior uveitis leading to secondary glaucoma, which can be either open angle or closed angle due to several underlying mechanisms (see Table 1).
Systemic malignancies such as juvenile xanthogranuloma and leukemia may be accompanied by uveitis, which can lead to secondary glaucoma.
Diabetes
Ocular manifestations of diabetic disease in children are currently uncommon, although they may be expected to increase as diabetes becomes more common in this age group. The likelihood of diabetic retinopathy is strongly correlated with duration of diabetes and overall glycemic control.48 Glaucoma secondary to diabetes is typically due to proliferative disease with angle neovascularization, causing secondary angle closure. Neovascular glaucoma (NVG) in young diabetic patients often portends an unfavorable outcome,57 and management is difficult and life-long. The approach to glaucoma is similar to that in adults, and recently, success with intravitreal anti-vascular endothelial growth factor (anti-VEGF) medications, coupled with trabeculectomy has been reported.58 A case of phacomorphic glaucoma in a child with newly diagnosed type 1 diabetes mellitus that resolved with cataract surgery has also been reported.59 Ultimately, careful ophthalmic monitoring of children with diabetes of longer than 5 years' duration, and especially those with elevated glycosylated hemoglobin, is required to prevent the devastating consequences of NVG.
Medications
Topical and systemic medications may lead to secondary OHT or glaucoma. It is important to inquire about medication use, even for seemingly remote health conditions, when assessing children with signs of glaucoma. There is often little awareness amongst other specialists of the potential of systemic medications to cause secondary glaucoma with possibly devastating effects on vision.60
Corticosteroids
Corticosteroids, whether topically applied to the eye or administered by other routes to the body, can cause raised IOP and lead to glaucoma. This is known as steroid-response OHT/glaucoma in that the eye “responds” to steroid administration with elevated IOP. In children, this response tends to be earlier (occurring within days to weeks from starting steroid), and more severe (higher IOPs) than in adults.61 In studies of topical ocular steroid preparations, the IOP response was greater in younger children (less than 6 years of age61) and greater with formulations of higher potency or higher frequency of administration.62,63
Pearl: Always ask for a history of steroid use in any form in children with signs of glaucoma, regardless of current IOP.
Mechanism
This is an open-angle mechanism in which corticosteroid induces raised IOP, mainly by its effect on the TM cells, in which upregulation of the myocilin (previously known as TIGR) gene leads to deposition of extracellular matrix (ECM) within the cells, cross-linking of actin fibers between the cells, and other effects to reduce aqueous outflow from the anterior chamber into Schlemm’s channel.64
Management
When possible, withdrawing the steroid (frequency of instillation, potency of dose), will often reverse the effects. Where protracted immunosuppressant treatment is required (eg, after PKP, or in some uveitides), “steroid sparing agents” may allow withdrawal of corticosteroids. This should be co-managed with a pediatric rheumatologist or physician experienced in DMARD therapy. Because the IOP response to withdrawal is not immediate, glaucoma medications (starting with aqueous suppressants) should also be initiated during the period of steroid weaning. If IOP normalizes, and steroids have been successfully weaned, glaucoma medications may also be withdrawn, with close monitoring of IOP.
When steroid withdrawal is not possible, or if IOP remains inadequately controlled despite steroid reduction, and glaucoma medications are not sufficient, surgery is required. The decision about when to intervene will depend on many factors, as will the choice of surgery. If the angle is otherwise uninvolved, and the glaucoma is caused primarily by steroid responsiveness rather than other ocular disease, angle surgery may be successfully undertaken. When this is not the case, or if angle surgery fails or other risk factors dictate it, filtering procedures such as trabeculectomy or GDD surgery are indicated.
Topiramate and other sulfa drugs
Idiosyncratic reactions to topiramate and other sulfa drugs (such as acetazolamide) can present with acute bilateral angle-closure glaucoma. This may present with blurred vision from a dramatic myopic shift, or with symptoms of acutely elevated IOP.
Mechanism
This is a form of secondary angle closure in which there is neither an underlying predisposing angle configuration, nor a contribution from pupillary block. The offending drug causes ciliary body edema, which relaxes the zonules, and the lens thickens. Anterior rotation of ciliary body from its attachment to the scleral spur encourages anterior displacement of the lens-iris diaphragm, which further shallows the anterior chamber and narrows the angle. In many cases, this is accompanied by ciliochoroidal effusions, which compound the “pushing” element of this mechanism.
Management
Acutely elevated IOP is managed with topical glaucoma medications (usually aqueous suppressants) and systemic acetazolamide, if necessary. The underlying mechanism is addressed by cycloplegia and stopping the offending drug. Its further use is contraindicated. PI is not indicated. If control cannot be achieved by these measures, drainage of the effusions may be considered. Rarely, a glaucoma procedure (trabeculectomy) may be required.12
Pearl: Be suspicious of bilateral acute onset angle closure as a possible drug reaction, and take a careful history of medication usage. B scan or ultrasound biomicroscopy (UBM) will show anterior cilio-choroidal effusions that may not be visible on clinical examination.
Drugs causing pupil block
Any medication with anti-cholinergic or adrenergic properties may contribute to angle closure in predisposed children.
Mechanism
Anticholinergic and/or adrenergic drugs cause some degree of pupillary dilation and anterior movement of the lens from their effect on the iris and ciliary body musculature. In children with conditions characterized by crowded anterior chambers (such as nanophthalmos) or narrowed angles (ROP), such medications may induce an angle-closure attack. There can be crowding of the angle by iris as well as secondary pupil block promoting further anterior displacement of the lens-iris diaphragm and peripheral iris, closing the angle further.
Management
Topical and systemic medications are used to lower IOP in the acute setting. Pilocarpine is not usually used, because this is not a primary pupil-block scenario. if anything, the situation may respond to mydriasis; each situation should be taken on its merits. The pupil block component is addressed by LPI or SPI. Further management will be dictated by the underlying ocular condition. Even with a patent PI, the child should continue to be monitored carefully and the angle assessed regularly, as the underlying mechanism is multifactorial, and progressive synechial closure may follow acute attacks.
References
- Sihota, R, Kumar S, Gupta V, Dada T, Kashyap S, Insan R, Srinavasan G. Early predictors of traumatic glaucoma after closed globe injury: trabecular pigmentation, widened angle recess, and higher baseline intraocular pressure. Arch Ophthalmol, 2008. 126(7): 921-926.
- Rocha KM, Martins EN, Melo LA Jr, Moraes NS, Outpatient management of traumatic hyphema in children: prospective evaluation. J AAPOS, 2004; 8:357-361.
- Joos K, et al., Glaucoma associated with acquired conditions. In: Childhood Glaucoma. WGA Consensus Series - 9., G.A. Weinreb RN, Papadopoulos M, Grigg J, Freedman S, eds. Kugler Publications: Amsterdam.
- Pandey P. Sung VC, Gonioaspiration for refractory glaucoma secondary to traumatic hyphema in patients with sickle cell trait. Ophthalmic Surg Lasers Imaging, 2010; 41(3): 386-389.
- Goldberg M. Sickled erythrocytes, hyphema, and secondary glaucoma: I. The diagnosis and treatment of sickled erythrocytes in human hyphemas. Ophthalmic Surg, 1979; 10:17-31.
- Nasrullah A, Kerr NC. Sickle cell trait as a risk factor for secondary hemorrhage in children with traumatic hyphema. Am J Ophthalmol. 1997; 123(6):783–790.
- Hooper CY, Fraser-Bell S, Farinelli A, Grigg JR. Complicated hyphaema: think sickle. Clin Experiment Ophthalmol, 2006. 34(4):377-378.
- De Leon-Ortega JE, Girkin CA. Ocular trauma-related glaucoma. Ophthalmol Clin North Am. 2002; 15:215-223.
- Salmon JF, Mermoud A, Ivey A, Swanevelder SA, Hoffman M. The detection of post-traumatic angle recession by gonioscopy in a population-based glaucoma survey. Ophthalmology. 1994; 101(11):1844-1850.
- Spirn M, Lynn MJ, Hubbard GB 3rd, Vitreous hemorrhage in children. Ophthalmology 2006; 113:848-852.
- Montenegro MH, Simmons RJ. Ghost cell glaucoma. Int Ophthalmol Clin, 1995;. 35(1):111-115.
- Razeghinejad MR, Pro MJ, Katz LJ. Non-steroidal drug-induced glaucoma. Eye (Lond). 2011; 25(8):971-980.
- Edwards AO. Ishihara Y. Retinal disorders and glaucoma. In: Glaucoma Science and Practice. Morrison JC, Pollack IP, eds. New York: Thieme Medical Publishers Inc. 2003; 291-304.
- Singh D, Chandra A, Sihota R, Kumar S, Gupta V. Long-term success of mitomycin-augmented trabeculectomy for glaucoma after vitreoretinal surgery with silicone oil insertion: a prospective case series. Retina. 2014; 34: 123-128.
- Ishida K, Ahmed II, Netland PA Ahmed glaucoma valve surgical outcomes in eyes with and without silicone oil endotamponade. J Glaucoma. 2009; 18(4):325-30.
- Low JR, Anshu A, Tan AC, Htoon HM, Tan DT., The outcomes of primary pediatric keratoplasty in Singapore. Am J Ophthalmol, 2014; 158(3):496-502.
- Wenick AS, Barañano, Evaluation and management of pediatric rhegmatogenous retinal detachment. Saudi J Ophthalmol, 2012; 26(3):255-263.
- Schaumberg DA, Dana MR, Christen WG, Glynn RJ. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology, 1998; 105(7):1213-1221.
- Hartnett ME, Gilbert MM, Hirose T, Richardson TM, Katsumi O., Glaucoma as a cause of poor vision in severe retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol, 1993; 231(8):433-438.
- Cryotherapy for Retinopathy of Prematurity Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001; 119(8):1110-1118.
- Pollard ZF. Secondary angle-closure glaucoma in cicatricial retrolental fibroplasia. Am J Ophthalmol, 1980; 89(5):651-653.
- Ueda N, Ogino N. Angle-closure glaucoma with pupillary block mechanism in cicatricial retinopathy of prematurity. Ophthalmologica, 1988; 196(1):15-18.
- Smith J, Shivitz I, Angle-closure glaucoma in adults with cicatricial retinopathy of prematurity. Arch Ophthalmol. 1984; 102(3):371-372.
- Uehara A, Kurokawa T, Gotoh H, Yoshimura N, Tokushima T. Angle closure glaucoma after laser photocoagulation for retinopathy of prematurity. Br J Ophthalmol. 88(8):1099-1100.
- Trigler L, Weaver RG Jr, O'Neil JW, Barondes MJ, Freedman SF. Case series of angle-closure glaucoma after laser treatment for retinopathy of prematurity. J AAPOS. 2005; 9(1):17-21.
- Moshfeghi DM, Silva RA, Berrocal AM. Exudative retinal detachment following photocoagulation in older premature infants for retinopathy of prematurity: description and management. 2014. 34(1):83-6.
- Ritch R, Forbes M Hetherington J Jr, Harrison R, Podos SM. Congenital ectropion uveae with glaucoma. Ophthalmology, 1984. 91(4):326-331.
- Hartnett, M.E., et al., Anterior segment evaluation of infants with retinopathy of prematurity. 1990; 97(1):122-130.
- Bekendam PJ, Bekendam PD, Carotid-cavernous fistula in a child after minimal orbital trauma. J AAPOS. 2004; 8(4):401-403.
- Paiva WS, de Andrade AF, Beer-Furtan A, et al. Traumatic carotid-cavernous fistula at the anterior ascending segment of the internal carotid artery in a pediatric patient. Childs Nerv Syst, 2013; 29(12):2287-2290.
- Rhee DJ, Gupta M, Moncavage MB, Moster ML, Moster MR. Idiopathic elevated episcleral venous pressure and open-angle glaucoma. Br J Ophthalmol, 2009; 93(2):231-234.
- Steidl S, Hartnett ME, eds. Clinical Pathways in Vitreoretinal Disease. New York: Thieme. 2003
- Pendergast SD, Trese MT, Shastry BS. Ocular findings in cutis marmorata telangiectatica congenita. Bilateral exudative vitreoretinopathy. Retina. 1997; 17:306-309.
- Shields JA, Kline MW, Augsburger JJ. Primary iris cysts: a review of the literature and report of 62 cases. Br J Ophthalmol, 1984; 68:152-166.
- Badlani VK, Quinones R, Wilensky JT, Hawkins A, Edward DP. Angle-closure glaucoma in teenagers. J Glaucoma, 2003; 12(3):198-203.
- Ritch R, Chang BM, Liebmann JM. Angle closure in younger patients. Ophthalmology, 2003; 110(10):1880-1889.
- Broughton WL, Zimmerman LE. A clinicopathologic study of 56 cases of intraocular medulloepitheliomas. Am J Ophthalmol. 1978; 85(3):407-418.
- Shields CL, Shields JA, Shields MB, Augsburger JJ. Prevalence and mechanisms of secondary intraocular pressure elevation in eyes with intraocular tumors. Ophthalmology, 1987; 94:839-846.
- Chantada GL, Gonzalez A, Fandino A, et al. Some findings at presentation can predict highrisk pathology features in unilateral retinoblastoma. J Pediatr Hematol Oncol. 2009; 31:325-329.
- Shields CL, Shields, JA, Baez KA, Cater J, De Potter PV. Choroidal invasion of retinoblastoma: Metastatic potential and clinical risk factors. Br J Ophthalmol, 1993; 77(9):544-548.
- Shields CL, Kaliki S, Shah SU, Luo W, Furuta M, Shields JA. Iris melanoma features and prognosis in children and adults in 317 patients. J AAPOS. 2012; 16:10-16.
- Shields CL, Materin MA, Shields JA, Gershenbaum E, Singh AD, Smith A. Factors associated with elevated intraocular pressure in eyes with iris melanoma. Br J Ophthalmol, 2001; 85(6):666-669.
- Sharkawi E, Oleszczuk JD, Bergin C, Zografos L. Baerveldt shunts in the treatment of glaucoma secondary to anterior uveal melanoma and proton beam radiotherapy. Br J Ophthalmol. 2012; 96(8):1104-1107.
- Vendal Z, Walton D, Chen T. Glaucoma in juvenile xanthogranuloma. Semin Opthalmol. 2006; 21(3):191-194.
- Liang S, Liu YH, Fang K. Juvenile xanthogranuloma with ocular involvement. Pediatr Dermatol., 2009; 26:232-234.
- Abramson A., Anterior chamber activity in children with acute leukemia. Ann Ophthalmol.; 12:53.
- Santoni G, Fiore C, Lupidi G, Bibbiani U. Recurring bilateral hypopyon in chronic myeloid leukemia in blastic transformation. A case report. Graefes Arch Clin Exp Ophthalmol, 1985; 223:211-213.
- Hoyt CT, Taylor D, eds. Pediatric Ophthalmology and Strabismus. Edinburgh: Elsevier.
- Heinz C, Mingels A, Goebel C, Fuchsluger T, Hiligenhaus A. Chronic uveitis in children with and without juvenile idiopathic arthritis: differences in patient characteristics and clinical course. J Rheumatol, 2008; 35(7):1403-1407.
- Chang JH, McCluskey P, Missotten T, Ferrante P, Jalaludin B, Lightman S, Use of ocular hypotensive prostaglandin analogues in patients with uveitis: does their use increase anterior uveitis and cystoid macular oedema? Br J Ophthalmol. 2008. 92(7):916-921.
- Ho CL,Wong EY, Walton DS. Goniosurgery for glaucoma complicating chronic childhood uveitis. Arch Ophthalmol, 2004; 122(6):838-844.
- Daniel E, Pistilli M, Pujari SS, et al. Risk of hypotony in noninfectious uveitis. Ophthalmology. 2012; 119(11):2377-2385.
- Woreta, F, Thorne JE, Jabs DA, Kedhar SR, Dunn JP. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol, 2007; 143(4):647-655.
- Foster, C.S., et al., Secondary glaucoma in patients with juvenile rheumatoid arthritis-associated iridocyclitis. Acta Ophthalmol Scand. 2000; 78(5):576-579.
- Givens KT, Lee DA, Jones T, Ilstrup DM. Congenital rubella syndrome: ophthalmic manifestations and associated systemic disorders. Br J Ophthalmol. 1993; 77(6):358-363.
- Grant WM. Late glaucoma after interstitial keratitis. Am J Ophthalmol, 1975. 79(1):87-91.
- Amaya LD, Taylor D, Russell-Egitt I, Nischal KK, Lengyel D, The morphology and natural history of childhood cataracts. Surv Ophthalmol, 2003; 48:125-144.
- Cornish KS, Ramamurthi S, Saidkasimova S, Ramaesh K. Intravitreal bevacizumab and augmented trabeculectomy for neovascular glaucoma in young diabetic patients. Eye (Lond). 2009; 23(4):979-981.
- Skrabic V, Ivanisevic M, Stanic R, Unic I, Bucan K, Galetovic D. Acute bilateral cataract with phacomorphic glaucoma in a girl with newly diagnosed type 1 diabetes mellitus. J Pediatr Ophthalmol Strabismus. 2010; 47 Online: p. e1-3.
- Gupta S, Shah P, Grewal S, Chaurasia AK, Gupta V. Steroid-induced glaucoma and childhood blindness. Br J Ophthalmol. 2015; 99:1454-1456.
- Lam, D., D. Fan, and J. Ng, Ocular hypertensive and anti-inflammatory responses to different dosages of topical dexamethasone in children: a randomized trial. Clin Experiment Ophthalmol, 2005. 33: p. 252-258.
- Fan D, Ng JS, Lam DS. A prospective study on ocular hypertensive and anti-inflammatory response to different dosages of fluorometholone in children. Ophthalmology. 2001; 108:1973-1977.
- Ng JS, Fan DS, Young AL, Yip NK, Tam K, Kwok AK, Lam DS. Ocular hypertensive response to topical dexamethasone in children: a dose-dependent phenomenon. 2000; 107:2097-2100.
- Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmol Ther, 2013; 2:55–72.