By Robert A. Clark, MD
Clinical Presentation
The sole function of the sixth cranial nerve is to innervate the lateral rectus muscle, so the hallmark of a sixth nerve palsy is weakness of the lateral rectus.
Important Anatomic Factors
Two anatomic factors can complicate the otherwise straightforward clinical presentation of a patient with horizontal diplopia, a face turn toward the paretic lateral rectus, impaired abduction toward the paretic lateral rectus, and esotropia in forced primary gaze.
The sixth nerve has a relatively long course from its nucleus in the pons to the lateral rectus (Figure 1) and, depending on the location of the pathology, other important neurologic or anatomic structures might also be compromised (Figure 2).
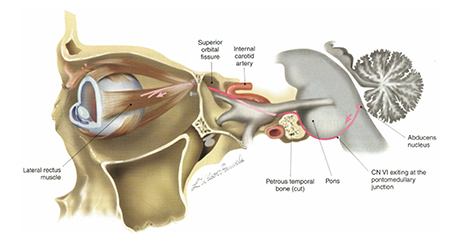
Figure 1. Route of the sixth nerve from the pons to the lateral rectus. (Reprinted with permission from Wilson-Pauwels L, Akesson E J, Stewart PA, et al. Cranial Nerves in Health and Disease, 2nd ed. Copyright 2002 BC Decker).
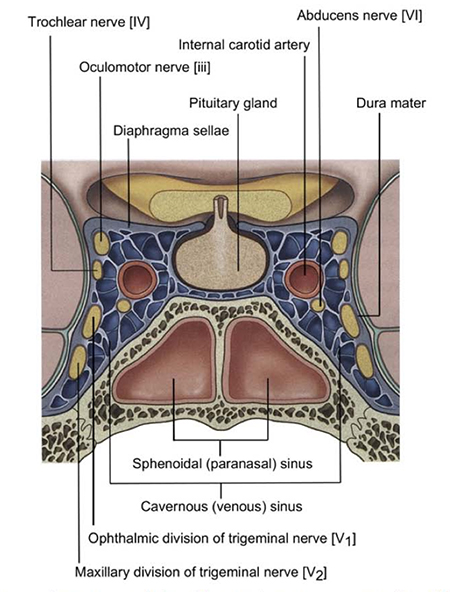
Figure 2. Coronal view of the cavernous sinus. The oculomotor nerve (cranial nerve 3) and trochlear nerve (cranial nerve 4) travel anteriorly along the lateral wall, along with the ophthalmic and maxillary divisions of the trigeminal nerve (cranial nerve 5). The abducens nerve (cranial nerve 6), however, floats in the middle of the cavernous sinus adjacent to the internal carotid artery. (Reprinted with permission from Drake R, Vogl W, Mitchell A. Gray’s anatomy for students, 2nd edition. London: Churchill Livingstone, 2010).
The sixth nerve itself contains a bifid, segregated structure of innervation to effectively create two vertically divided neuromuscular compartments within the lateral rectus (Figure 3). The two lateral rectus compartments have been shown to have differential contraction (Figure 4) during ocular counter-rolling, vertical vergence, and vertical ductions, endowing the lateral rectus with potential vertical and torsional actions in addition to abduction (Figure 5).
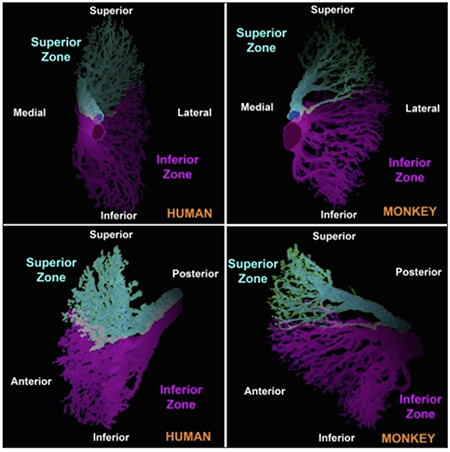
Figure 3. Three-dimensional reconstructions in two perspectives each in human and monkey of the histology of intramuscular branches of cranial nerve 6, demonstrating segregation of innervation into superior and inferior zones. (Reprinted with permission from Peng M, Poukens V, da Silva Costa RM, et al. “Compartmentalized innervation of primate lateral rectus muscle.” Invest Ophthalmol Vis Sci. 2010: 51 (9), 4612-4617).
Important Elements of Patient History
Particular attention must be paid to three key clinical factors in addition to the standard historical elements included in all ophthalmology exams.
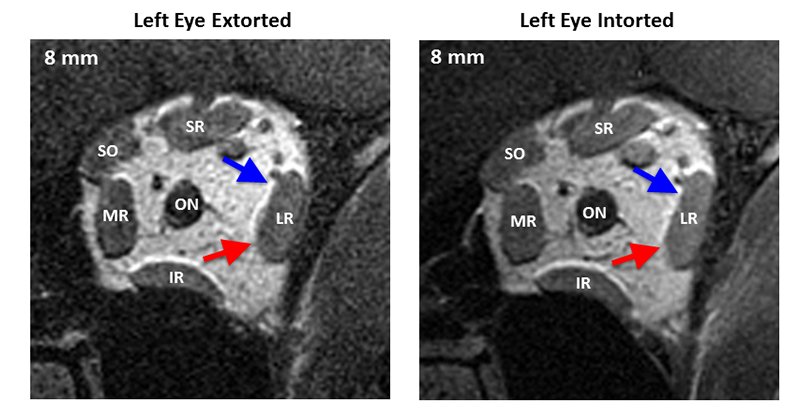
Figure 4. Quasi-coronal magnetic resonance imaging of the left orbit with the left eye extorted (left eye in the up position) and intorted (left eye in the down position). Despite no change in the horizontal or vertical eye position (torsional rotation of the globe only), the inferior compartment of the lateral rectus (red arrow) shows contractile thickening in the posterior orbit, 8 to 14 mm posterior to the globe, while the superior compartment (blue arrow) does not. LR = lateral rectus, SR = superior rectus, SO = superior oblique, MR = medial rectus, IR = interior rectus. [FIGURE 4 POWERPOINT TO BE ENTERED.]
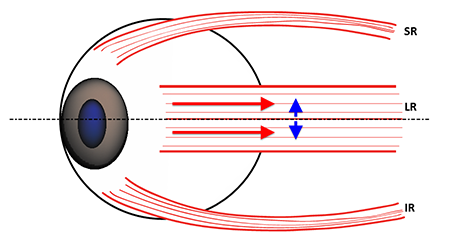
Figure 5. Lateral view of the vertical extent of the lateral rectus (LR) insertion across the horizontal globe meridian (dashed black line). SR = Superior Rectus; IR = Inferior Rectus. If both compartments contract symmetrically, the vertical forces (blue arrows) caused by the offset of the compartments from the horizontal globe meridian are opposite and equal, canceling each other and leaving the LR with only abducting force (red arrows).
The age of the patient is important in determining the need for an extensive workup, including neuro-imaging. Patients older than 50 years with known vasculopathic risk factors, especially diabetes mellitus, usually can be followed clinically in the absence of other neurological symptoms. If, after three months, there are no signs of clinical improvement for isolated sixth nerve palsies, it is appropriate to initiate a neurologic workup to look for the nonvasculopathic etiologies (Table 1). Sixth nerve palsies in younger patients, however, have been associated with high rates of increased intracranial pressure, vascular anomalies, and neoplasms (Table 2). Patients younger than 50 years should undergo a neurologic workup at the time of initial diagnosis unless there is a clear history of significant antecedent trauma, with idiopathic or post-viral etiologies remaining diagnoses of exclusion pending a normal workup.
| Table 1 - Causes of Sixth Nerve Palsies in Adults |
|---|
| Vasculopathies |
Diabetes Mellitus
Atherosclerosis
Hypertension
Preeclampsia |
| Trauma |
Skull Fracture
Head Injury
Cervical Spinal Fracture |
| Neoplasm |
Benign
Malignant
Metastatic |
| Infections |
Lyme Disease
Syphilis
Varicella Zoster
Bacterial Infection
Fungal Infection |
| Hematologic |
Leukemia
Lymphoma
Interferon Therapy |
| Systemic Disorders |
Systemic Lupus Erythematosus
Sarcoidosis
Amyloidosis
Collagen Vascular Disease |
| Intracranial Vascular Causes |
Aneurysm
Arteriovenous Malformation
Cerebrovascular Embolism
Giant Cell Arteritis
Carotid Artery Dissection |
| Neurologic Disorders |
Pseudotumor Cerebri
Multiple Sclerosis
Cluster Headache
Lithium Toxicity |
| Iatrogenic |
Neurosurgical Trauma
Nerve Block
Lumbar Puncture
Spinal or Epidural Anesthesia
Myelography |
| Table 2 - Causes of Sixth Nerve Palsies in Children |
|---|
| Trauma |
Skull Fracture
Head Injury |
| Neoplasm |
Benign
Malignant
Metastatic |
| Infections |
Abscess
Gradenigo syndrome
Varicella Zoster
Meningitis
Lyme Disease
Cytomegalovirus
Epstein Barr Virus |
| Vasculopathies |
Diabetes Mellitus
Atherosclerosis
Hypertension |
| Intracranial Disorders |
Aneurysm
Arteriovenous Malformation
Arnold-Chiari Malformation
Hydrocephalus |
| Neurologic Disorders |
Pseudotumor Cerebri
Migraine
Multiple Sclerosis |
| Iatrogenic |
Neurosurgical Trauma
Immunization
Birth Trauma |
The presence of other localizing signs and symptoms can provide both a location for the site of the pathology and a diagnosis. Moebius syndrome, the classic association of congenital concurrent sixth and seventh nerve palsies, is often accompanied by other neurodevelopmental defects, including abnormalities of other cranial nerves. A similar acquired syndrome in adults is ventral pontine syndrome from an infarct in the ventral pons that affects the nuclei of the sixth and seventh cranial nerves (Figure 1). Cavernous sinus pathology typically involves cranial nerves three and four, and the superior two divisions of five (Figure 2). Gradenigo syndrome from severe bacterial infections of the middle ear and adjacent petrous portion of the temporal bone can create periorbital pain from involvement of the fifth cranial nerve, in addition to the sixth nerve palsy. Finally, isolated sixth nerve palsies are often accompanied by small (< 5 prism diopter) hypertropias, possibly from asymmetric involvement of the lateral rectus compartments, but the presence of larger hypertropias should prompt consideration of concurrent fourth nerve palsies, skew deviations, or, in children, dissociated vertical deviations.
The magnitude of the lateral rectus deficit provides important diagnostic, prognostic, and therapeutic information. Complete palsies with symmetrical atrophy of both compartments of the lateral rectus (Figure 6) are the most straightforward to diagnose because of the accompanying large esotropia and severely restricted abduction. Most patients with complete sixth nerve palsies will be unable to even abduct the involved eye to the midline.
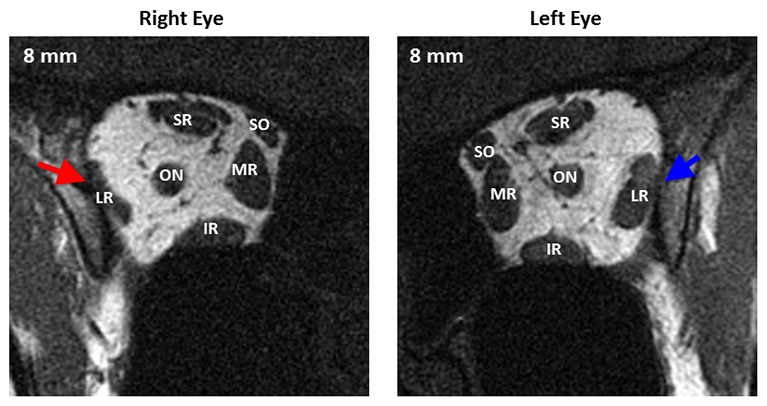
Figure 6. Quasi-coronal magnetic resonance imaging of both orbits in central gaze showing profound symmetric atrophy of the paretic right lateral rectus muscle belly (red arrow) compared with the uninvolved left lateral rectus belly (blue arrow). Distances are scaled by 2mm slices posterior or anterior to the globe-optic nerve junction. LR = lateral rectus, SR = superior rectus, SO = superior oblique, MR = medial rectus, IR = inferior rectus.
Partial lateral rectus function is associated with a smaller primary gaze esotropia and retained ability to abduct to the midline and beyond (Figure 7), but the preservation of some sixth nerve function raises the possibility of other confounding diagnoses (Table 3). Partial sixth nerve palsies, however, are more amenable to surgical correction if spontaneous recovery does not occur.
| Table 3 –Differential Diagnosis of Sixth Nerve Palsy |
|---|
| Neurologic Disorders |
Duane Syndrome
Myasthenia Gravis
Progressive External Ophthalmoplegia
Horizontal Gaze Palsy |
| Restrictive Disorders |
Dysthyroid Orbitopathy
Medial Wall Orbital Fracture
Congenital Fibrosis Syndromes |
| Post-Surgical Disorders |
Lost Lateral Rectus
Disinsertion of the Lateral Rectus
Excessive Lateral Rectus Recession
Medial Rectus Scarring and Contracture
Excessive Medial Rectus Resection |
| Miscellaneous |
High Myopia with Inferior Lateral Rectus Displacement
Inflammatory Orbital Disease
Orbital Amyloidosis |
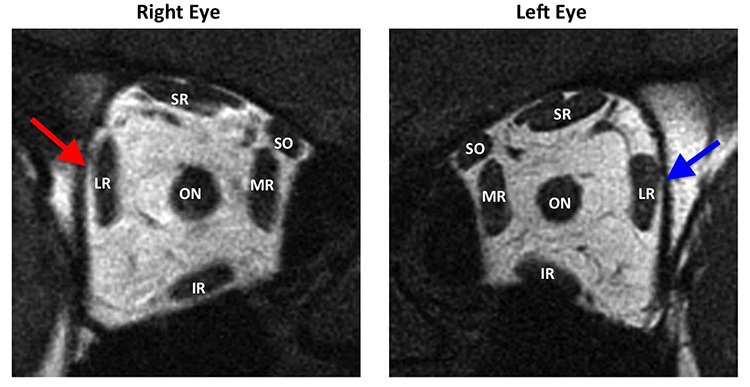
Figure 7. Quasi-coronal magnetic resonance imaging of both orbits in central gaze showing partial atrophy of the paretic right lateral rectus muscle belly (red arrow) compared with the uninvolved left lateral rectus belly (blue arrow) in the image plane 4 mm posterior to the globe-optic nerve junction. LR = lateral rectus, SR = superior rectus, SO = superior oblique, MR = medial rectus, IR = inferior rectus.
A recently discovered subtype of sixth nerve palsy, lateral rectus superior compartment palsy (Figure 8), demonstrates asymmetrical atrophy of primarily the superior compartment of the lateral rectus. This subtype may be associated with vertical and torsional defects in addition to the abduction deficit in the absence of concurrent cyclovertical muscle palsies. The prevalence of asymmetric partial sixth nerve palsies remains to be determined.
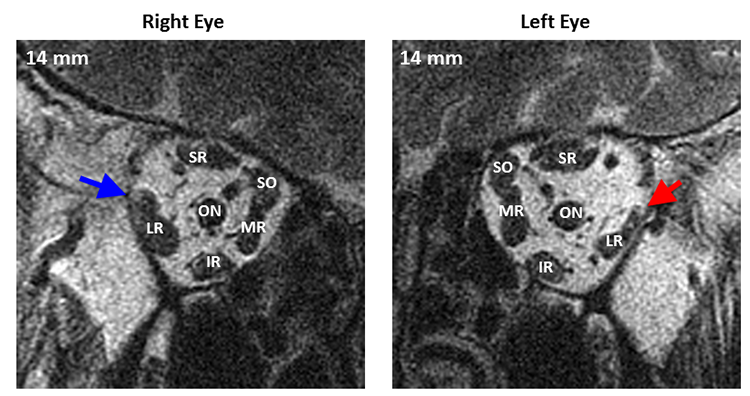
Figure 8. Quasi-coronal magnetic resonance imaging of both orbits in central gaze showing marked asymmetric atrophy of the superior compartment of the left lateral rectus (red arrow) compared with the superior compartment of the uninvolved right lateral rectus (blue arrow). The inferior compartment of the left lateral rectus also has mild atrophy, not as pronounced as the superior compartment. Distances are scaled by 2 mm slices posterior or anterior to the globe-optic nerve junction. LR = lateral rectus, SR = superior rectus, SO = superior oblique, MR = medial rectus, IR = interior rectus.
Diagnostic Examination
In addition to the standard ophthalmic examination, the following elements should be emphasized:
- Visual Acuity: In children, paralytic esotropia might cause strabismic amblyopia.
- Motility: The diagnostic positions of gaze will uncover the incomitant esotropia that increases with attempted gaze toward the involved lateral rectus, but the presence of decreased adduction, especially with globe retraction, should raise the possibility of Duane syndrome. In addition, the presence of superimposed vertical or torsional defects should raise the possibility of an asymmetric sixth nerve palsy if the secondary deviations are small (< 5 prism diopters), or concurrent third or fourth nerve palsies or skew deviations if the secondary deviations are large (> 5 prism diopters).
- Neurologic Signs: The presence of papilledema, nystagmus, multiple cranial nerve neuropathies or systemic neurologic signs such as paresthesia or paresis should raise the possibility of a more diffuse intracranial process (Video 1).
Video 1. Partial right sixth nerve palsy and gaze-induced nystagmus in a young girl with an intracranial mass.
- Special Clinical Testing: Forced duction and force generation testing can determine the relative contributions of medial rectus restriction and lateral rectus weakness to the abduction deficit. Although rarely needed, saccadic velocity testing can also help distinguish between restriction and paresis (Figure 9).
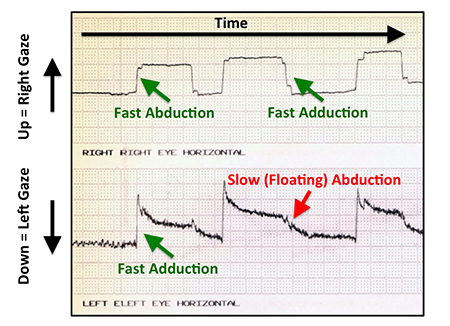
Figure 9. Saccadic velocity tracing for a patient with a complete left lateral rectus palsy. Vertical displacements represent changes in eye positions to the right (up) and left (down) for the right eye (top tracing) and left eye (bottom tracing). Green arrows point to linear, fast changes in gaze position, while the red arrow points to a slow, drifting saccade.
Additional Workup
The need for laboratory studies or imaging depends on the findings uncovered during the clinical presentation and diagnostic exam. For isolated sixth nerve palsies in older (> 50) patients with diabetes mellitus or other vasculopathic risk factors, careful clinical follow-up is indicated, with additional workup only for new neurologic symptoms or for lack of any clinical signs of early resolution after three months of follow-up. In younger patients (< 50) and patients with other concurrent neurologic or systemic symptoms, however, a more aggressive workup is indicated at presentation.
Neuro-imaging should be performed for younger patients and/or those with concurrent neurologic symptoms. The neuro-imaging should be performed urgently in the presence of other neurologic signs such as papilledema, but can be performed nonurgently within one or two weeks for isolated sixth nerve palsies in younger patients. Neuro-imaging should also be performed in older patients at the time of initial diagnosis if there are other neurologic findings, and for suspected vasculopathic sixth nerve palsies if no clinical signs of spontaneous improvement have occurred after three months of follow-up.
Erythrocyte sedimentation rate (ESR) and temporal artery biopsy should be considered for elderly patients at risk for giant cell arteritis.
Thyroid studies, including the sensitive thyroid-stimulating hormone and anti-thyroglobulin antibody tests, should be considered for patients with clinical signs of dysthyroid orbitopathy and restrictive strabismus.
Orbital imaging should be considered for patients with possible orbital trauma and extraocular muscle entrapment and for those with partial sixth nerve palsies to assess the symmetry and extent of muscle atrophy. Orbital imaging can also aid the diagnosis of patients with high myopia to help determine if an abnormal location of the lateral rectus muscle within the orbit, not lateral rectus paresis, is the cause of the esotropia.
Treatment
The treatment can be divided into two phases.
First six months after onset
- Because of the high rate of spontaneous recovery, most sixth nerve palsies should initially be managed conservatively, unless recovery is unlikely due to the type of pathology (e.g., total resection of the sixth nerve during a neurosurgical procedure).
- Occlusion should be used to treat amblyopia in young (< 8) children. To prevent amblyopia, the occlusion can be utilized on an alternating daily basis between the two eyes both to preserve vision and to stimulate movement in the paretic eye to reduce the potential for medial rectus contracture. Occlusion can also eliminate diplopia in older children and adults who cannot adopt a face turn to achieve single binocular vision.
- Temporary (Fresnel) prisms can be used to treat diplopia in patients with eyeglasses. The prisms create optical blur in direct proportion to the strength of the prism, so for large angles of esotropia they may reduce visual acuity 2-3 lines or more.
- Medial rectus chemodenervation (botulinum toxin) can be useful for patients with complete sixth nerve palsies when single binocular vision cannot be achieved even with a face turn. There may also be a role for chemodenervation when no clinical signs of recovery emerge within the first month or two to prevent contracture in the medial rectus while awaiting recovery of lateral rectus function. Early chemodenervation can also reduce the amount of surgery required to correct persistent sixth nerve palsies, although the evidence in the literature is not conclusive on this point.
Greater than six months after onset
The possibility of spontaneous recovery diminishes dramatically if the sixth nerve palsy persists beyond six months. In this situation, surgical intervention is indicated to establish or broaden the field of single binocular vision, with the surgical approach highly dependent on the presence of residual lateral rectus function.
A complete sixth nerve palsy is characterized by a floating saccade toward central gaze from the resting esotropic, adducted eye position. Force generation testing reveals the absence of lateral rectus force, formal saccadic testing demonstrates diminished saccadic velocity (Figure 9), and orbital imaging reveals profound, symmetric atrophy of the lateral rectus belly (Figure 6). Because the lateral rectus is incapable of generating abducting force to maintain eye alignment, lateral rectus resection or plication rarely produces any sustained clinical improvement. Instead, the lateral rectus attachment to the globe is left intact to provide ciliary artery blood flow to the anterior segment and a surgical transposition of the vertical rectus extraocular muscles is performed to create abducting force (Figure 10).
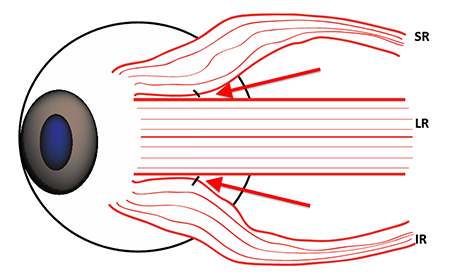
Figure 10. Lateral view of the lateral rectus (LR), superior rectus (SR), and inferior rectus (IR). Augmentation of the full tendon transposition can be performed by placing permanent fixation sutures (red arrows) near the superior and inferior borders of the LR to pull the posterior vertical rectus bellies temporally, increasing the magnitude of abducting force.
- An incomplete sixth nerve palsy is characterized by persistent esotropia in primary gaze, but some lateral rectus abducting force is present that is capable of rotating the eye into abduction past the midline or, in the presence of significant medial rectus restriction, capable of abducting force generation toward the midline (Figure 7). The surgical treatment is typically a combination of medial rectus recession (Video 2), possibly with adjustable suture placement or concurrent chemodenervation, combined with lateral rectus resection (Video 3).
- Postoperative prisms may be required to provide single binocular vision in primary gaze, especially in patients with complete sixth nerve palsies.
- Because corrective strabismus surgeries are typically performed in older patients with vasculopathic risk factors, there is a postoperative risk of anterior segment ischemia. The risk is minimized by limiting the surgery to disinsertion of, at most, two muscles in a single operation and/or by using split-tendon procedures on the vertical rectus muscles during transpositions (Figure 10).
Video 2. Medial rectus recession.
Video 3. Lateral rectus resection.
References
- Britt MT, Velez FG, Thacker N, Alcorn D, Foster RS, Rosenbaum AL. Partial rectus muscle-augmented transpositions in abduction deficiency. J AAPOS. 2003; 7: 325-332.
- Cinciripini GS, Donahue S, Borchert MS. Idiopathic intracranial hypertension in prepubertal pediatric patients: characteristics, treatment, and outcome. Am J Ophthalmol. 1999; 127: 178-182.
- Clark RA, Demer JL. Lateral rectus superior compartment palsy. Am J Ophthalmol. 2014; 157: 479-487.
- Couser NL, Lenhard PD, Hutchinson AK. Augmented Hummelsheim procedure to treat complete abducens nerve palsy. J AAPOS. 2012; 16: 331-335,.
- Demer JL, Clark RA, Kono R, Wright W, Velez FG, Rosenbaum AL. A 12-year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2002; 6: 337-347,.
- Dutton JJ, Fowler AM. Botulinum toxin in ophthalmology. Surv Ophthalmol. 2007; 52: 13-31,.
- Forrozan R, Bhatti MT, Falardeau J, Gordon LK, Lee MS, Subramanian PS, and Kawasaki A. Basic and Clinical Science Course, Section 5: Neuro-Ophthalmology. San Francisco, CA: American Academy of Ophthalmology, 2014. 424.
- Foster RS. Vertical muscle transposition augmented with lateral fixation. J AAPOS. 1997: 1: 20-30,.
- Goodwin D. Differential diagnosis and management of acquired sixth cranial nerve palsy. Optometry. 2006; 77: 534-539,.
- Holmes JM, Beck RW, Kip KE, Droste PJ, Leske DA, Pediatric Eye Disease Investigator Group. Predictors of nonrecovery in acute traumatic sixth nerve palsy and paresis. Ophthalmology. 108: 1457-1460, 2001.
- Holmes JM, Droste PJ, Beck RW. The natural history of acute traumatic sixth nerve palsy or paresis. J AAPOS. 1998; 2: 265-268,.
- Holmes JM, Leske DA. Long-term outcomes after surgical management of chronic sixth nerve palsy. J AAPOS. 2002; 6: 283-288,.
- Holmes JM, Leske DA, Christiansen SP. Initial treatment outcomes in chronic sixth nerve palsy. J AAPOS 5: 370-376, 2001.
- Krzizok TH, Kaufmann H, Traupe H. New approach in strabismus surgery in high myopia. Br J Ophthalmol. 1997; 81: 625-630,.
- Krzizok TH, Kaufmann JM, Traupe H. Elucidation of restrictive motility in high myopia by magnetic resonance imaging. Arch Ophthalmol. 1997; 115: 1019-1027.
- Lee MS, Galetta SL, Volpe NJ, Liu GT. Sixth nerve palsies in children. Pediatr Neurol. 1999; 20: 49-52,.
- Leiba H, Wirth GM, Amstutz C, Landau K. Long-term results of vertical rectus muscle transposition and botulinum toxin for sixth nerve palsy. J AAPOS. 2010; 14: 498-501,.
- Leiderman YL, Lessell S, Cestari DM. Recurrent isolated sixth nerve palsy after consecutive annual influenza vaccinations in a child. J AAPOS. 2009; 13: 317-318.
- Leuder GT, Archer SM, Hered RW, Karr DJ, Kodsi SR, Kraft SP, Paysse EA, and Nischal K. Basic and Clinical Science Course, Section 6: Pediatric Ophthalmology and Strabismus. San Francisco, CA: American Academy of Ophthalmology, 2014, p. 496.
- Merino P, Gómez de Liaño P, Villalobo JMC, Franco G, Gómez de Liaño R. Etiology and treatment of pediatric sixth nerve palsy. J AAPOS. 2010; 14: 502-505,.
- Osako M, Keltner JL. Botulinum A toxin (Oculinum) in ophthalmology. Surv Ophthalmol. 1991; 36: 28-46,.
- Patel SV, Holmes JM, Hodge DO, Burke JP. Diabetes and hypertension in isolated sixth nerve palsy: a population-based study. Ophthalmology. 2005; 112: 760-763,.
- Patel SV, Mutyala S, Leske DA, Hodge DO, Holmes JM. Incidence, associations, and evaluation of sixth nerve palsy using a population-based method. Ophthalmology. 2004; 111: 369-375,.
- Peng M, Poukens V, da Silva Costa RM, Yoo L, Tyschen L, Demer JL. Compartmentalized innervation of primate lateral rectus muscle. Invest Ophthalmol Vis Sci. 2010; 51: 4612-4617.
- Peters GB 3rd, Bakri SJ, Krohel GB. Cause and prognosis of nontraumatic sixth nerve palsies in young adults. Ophthalmology. 2002; 109:1925-1928.
- Phamonvaechavan P, Anwar D, Guyton DL. Adjustable suture technique for enhanced transposition surgery extraocular muscles. J AAPOS. 2010; 14: 399-405.
- Pihlblad MS, Demer JL. Hypertropia in unilateral isolated abducens palsy. J AAPOS. 2014; 18: 235-240,.
- Prasad S, Volpe NJ. Paralytic strabismus: third, fourth, and sixth nerve palsy. Neurol Clin. 2010; 28: 803-833,.
- Rosenbaum AL. Costenbader Lecture. The efficacy of rectus muscle transposition surgery in esotropic Duane syndrome and VI Palsy. J AAPOS. 2004; 8: 409-419,.
- Rosenbaum AL, Santiago AP. Clinical Strabismus Management: Principles and Surgical Techniques. Philadelphia: W.B. Saunders Company, 1999.
- Sanders SK, Kawasaki A, Purvin VA. Long-term prognosis in patients with vasculopathic sixth nerve palsy. Am J Ophthalmol. 134: 81-84, 2002.
- Shewakramani S, McCann DJ, Thomas SH, Nadel ES, Brown DF. Sixth cranial nerve palsy. J Emerg Med. 2005; 29: 207-211,.
- Shioya A, Takuma H, Shiigai M, Ishii A, Tamaoka A. Sixth nerve palsy associated with obstruction in Dorello's canal, accompanied by nodular type muscular sarcoidosis. J Neurol Sci. 2014; 343: 203-205,.
- Silva MN, Saeki N, Hirai S, Yamaura A. Unusual cranial nerve palsy caused by cavernous sinus aneurysms. Clinical and anatomical considerations reviewed. Surg Neurol. 1999; 52: 143-149,.
- Simon JW, Grajny A. Anterior segment ischemia following augmented 2-muscle transposition surgery. J AAPOS. 2004; 8: 586-587,.
- Struck MC. Augmented vertical rectus transposition surgery with single posterior fixation suture: modification of Foster technique. J AAPOS. 2009; 13: 343-349,.
- Tornabene S, Vilke GM. Gradenigo's Syndrome. J Emerg Med. 2010; 38:449-451,.
- Velez FG, Oltra E, Isenberg SJ, Pineles SL. Assessment of torsion after superior rectus transposition with or without medial rectus recession for Duane syndrome and abducens nerve palsy. J AAPOS. 2014; 18:457-460.
- Vijayalakshmi P, Muralidhar R, Shetty S, Sane M. Resolution of anterior segment ischemia after the removal of lateral fixation sutures. 2008; J AAPOS 12: 531-532.
- Wong A, Tweed D, Sharpe J. Vertical misalignment in unilateral sixth nerve palsy. Ophthalmology. 2002; 109: 1315-1325.