By Anna M. Horwood, PhD, DBOT
Overview and Context
These pages cover the results of research into the typical motor aspects of development of binocular vision in very early childhood. A chapter on the Clinical Examination of Ocular Alignment and Binocular Vision in Infants Under Six Months of Age deals with these studies’ clinical assessment and the recognition of atypical signs.
The development and assessment of visual acuity, stereopsis, refraction, and the process of emmetropization is a large body of literature, and will only be covered here where it affects motor systems. Specific ocular motor anomalies such as esotropia, exotropia, incomitant strabismus, congenital dysinnervation syndromes, and paralytic strabismus will also not be covered as their management extends well beyond infancy.
The First Months
Infants are born with practically no visual experience, but by around 4 months of age, they have learned or will have developed a complex range of interrelated visual skills.
Many visual processes–fixation, ocular alignment, visual acuity (VA), mature motion detection, convergence (C), accommodation (A), convergence/accommodation linkages, stereopsis, and emmetropization--develop concurrently postnatally, and if all goes well, these processes reinforce each other as they develop (Figure 1A). It is impossible for clinicians to consider any of these systems in isolation. If there is an abnormality or even a mismatch of the developmental trajectories or critical periods in any of these systems, it can quickly lead to concurrent or subsequent maldevelopment of many others (Figure 1B). In very early infancy, it is important to be able to differentiate typical from atypical development in these interrelated systems so that only appropriate interventions are applied.
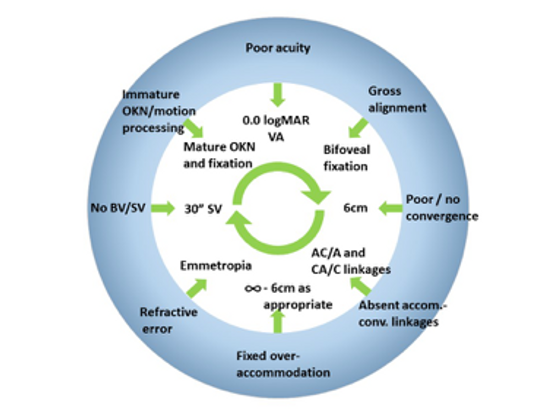
Figure 1A. Typical development. Outer ring: state of visual skills at birth. Inner section: mature visual skills that reinforce each other
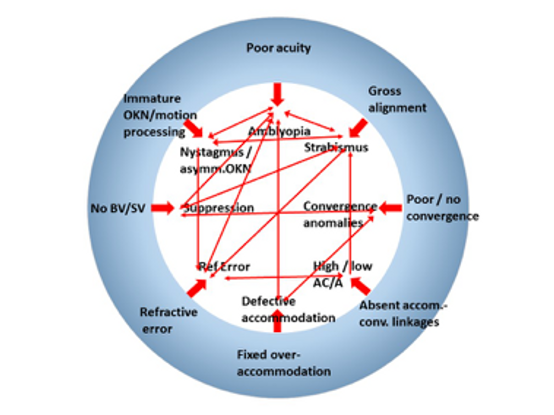
Figure 1B. Atypical development. Outer ring – state at birth. Inner section – abnormal consequences of maldevelopment and examples where abnormal feedback affects other systems
Coordinated systems
We have 2 horizontally separated eyes that need to be coordinated to line up when fixating objects anywhere in a visual scene. In addition, they must also cope with binocular fixation of objects as they move in depth between near and distance fixation (convergence) and must also concurrently alter focus of the lenses to keep the images clear in each eye. These interlinked processes of alignment, ocular vergence and accommodation are fundamental to mature binocular single vision in order to take advantage of fine stereopsis, which requires 2 clear retinal images to fall within Panum’s area (Figure 2).
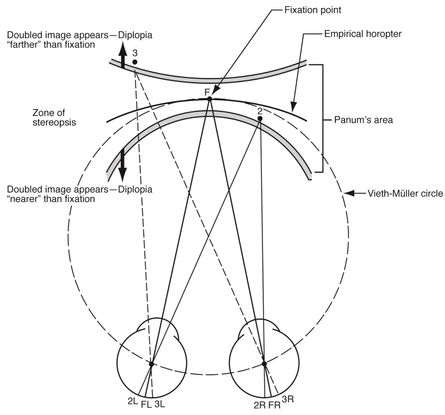
Figure 2. Empirical horopter. F = fixation point; FL and FR = left and right foveae, respectively. Point 1, falling within Panum’s area, is seen singly and stereoscopically. Point 2 falls outside Panum’s area and is therefore seen doubly. (American Academy of Ophthalmology, 2018)
Most of the literature on these topics supports the notion that ocular versions, convergence, and accommodation are yoked systems that act in fixed relationships, with limited flexibility to act independently, although no clear anatomical pathways have been identified. What is less clear is whether these relationships are learned or hard wired. More recent research suggests that these linkages may be less fixed than previously thought.
Deficiencies and abnormalities of alignment, vergence, and accommodation result in the common childhood visual problems of concomitant strabismus (which in turn is associated with suppression, loss of stereopsis, amblyopia, nystagmus, and dissociated vertical deviation [DVD]), heterophoria, and convergence and accommodation anomalies. Some of these conditions emerge and present well beyond infancy, but understanding the visual skills and associations learned in infancy are fundamental in managing these patients.
Concurrent Development
Visual acuity
Infants have poor visual acuity at first.1 A useful rule of thumb is that acuity improves by approximately 1 cycle per degree per month.2 Newborn acuity is no more than 20/600 or 6/180 and reaches 20/200 or 6/60 at 1 year of age. Contrast sensitivity is also poor at first3, but infants have sufficient vision to be able to recognize their own mother’s face within the first days of life.4 Visual acuity develops very rapidly in the first 6 months of life, then more slowly as infancy progresses. Teller gave a good overview of visual acuity 5, but for the purposes of these pages, it is reasonable to assume that typical infants have sufficient acuity to fixate and follow slowly objects of interest such as faces and large bright unambiguous targets from birth (Figure 3).

Figure 3. Simulation of adult compared with newborn visual acuity of a face at 30 cm. (Image cropped from larger illustration in von Hofsten et al., 2014)6
Emmetropization
The majority of infants will demonstrate hyperopia when refracted under cycloplegia, but otherwise they generally show a myopic refraction7 as accommodation frequently fails to relax into the distance. The range of cycloplegic errors is wide, but with a high prevalence of hyperopia and astigmatism, and has a much flatter distribution curve than that of older children and adults.
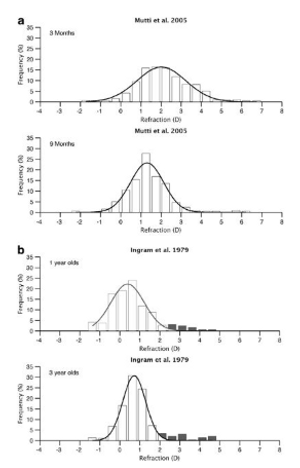
Figure 4. Four distributions of refraction from two different studies8,9 from 3 months of age to 3.5 years. (a) 3–9 months. (b) 1–3 years. (Illustration reprinted by permission from Springer Nature, Eye, Emmetropisation and the aetiology of refractive errors. Flitcroft DI. ©2014.10)
The process of emmetropization means that, for a population of typical infants, the distribution of refractive errors becomes more closely bunched around emmetropia as they grow.10,11 Within moderate limits, the closer to zero the refraction starts, the lower the rate of change towards emmetropia.12 Emmetropization appears to be an active growth process in response to visual input, with blur, light levels and genetic factors being implicated.10,13 Some specific groups are known not to emmetropize as well as others, and these groups comprise much of the pediatric ophthalmology population, for example, children with strabismus, high refractive error, amblyopia, Down syndrome other developmental delays, albinism, and nystagmus.
Ocular alignment
Neonatologists, midwives and “early days” professionals generally tell parents not to worry if their infant has short periods of ocular misalignment in their first weeks, because they will resolve. In contrast, the older clinical ophthalmology literature suggested that any neonatal strabismus was a sign of “congenital” esotropia.
Sondhi and colleagues14 assessed the ocular alignment in infants on a neonatal unit and reported that 70% showed a manifest exotropia at some time, but also commented that some infants showed “convergence spasm” at times. Other studies suggest infants’ eyes are broadly aligned.15 Another study asked orthoptist mothers, trained in the assessment of strabismus,16 to observe their own newborn infants from birth throughout their first year. Few of these mothers reported exotropia in their infants, even in their first days, but many reported that fleeting esotropias or over-convergence were common, and these appeared to be most common as infants attempted fixation on a near object (Figure 5). Most deviations were momentary and soon corrected, but 8% of infants’ eyes were misaligned for more than 15% of their waking hours. These neonatal misalignments generally were becoming less frequent by 2 months of age and, in typical children, had disappeared by 4 months.
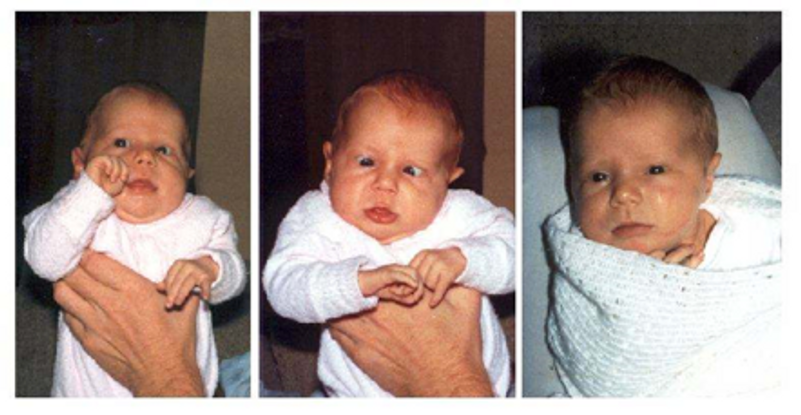
Figure 5. Intermittent neonatal misalignments in the same infant in the first month of life, which resolved by 3 months of age.
The difference between the Sondhi and Horwood studies may be that many newborns may look divergent to eye professionals but not parents, that exodeviations are only present in the first few days, or that infants interact differently with professionals and their main caregiver, but parents generally only notice intermittent esodeviations. Initial speculation that these eso-misalignments were a sign of an emerging accommodation/convergence linkage was disproved by a subsequent laboratory study,17 which showed that they were rarely associated with concurrent accommodation. Many typically developing infants appeared to “get stuck” in convergence and lacked the ability to relax their vergence appropriately after fixating a target that had loomed towards them.
Epicanthus. Prominent epicanthal folds can be a major cause of pseudostrabismus, particularly noticeable if the infant is fixating slightly to the side or is fixating a fixation light but with a small head turn. The reduced nasal scleral show caused by the epicanthus as the eye adducts can give a very strong illusion of esotropia (Figure 6).
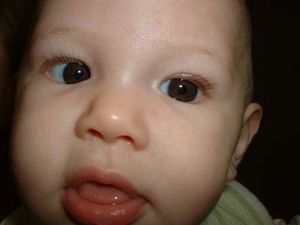
Figure 6. Pseudostrabismus due to epicanthus
Angle kappa
Angle kappa is a measure of the displacement of the corneal reflex (first Purkinje image) from the center of the pupil. It is sometimes also referred to as angle alpha or lambda—angles that are mathematically very similar, but refer to slightly different measurement points within the eye. It occurs because the fovea is temporal to the optic disc, so there is an angle between the optic and visual axes. A typical angle kappa is positive (corneal reflex nasal to the center of the pupil). Angle kappa relates to axial length, and so a shorter eye (neonatal or hyperopic) will have a larger positive angle. The posterior chamber of the neonatal eye develops very rapidly in the first 6 months of life, so the neonatal angle kappa of around 17° per eye at birth declines rapidly in the first 9 months of life. In older children it is around 5° per eye.18 A large positive angle kappa is a cause of pseudoexotropia and can be especially misleading for clinicians accustomed to using corneal reflection position to assess a strabismus angle in older children (Figure 7 and Video 1).
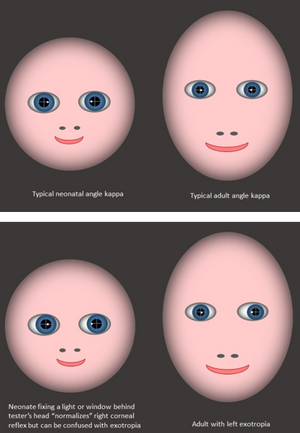
Figure 7. The large angle kappa of infancy makes corneal reflections appear more nasal than in adults. The effect is especially misleading if the infant fixes a bright light behind the examiner’s head, when corneal reflections are identical to those of an adult with exotropia.
Video 1. Infant angle kappa as compared to adult angle kappa
Fixation and following
Infants will fixate and follow a very slowly moving face or unambiguous bright target from soon after birth. They are rarely engaged by distant targets except bright windows or lights. Once engaged with a target, very young infants may exhibit “sticky fixation,” where they appear to have difficulty disengaging fixation from one target to the next.19
Binocularity and Stereoacuity
There is a large body of literature on the development of cortical binocularity and stereopsis (and the subtle neurological and technical differences between the 2 terms).20,21 The consensus is that adult-like binocular vision emerges relatively rapidly between 12 and 16 weeks of age.22 Before this, infants prefer rivalrous (simultaneous but different) targets in each eye, before switching to a preference for fusible (similar) ones.23
Normal binocularity depends on the postnatal development of binocular cells in the visual cortex, and their connections are acutely sensitive to disruption by unequal visual input from the eyes in the critical period. The critical period for binocularity is thought to start a few weeks after birth and its onset appears concurrent with the development of cortical binocular cells and stereopsis. Before this, frequent neonatal misalignments or even genuine sixth cranial nerve palsy24 do not appear to have the damaging effects that they would have later in infancy. The acquisition of binocular connections at a cellular level is also the first time that pathological suppression of 1 eye is likely to be possible.
Motion detection
Infant motion sensitivity and response is another area with a large body of literature, but for clinicians it is useful to know that slow (probably subcortical) binocular optokinetic nystagmus can usually be elicited from birth. Monocular optokinetic nystagmus (OKN) and response to motion are asymmetrical in the first year of life,25,26 with much better temporal to nasal responses then nasal to temporal. Before (or in the absence of) the onset of binocularity, this monocular bias towards following a target approaching the nose, rather than receding, may be one reason that neonatal misalignments and infantile strabismus are typically convergent.17 Some asymmetry is still detectable in the second year,27 and larger asymmetries persist into later life in infantile esotropia.
Vergence eye movements and motor fusion
Infants have not been shown to be able to converge to near targets at birth, but conversely, parents do not comment on significant lack of convergence for near fixation (when feeding, for example). Thorn and colleagues15 studied the development of vergence and random dot stereopsis and found that “first” convergence to an illuminated toy developed at around 12 weeks of age (just before preference for fusional targets), but “full,” more precise, vergence developed approximately a week later. Riddell and colleagues28 found that younger infants from 5 weeks of age could overcome a 20∆ base-out prism if enough time was given and if the target was isolated and unambiguous (an illuminated toy in a darkened room), demonstrating that vergence movements were possible much earlier. These very young infants would occasionally overcome the prism, then get stuck in a convergent deviation and even appear to try to correct their misalignment in the wrong direction (becoming more convergent) before eventually correcting to normal binocular alignment. This behavior often occurred in the same infants, and at the same time that their parents were noting occasional esotropias (neonatal misalignments) at home.
A more detailed longitudinal study by Horwood and Riddell found that infants could converge to a single unambiguous target with a gain not significantly different from adult levels by 8 to 9 weeks of age.29 Before the onset of binocularity, it is not clear whether simultaneous alignment of both eyes on a near target is “motor fusion” or just 2 simultaneous monocular adduction movements to achieve fixation on the macular regions of each eye.
Accommodation
A myopic noncycloplegic refraction6 but hyperopia when under cycloplegia, demonstrates that accommodation is being exerted from the first days of life, but it is not necessarily related to target distance or necessarily under any visual control. Horwood and Riddell’s group29,30 found that in the first weeks of life, accommodation varies little with fixation distance, in agreement with earlier literature.31 By 8 to 9 weeks, mean response gains across a group of infants to a dynamic, engaging, binocular target were not significantly different from those of adults.29,30 These mean data mask the much wider variability within the accommodation data in comparison to the simultaneously collected vergence data in the same infants. Many young infants demonstrated a characteristic “all or nothing” accommodation response, with flat accommodation slopes for 3 more distant targets, then accommodating for the closest target only, while vergence responses were largely linear for target demand (Figure 8).
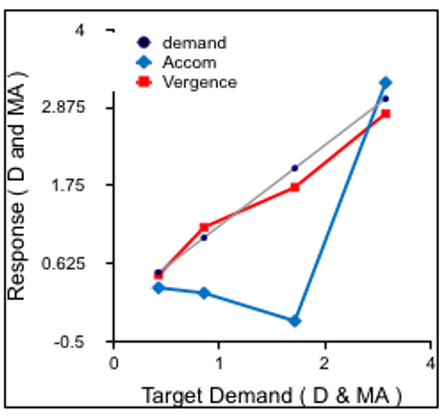
Figure 8. Typical “all or nothing” accommodation response (with concurrent appropriately linear vergence) to targets moving in depth from 2m to 33cm in a 2-month-old infant. Accommodation shown in diopters (D) and vergence in metre angles (MA) so equivalent responses for the stimulus demand are plotted on the same chart.
Accommodation-convergence linkages
Older literature suggests that the accommodation and convergence systems are related in a fixed and innate relationship—usually expressed as the accommodative convergence to accommodation (AC/A) ratio. There is very limited data on vergence and accommodation in the very first weeks, but if accommodation is unresponsive to fixation distance but convergence is more active, there is unlikely to be a link at first. Although Turner and colleagues30 found that beyond 8 weeks of age mean AC/A ratios did not change significantly over development, this does not mean that linkages are similar in infants and adults. Accommodation is much less precise than convergence at all ages, but particularly so in infants, where linear stimulus/response curves are common for convergence, but “all or nothing” accommodation responses occur simultaneously (Figure 7). The poor acuity of infants may make them insensitive to small changes in blur cues, while the precision of the main vergence cue (disparity) is much greater. This suggests that the accommodation and vergence systems are relatively independent at first, although driven by common cues.
There is a large body of literature on the stability of the AC/A ratio in (often highly experienced) adults, but evidence from more naturalistic experiments, or using clinical methods, on infants, children and adults suggest that fixed ratios are rarely repeatable using different methods.32,33,34 On an individual basis, they are not nearly as fixed as reported means data suggest. It seems quite possible that AC/A relationships are learned associations because accommodation and convergence need to work in parallel for any near task and become broadly associated but could still remain 2 more separate systems driven by the same cues.
Visual Cues
Professionals often think in terms of “accommodation driving vergence” or “vergence driving accommodation,” but this implies that internal drives and cross-linkages are stronger than cues from the environment. It may be more useful to think of how common cues can drive both systems.
The 3 main cues to accommodation and convergence are blur, binocular disparity (in its broadest sense that if the eyes are not directed at the target, they get displaced images on the retinae), and proximal cues such as looming, motion parallax, perspective, size, etc. Each of these cues is capable of driving a significant amount of convergence and accommodation when presented in isolation. If these individual response gains were added, the total response would be excessive, so they must be weighted. Older literature suggests that blur drives accommodation and also two-thirds of convergence (leading to the typical AC/A ratio of around 4D:1D), with proximal and disparity cues making up the difference. The role of disparate images driving vergence and accommodation (the CA/C – convergence accommodation to convergence relationship) is rarely considered in the clinical literature because it is technically very difficult to measure.
More recent research has shown that the disparity drive to both convergence and accommodation may be the most important, at least in typical older children and adults, with minor roles for blur and proximal cues. Horwood and Riddell29 studied the development of these cue weightings over infant development. In the first weeks of life, infants respond best to proximal /looming cues. This may explain why neonatal misalignments are more common just after infants watch a target which has loomed towards them. In “middle infancy” between 3 months and one year, blur disparity and proximal cues can all drive responses equally. By 5 years of age disparity drives the best responses, and proximal cues wane significantly in influence. Although blur might be expected to become a more important cue as visual acuity improves and emmetropization occurs, its weighting does not appear to change throughout development.
Many other biological systems follow a parsimonious developmental model, pruning back the least efficient parts of system and reinforcing the most efficient or precise. Vergence and accommodation behave similarly, using the best cue available at first (proximal/looming), but shifting to disparity, with much smaller dead-zones, as it is made available by emerging binocularity and fine acuity. Bharadwaj and Candy35 also support a strong role for disparity cues in driving vergence and accommodation in infants beyond 1.9 months of age.
If something should occur to interfere with this typical developmental trajectory—suppression to disrupt BV, or refractive error to give excessive blur, different weightings may emerge.36 This may help explain the wide clinical variation in children with strabismus or heterophoria. If some children can vary in their responses to blur, disparity and proximal visual cues, this might explain why spectacles help one child’s strabismus and not another.
Hard wired or learned?
Most aspects of infant visual development appear to be pre-programmed (hard-wired), so in premature infants they will develop according to gestational (corrected), not chronological, age. Convergence and accommodation appear similarly pre-programmed,37 but a clue to a possible cause of infantile esotropia may be provided by a paper by Jando and colleagues,38 who reported that development of the response to dynamic random dot correllograms (a marker for cortical binocularity) appears experience dependent. Convergence and accommodation are normally fairly stable by 8 to 9 weeks, well before cortical binocularity emerges at 12 to 16 weeks. But in premature infants, they may still be more erratic (so the eyes less well aligned and focused) at a time when experience-dependent binocular connections (and so the potential for suppression) are emerging—providing a possible risk factor for strabismus.
When does neonatal misalignment become infantile esotropia?
At what point can normal, but frequent, neonatal misalignments be differentiated from pathological infantile esotropia? The tipping point seems to be in the third month of age. Typical neonatal misalignments are reducing by 2 months and most have resolved completely by 4 months, while children destined to develop infantile esotropia are seen to be strabismic more frequently and the esotropic angle gradually increases.39 It is probably no coincidence that this tipping point is the time that stereopsis is typically emerging. Motion detection and OKN are asymmetrical in early infancy, and infants converge best to looming targets which involve motion cues, resulting in a bias toward convergence rather than divergence. As typical binocularity emerges, the adult-like disparity control increasingly corrects for this eso-bias. If binocularity does not emerge or is extinguished by suppression, neonatal biases to esodeviation and convergence (or maybe 2 monocular adductions), and better responses to looming cues as an object approaches rather than recedes, may persist without any counteracting influence.
- Dobson V, Teller DY. Visual acuity in human infants: a review and comparison of behavioral and electrophysiological studies. Vision Res. 1978;18:1469-1483.
- Atkinson J. The Developing Visual Brain, Oxford, Oxford Medical Publications. 2000.
- Banks MS, Salapatek P. Acuity and contrast sensitivity in 1-, 2-, and 3-month-old human infants. Invest Ophthalmol Vis Sci. 1978;17:361-365.
- Bushnell IWR, Sai F, Mullin JT. Neonatal recognition of the mother's face. Brit J Devel Psychol, 1989;7:3-15.
- Teller DY. First glances: the vision of infants. the Friedenwald lecture. Invest Ophthalmol Vis Sci, 1997;38:2183-2203.
- Von Hofsten O, von Hofsten C, Sulutvedt U, Laeng B, Brennen T, Magnussen S. Simulating newborn face perception. Journal of Vision, 2014;14, 16.
- Mohindra I, Held, R. Refractions in humans from birth to 5 years. Documenta Ophthalmologica Proceedings Series. 1981;28:19-27.
- Mutti DO, Mitchell GL, Jones LA, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074-3080.
- Ingram RM, Barr A. Changes in refraction between the ages of 1 and 3 1/2 years. Brit J Ophthalmol, 1979;63:339-342.
- Flitcroft DI. Emmetropisation and the aetiology of refractive errors. Eye. 2014;28:169.
- Gwiazda J, Thorn F, Bauer J, Held R. Emmetropisation and the progression of manifest refraction in children followed from infancy to puberty. Clin Vis Sci. 1993;8:337-344.
- Saunders K, Woodhouse J, Westall C. Emmetropisation in human infancy: rate of change is related to initial refractive error. Invest Ophthalmol Vis Sci. 1995;35:1325-1328.
- Troilo D. Neonatal eye growth and emmetropisation--a literature review. Eye. 1992;6:154-160.
- Sondhi N, Archer SM, Helveston EM. Development of normal ocular alignment. J Pediatr Ophthalmol Strabismus. 1988;25:210-211.
- Thorn F, Gwiazda J, Cruz AA, Bauer JA, Held R. The development of eye alignment, convergence, and sensory binocularity in young infants. Invest Ophthalmol Vis Sci, 1994;35:544-553.
- Horwood AM. Maternal observations of ocular alignment in infants. J Pediatr Ophthalmol Strabismus. 1993;30:100-105.
- Horwood AM, Riddell PM. Can misalignments in typical infants be used as a model for infantile esotropia? Invest Ophthalmol Vis Sci. 2004;45:714-720.
- Riddell PM, Hainline L, Abramov I. Calibration of the Hirschberg test in human infants. Invest.Ophthalmol Vis Sci. 1994:35, 538-543.
- Hood, BM, Atkinson J. Disengaging visual attention in the infant and adult. Infant Behavior and Development. 1993;16:405-422.
- Birch E, Gwiazda J, Held R. Stereoacuity development for crossed and uncrossed disparities in human infants.Vision Res. 1982;22:507-513.
- Braddick O, Wattam-Bell J, Day J, Atkinson J. The onset of binocular function in human infants. Human Neurobiology. 1983;2:65-69.
- Birch E. Stereopsis in infants and its developmental relation to visual acuity. In: Simons K. (ed.) Early Visual Development, Normal & Abnormal. New York: Oxford University Press. 1993.
- Shimojo S, Bauer J, O'Connell K, Held R. Pre-stereoptic binocular vision in infants. Vision Res. 1986;26:501-510.
- Elston JS, Timms C. Clinical evidence for the onset of the sensitive period in infancy. Br J Ophthalmol. 1992;76:327-328.
- Atkinson J. Development of optokinetic nystagmus in the human infant and monkey infant: an analogue to development in kittens. In: Freedman RD (ed.) Developmental neurobiology of vision. New York: Plenum Press. 1979.
- Wattam-Bell J. Motion processing asymmetries and stereopsis in infants. Vision Research. 2003;43:1961-1968.
- Lewis TL, Maurer D, Chung JYY, Holmes-Shannon R, van Schaik CS. The development of symmetrical OKN in infants: quantification based on OKN acuity for nasalward versus temporalward motion. Vision Research. 2000;40:445-453.
- Riddell P, Horwood A, Houston S, Turner J. The response to prism deviations in human infants. Current Biology. 1999;9:1050-1052.
- Horwood A, Riddell P. Developmental changes in the balance of disparity, blur and looming/proximity cues to drive ocular alignment and focus. Perception. 2013b;42:693-715.
- Turner JE, Horwood AM, Houston SM, Riddell PM. Development of the response AC/A ratio over the first year of life. Vision Res. 2002;42:2521-2532.
- Aslin R. Infant accommodation and convergence. In: SIMONS, K. (ed.) Early Visual Development, Normal & Abnormal. New York: Oxford University Press. 1993.
- Gage J. A comparison of AC/A ratio measurement using the gradient method at near and distance fixation. Brit Orthoptic J. 1996:53, 25.
- Horwood A, Riddell P. The Clinical Near Gradient Stimulus AC/A ratio correlates better with the response CA/C ratio than with the response AC/A ratio. Strabismus, 2013a;21:140-144.
- Murray C, Newsham D. Normative Values for the Accommodative Convergence to Accommodation Ratio (AC/A). Invest Ophthalmol Vis Sci. 2010;51:801.
- Bharadwaj S, Candy T. Cues for the control of ocular accommodation and vergence during postnatal human development. Journal of Vision. 2008;8:1-16.
- Horwood AM, Riddell PM. Disparity-driven vs blur-driven models of accommodation and convergence in binocular vision and intermittent strabismus. Journal of American Association for Pediatric Ophthalmology and Strabismus {JAAPOS}. 2014;18:576-583.
- Horwood AM, Toor SS, Riddell PM. Convergence and Accommodation Development Is Preprogrammed in Premature Infants. Invest Ophthalmol Vis Sci. 2015;56:5370-5380.
- Jando G, Miko-Barath E, Marko K, Hollody K, Torok B, Kovacs I. Early-onset binocularity in preterm infants reveals experience-dependent visual development in humans. Proc Natl Acad Sci U S A. 2012;109:11049-11052.
- Horwood A. Neonatal ocular misalignments reflect vergence development but rarely become esotropia. Br J Ophthalmol, 2003;87:1146-1150.