Three large studies published in the February edition of Ophthalmology add to the evidence that intracameral antibiotics are the most effective option for endophthalmitis prevention in cataract surgery, making a strong case for universal adoption of intracameral antibiotic prophylaxis.
The studies from United States, India and Iran examined outcomes from more than 600,000 patients and found a 2- to 4-fold reduction in the infection rate when intracameral antibiotics were used compared with topical antibiotics alone. Additionally, the U.S. study found that adding topical antibiotics to an intracameral regimen did not increase efficacy. All 3 studies identified posterior capsule rupture and vitreous loss during surgery as significant risk factors for postop infection.
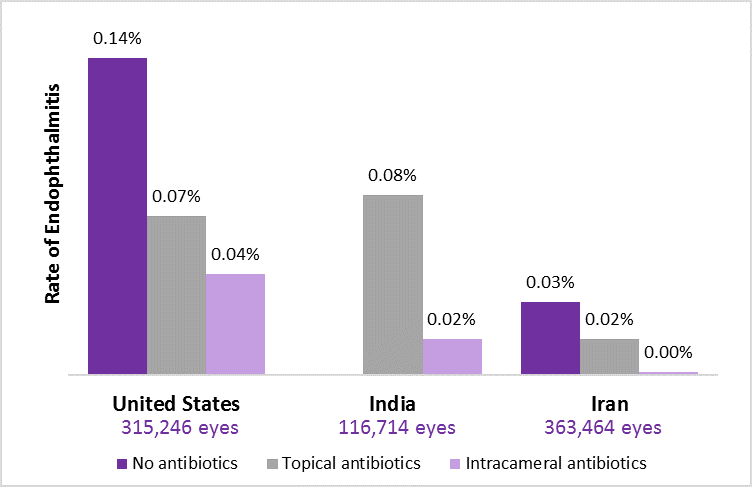
The Indian study showed the most dramatic drop in the infection rate, with a 4-fold decrease occurring among the charity cases receiving manual sutureless small-incision cataract surgery. While the baseline rate of endophthalmitis in the United States is substantially lower than that of the charity cases receiving care at Aravind Hopsital, there is room for incremental improvement. The U.S. study found that potentially 2,000 cases of endophthalmitis per year could have been avoided had intracameral antibiotics been used.
In an accompanying editorial, Jonathan Javitt, MD, makes a strong case for universal adoption of intracameral antibiotic prophylaxis after cataract surgery and, by extension, other forms of intraocular surgery. This practice already has been adopted in Europe and elsewhere. He identifies current financial and regulatory mechanisms that block universal adoption in the United States, but also provides a path forward.
Because there is no FDA-approved antibiotic formulation for intracameral use, U.S. ophthalmologists must take the risk, liability, and financial, legal, and logistical challenges of using unapproved antibiotic formulations. Also, such a product would likely have little economic incentive for a commercial sponsor to take on the expensive and lengthy randomized trial required to demonstrate a statistically significant reduction in the rate of endophthalmitis to support FDA approval.
However, Dr. Javitt poses a possible regulatory path forward. The law does provide for situations in which the FDA may allow historical controls to demonstrate efficacy in exceptional circumstances where randomized prospective studies are simply infeasible. He writes that this does not solve the problem of motivating a commercial entity to supply properly manufactured intracameral antibiotics, “However, if the FDA regulatory hurdle is lowered to the point where a commercial sponsor merely needs to prove safety of the product and compliance with good manufacturing practices, rather than efficacy of the product in preventing endophthalmitis, it is likely that commercial entrants will emerge.”