Investigators document a case of retinal necrosis with counting fingers vision in a patient who received an injection of methylprednisolone acetate (Depo-Medrol) during complicated cataract surgery.
Depo-Medrol (Pfizer, New York) is a formulation of methylprednisolone acetate that is FDA-approved for periocular injection for sympathetic ophthalmia, temporal arteritis, uveitis and ocular inflammatory conditions. Inadvertent injection of Depro-Medrol into the vitreous has been associated with rapid retinal toxicity, including retinal atrophy, detachment and hemorrhage. It’s packaging and appearance are very similar to triamcinolone acetonide (Kenalog, Triesence).
Study design
This is the first case report of intentional Depo-Medrol injection to stain vitreous during complicated cataract surgery. The patient was a 68-year-old man who complained of vision loss in the left eye immediately following cataract surgery. HIs operative note described cataract surgery complicated by a torn posterior capsule and prolapse of vitreous into the anterior chamber. The cataract surgeon injected 0.5 mL of Depo-Medrol into the anterior chamber to aid in visualizing the vitreous. An anterior vitrectomy was then performed prior to completion of the cataract surgery and IOL placement. Unfortunately, the first time the surgeon learned about the potential retinal toxicity from intraocular Depo-Medrol occurred during communication with the authors following consultation.
Outcomes
On exam, his vision was count fingers in the left eye, and 20/30 in the right eye; IOP was 16 mmHg bilaterally. Anterior segment exam showed mild corneal edema with rare anterior segment cell. Posterior segment exam revealed a normal appearing right macula and a whitened, translucent, atrophic central macula in the left eye. Spectral domain OCT showed central macular thinning with loss of all retinal layers in the central macula.
Limitations
Case reports have inherent limitations as they are not research but a starting off point to raise awareness. This report highlights a potentially blinding intraoperative medication substitution.
Clinical significance
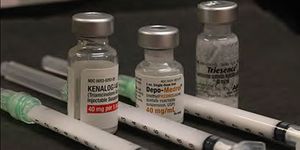 Depo-Medrol has similar packaging to Kenalog (triamcinolone acetonide) and both are commonly stocked in operating rooms. It’s inadvertent use in intraocular surgery can have devastating consequences, including acute retinal necrosis likely from the preservative.
Depo-Medrol has similar packaging to Kenalog (triamcinolone acetonide) and both are commonly stocked in operating rooms. It’s inadvertent use in intraocular surgery can have devastating consequences, including acute retinal necrosis likely from the preservative.
Although labeled properly as not for intraocular use, once drawn into a syringe, it is nearly indistinguishable from safe medications. Surgeons should be aware of exactly which medications are safe for vitreous staining, and may want to consider removing unsafe medications from stock to avoid this major complication.