The Endophthalmitis Vitrectomy Study (EVS) has strongly influenced the management of acute postoperative endophthalmitis since the release of its findings in 1995.1 The EVS confirmed the visual benefit of pars plana vitrectomy in patients presenting with visual acuity of light perception only in which acute postoperative endophthalmitis occurs within six weeks of cataract extraction or secondary IOL implantation. The study also showed that when intravitreal antibiotics are given, systemic antibiotics-in this case intravenous ceftazidime and amikacin-provide no added visual benefit. However, a re-evaluation of the EVS recommendations seems appropriate, since treatment modalities have changed since the EVS results were released.
Although intravitreal vancomycin remains a standard choice for Gram-positive organisms, intravitreal ceftazidime is now favored over amikacin, the drug used in the EVS, due to safety concerns about amikacin's retinal toxicity. The availability of fourth-generation fluoroquinolones with excellent intraocular penetration, such as moxifloxacin, have led some to treat systemically in spite of the EVS findings, attributing the absence of benefit observed in the EVS to inadequate ocular penetration of the drugs used in the study. Should these developments affect how we implement EVS recommendations for postoperative endophthalmitis? Are they applicable to bleb-related, posttraumatic and endogenous endophthalmitis? What are some of the pitfalls in applying the EVS findings? What is the role of the comprehensive ophthalmologist? This article explores these issues.
EVS findings and vitrectomy
The EVS findings were definitive for the treatment of acute postoperative endophthalmitis (Figure 1). The study showed unequivocal benefit for vitrectomy in patients with visual acuity of light perception only. Hence, the greatest potential pitfall in implementing the EVS findings is the inadvertent overestimation of a patient's visual acuity as hand motions when only light perception exists, resulting in a needed vitrectomy not being performed. For correct assessment, hand movement testing as performed in the EVS should be made with the light source 2 feet (60 cm) behind the patient with at least four out of five presentations identified correctly by the patient.
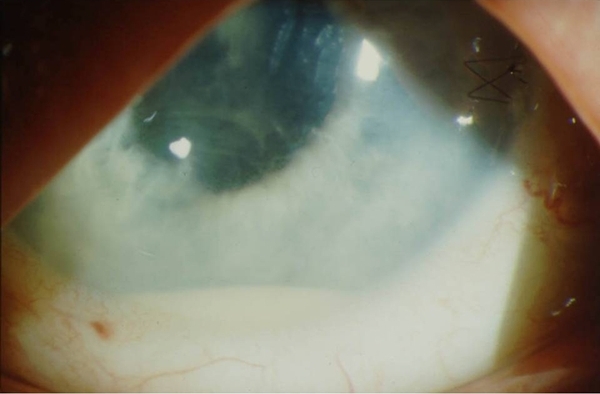
Image courtesy of Dennis P. Han, MD.
Figure 1. Hypopyon and anterior chamber fibrin deposition in a patient with acute postoperative endophthalmitis.
The EVS did not directly address the role of vitrectomy in bleb-related, posttraumatic and endogenous endophthalmitis, conditions associated with a more virulent spectrum of microorganisms than those observed in postoperative endophthalmitis. However, the study did provide some clues. When multivariate analysis of microbiological factors was performed in the EVS, poor presenting visual acuity seemed to be more important than type of infecting organism at predicting benefit from vitrectomy. Thus intervention prior to severe visual loss appeared to have a greater bearing on the value of vitrectomy than the infecting agent itself.2 One may then speculate that presenting visual acuity may be a valuable indicator regardless of organism type or perhaps endophthalmitis cause. Nevertheless, the marked predominance in the EVS of coagulase-negative staphylococci, which comprised about 70 percent of all isolates in the study, makes it difficult to apply the EVS findings to other forms of endophthalmitis.
Intravitreal antibiotic options and vitrectomy
The effect on visual outcome of the shift of intravitreal therapy from amikacin to ceftazidime for empiric Gram-negative coverage is uncertain. More rapid bacterial killing, greater synergy with vancomycin and less susceptibility to "inoculum effect" (in which larger inocula of bacteria reduces efficacy of bacterial killing) has been attributed to the use of amikacin versus ceftazidime. However, in postoperative endophthalmitis cases, in which fewer than five to 10 percent of cases are Gram-negative bacteria and about 70 percent are vancomycin-susceptible coagulase-negative staphylococci, these benefits may not translate to a significant clinical effect. Whatever the case, our current intravitreal antibiotic choices do not appear to diminish the role of vitrectomy for acute infectious endophthalmitis.
The importance of the diagnostic role of vitrectomy
In endogenous endophthalmitis cases for which the primary agent infection has not been isolated, the vitreous specimen obtained may provide the only opportunity to find the cause of a life-threatening infection elsewhere in the body. A vitrectomy or mechanized biopsy using a vitrectomy cutter may assure collection of such a specimen and allow potentially life-saving treatment, whereas a vitreous tap might yield an inadequate specimen or none at all. In contradistinction, the role of cultures in the usual case of postoperative endophthalmitis after cataract surgery is uncertain, since the current regimen of vancomycin and ceftazidime cover the infecting agents in most cases (99 percent in the EVS).3
Conditions requiring alternative interpretations of the EVS
A subgroup analysis in the EVS identified a trend suggesting that diabetic patients might benefit from vitrectomy regardless of their presenting visual acuity.4 Because the study lacked statistical power, either a tap-biopsy or a more aggressive approach with vitrectomy was deemed appropriate for patients with diabetes.
Complications following cataract extraction aside from endophthalmitis may coexist and justify pars plana vitrectomy. In eyes with vitreous incarceration in the wound or significant amounts of retained lens material, vitrectomy might reduce the severity or duration of inflammation and lessen the chances of pseudophakic cystoid macular edema (CME). As to whether vitrectomy for these indications should be performed immediately or delayed remains unknown. A randomized trial of vitrectomy for established chronic CME and vitreous incarceration in eyes without endophthalmitis favored the use of vitrectomy to improve visual outcome.5
Severe choroidal detachments or opacification of the cornea may make vitrectomy difficult or impossible. In such instances, a vitreous biopsy or anterior chamber tap and intraocular antibiotic injection may be all that can be accomplished.
Systemic, subconjunctival and topical antibiotics for acute endophthalmitis
The impact of the newer-generation oral antibacterial and antifungal agents on the management of acute-onset forms of endophthalmitis remains unclear based on what we know of the pharmacokinetics of intravitreal versus systemic administration.6 With intravitreal injection, the intraocular drug concentration is achieved so rapidly and at such a high concentration as to render insignificant the addition of systemically-administered antibiotics. In the subgroup analysis of EVS cases infected with bacteria susceptible to ceftazidime, the EVS intravenous agent with the best intravitreal penetration, no visual outcome benefit was observed in eyes that received ceftazidime compared with those that did not.7
Another rationale for systemic antimicrobial use has been to provide a longer antibiotic presence after the intravitreal antibiotic has cleared. However, the outcome of acute endophthalmitis is usually determined while intravitreally administered antibiotics persist in the eye, and a later therapeutic effect of systemic antibiotics is unlikely. In addition, neither topical nor subconjunctival antibiotic administration has been of proven benefit in the eradication of established acute postoperative endophthalmitis also treated with intravitreal therapy. Their use in the EVS and after its publication could be considered a vestige of so-called "conventional" therapy (using intravenous, subconjunctival and topical antibiotics only) that predates the advent of intravitreal administration. However, cases of endophthalmitis that originate from contiguous ocular structures, such as infected filtration blebs and corneal ulcers, are exceptions and may benefit from some of these modalities.
In contrast to acute forms of endophthalmitis, chronic endophthalmitis cases involving relatively indolent organisms that are difficult to eradicate (such as fungi and P. acnes) are candidates for systemic antimicrobial agents whose use as adjuncts to vitrectomy has been described.8 In endogenous endophthalmitis, systemic antimicrobials are often critical for treating the initial site of infection, and adjunctive vitrectomy and intravitreal antimicrobials may be needed. For fungal endophthalmitis, newer oral and intravitreal antifungal agents, such as voriconazole, represent useful additions to the previously available therapy, amphoteracin, a more toxic agent when given either intravitreally or systemically.9
Rarely, life-threatening systemic infection may result from uncontrolled endophthalmitis.10 In cases progressing to fulminant panophthalmitis, in which there is a risk that the infection may spread to the orbit or other body sites, both systemic and intravitreal antibiotics should be administered and enucleation considered.
The role of comprehensive ophthalmologists in treating postoperative endophthalmitis
Although pars plana vitrectomy remains firmly in the realm of the vitreoretinal specialist, whether the comprehensive ophthalmologist should administer intravitreal antimicrobial therapy hinges on several factors, foremost being the ophthalmologist's expertise in administering intravitreal drugs. The treating ophthalmologist should be well-versed in the findings of the EVS and the potential pitfalls of their implementation. Proximity to an accredited microbiology laboratory and a pharmacy familiar with intravitreal drug preparation are important considerations. Determination of the etiologic agent in acute postoperative endophthalmitis cases is desirable but should not delay treatment.
The intravitreal antibiotic choices of vancomycin 1.0 mg and ceftazidime 2.25 mg, each in the usual volume of 0.1 ml, currently provide excellent antimicrobial coverage for such cases and are usually delivered on an empiric basis without culture results being known. These choices are currently suited to cases in the United States and Western Europe, where the infecting microbiological spectra mirror those of the EVS and severe multidrug resistance remains uncommon.
Summary
Intravitreal injection of antimicrobial agents is the mainstay of endophthalmitis treatment, with the role of systemic antimicrobials limited to treating the primary source of endogenous endophthalmitis and perhaps chronic forms of endophthalmitis involving indolent or hardy organisms. In cases of fulminant endophthalmitis or panophthalmitis, systemic antibiotics are appropriate prophylaxis against the spread of infection. In most instances, systemic antimicrobials are adjuncts to intravitreal agents.
Pars plana vitrectomy should be performed in cases of acute postoperative endophthalmitis with visual acuity of light perception only and perhaps in diabetic patients even if visual acuity is better. It remains unclear whether to extend this recommendation to bleb-related, posttraumatic and endogenous endophthalmitis, in which the microbiological spectrum includes more virulent organisms, since the EVS did not address these conditions specifically. Vitrectomy may also address concurrent conditions, such as severe vitreous incarceration or retained lens material, that can produce CME or chronic postoperative inflammation. Severe corneal opacification and choroidal detachment may preclude vitrectomy.
Although the role of the comprehensive ophthalmologist in postoperative endophthalmitis management can be debated, we can state definitively that early endophthalmitis detection is key. It is critical that postsurgical patients are educated about the need for immediate evaluation if they experience increasing discomfort or worsening vision, which is the most common manifestation of early endophthalmitis.
This article was supported in part by an unrestricted grant to the Medical College of Wisconsin from Research to Prevent Blindness, Inc., New York, NY.
References
1. The Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study: A randomized trial of vitrectomy and intravenous antibiotics for the treatment of post-operative bacterial endophthalmitis. Arch Ophthalmol. 1995;113(12):1479-1496.
2. The Endophthalmitis Vitrectomy Study Group. Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122(6):830-846.
3. Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122(1):1-17.
4. Doft BH, Wisniewski SR. Kelsey SF, Fitzgerald SG, and the Endophthalmitis Vitrectomy Study Group. Diabetes and postoperative endophthalmitis in the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 2001;119(5):650-656.
5. Fung WE. Vitrectomy for chronic aphakic cystoid macular edema. Results of a national, collaborative, prospective, randomized investigation. Ophthalmology. 1985;92(8):1102-1011.
6. Doft BH. Treatment of postcataract extraction endophthalmitis: a summary of the results from the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 2008;126(4):554-556.
7. Barza M, Han DP, Doft BH. Microbiologic factors and visual outcome in the Endophthalmitis Vitrectomy Study. Author reply. Am J Ophthalmol. 1997;124(1):128-130.
8. Kroll P, Emerich KH, Fegeler W. Candida albicans endophthalmitis: results of pars plana vitrectomy without intraocular antimycotic therapy. Klin Monatsbl Augenheilkd. 1984;184(2):104-108.
9. Breit SM, Hariprasad SM, Mieler WF, Shah GK, Mills MD, Grand MG. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol. 2005;139(1):135-140.
10. Duke-Elder S, Perkins ES. In:Duke-Elder S, Perkins, ES, eds. Diseases of the Uveal Tract. Vol IX. System of Ophthalmology. St. Louis: C.V. Mosby Co.; 1966:48.
Author Disclosure
Dr. Han has no financial interests to disclose.