Authors: Ruwan A. Silva, MD; Mark S. Blumenkranz, MD
Background
With an annual incidence of approximately 1 in 10,000 people, rhegmatogenous retinal detachments are the most common type of retinal detachment. The term itself is derived from the Greek word for "break" and speaks to these detachments being rooted in full thickness deficits in the neurosensory retina.
General risk factors for rhegmatogenous retinal detachments include axial myopia (the magnitude of which is directly proportional to the risk of retinal detachment and which is present in the majority of nontraumatic retinal detachments) cataract surgery (particularly cases with vitreous loss, vitreous prolapse, or surgery in younger patients), ocular trauma and a contralateral rhegmatogenous retinal detachment and certain peripheral retinal degenerations including lattice degeneration. A common basis underlying these risk factors is the resultant increase in vitreous traction they impart upon the retina thus predisposing patients to developing retinal breaks. The example of blunt ocular trauma is illustrative in that, mechanistically, the putative development of a retinal break appears to be globe compression with subsequent contraction and distention of the vitreous particularly at the equator and ora serrata. The subsequent globe expansion then produces an acute increase in vitreoretinal traction, which often results in a retinal break-most oftentimes a retinal dialysis. Thus, with the exception of the retina itself, no structure is more responsible for the development of rhegmatogenous retinal detachments than the vitreous humor.
Most relevant are areas of interface to which the vitreous is particularly adherent to the retina. These regions include the vitreous base (which is 2-6 mm wide and ‘straddles’ the ora serrata), retinal vessels and pre-existing developmental lesions in the peripheral retina (including cystic retinal tufts, lattice degeneration, zonular traction tufts and enclosed oral bays). These points of adhesion are most likely to cause retinal breaks during the pathologic event most critical in causing the majority of retinal detachments: the posterior vitreous detachment. Generally occurring between ages 50 to 70 years of age, this separation of the posterior hyaloid face from the inner retina exerts traction on the retina at sufficient levels to yield a retinal break. The regions of firm vitreoretinal adhesion are particularly susceptible. Traction at the vitreous base often causes retinal breaks at its posterior most aspect, and paravascular traction often yields retinal tears through shearing bridging vessels or avulsing adjacent vasculature from the retinal surface. A caveat is that retinal breaks are found in approximately 10% of the population meaning that only a small fraction of them produce actual retinal detachments. The most important factors generating a detachment from a retinal break include centripetal vitreoretinal traction at the edge of a retinal break and the flow of liquefied vitreous through a retinal break into the subretinal space. Since the presence of these factors varies considerably depending on the type of retinal break present, any discussion of laser retinopexy most appropriately begins with identifying which retinal breaks require treatment.
Asymptomatic Breaks
One large category of retinal breaks is termed “asymptomatic” and includes mainly operculated and atrophic round holes. Since there is no vitreoretinal traction at the edge of these breaks, retinal detachments are unlikely to occur secondarily to them. Clinical data from large observational case series demonstrate the minimal risk of retinal detachment from these lesions. Additionally, there are no prospective or randomized trials evaluating prophylactic treatment of these lesions, thus no evidence supports any benefit of prophylactic therapy. In contrast, asymptomatic horseshoe retinal tears have a 5% risk of retinal detachment, which though lower than the risk of retinal detachment with symptomatic horseshoe tears, is not quite equivalent to the loer risk associated with operculated or atrophic round holes. Current recommendations are close follow-up without prophylactic treatment. Asymptomatic retinal detachments have also been described with the estimate of progression to clinical retinal detachment varying from 11% to 31%. As an equivalent percentage of asymptomatic detachment cases instead achieve spontaneous resolution before a clinical detachment occurs, close observation or initial prophylactic treatment may both be considered.
Symptomatic Breaks
Symptomatic retinal breaks constitute another category of retinal breaks for which intervention is generally indicated. These breaks are characterized by the perception of increased ‘flashers’ or ‘floaters’ and usually occur due to increased vitreoretinal traction exerted during an acute posterior vitreous detachment. Posterior vitreous detachments putatively carry a 10% to 20% risk overall of causing acute breaks. Two important prognostic signs are pigmented cell or hemorrhage in the vitreous at the time of initial evaluation. In the latter case, approximately 70% of these patients will have at least one retinal tear (with nearly 1/3 of these patients having multiple breaks) and almost 90% of these tears are located in the superior quadrants. Additionally, the denser the vitreous hemorrhage, the higher the likelihood a retinal tear is present. In the absence of vitreous pigmented cell or vitreous hemorrhage, the risk of a retinal tears at the time of an acute posterior vitreous detachment is only 2% to 4% though there is also a 2% to 5% risk of patients developing retinal breaks in subsequent weeks. Retinal breaks with persistent vitreous traction at their edge (which denotes nearly all tears and retinal dialyses though rarely includes operculated holes) yield retinal detachments 50% of the time and therefore should be treated with immediate laser retinopexy, which reduces the risk of retinal detachment to less than 5%.
Lattice Degeneration
An important developmental lesion related to retinal breaks and posterior vitreous detachments is lattice degeneration, the margins of which have firm vitreoretinal adhesions. Traction on these lesions typically produce retinal tears at the posterior or lateral edges. While lattice degeneration is seen in only 6% to 8% of the general population, up to 30% of all rhegmatogenous retinal detachments occur in these eyes.Inversely, the lifetime risk of a retinal detachment in a patient with lattice is less than 1%.Lattice degeneration generally occurs at the vertical meridians of the peripheral retina and produces retinal detachments in two ways. First, through progressive retinal atrophy (and not posterior vitreous detachments), retinal holes may develop in as many as 35% of these lesions and tends to occur in young myopic patients. Localized retinal detachments occur in 2% of cases and, if they do become clinical, expand relatively slowly. Though treatment is only warranted in cases of expanding retinal detachments, regular follow-up for all patients with such atrophic holes is critical. Second, lattice is also associated with symptomatic retinal tears, though such retinal breaks are associated with posterior vitreous detachments. In these cases (or in asymptomatic tears occurring in patients who are either aphakic or have suffered a contralateral retinal detachment), treatment is indicated as the risk of retinal detachment is high. An important consideration is that lattice degeneration occurs bilaterally in 45% of patients which raises the question of whether to treat asymptomatic lattice prophylactically in the contralateral eye of a patient who has suffered a lattice associated retinal detachment. Though no consensus guidelines exists, the estimates of retinal detachment risk in the contralateral eye is 5% (and up to 25% in high myopes with extensive lattice) with prophylactic therapy reducing the risk approximately three-fold (Figure 1).
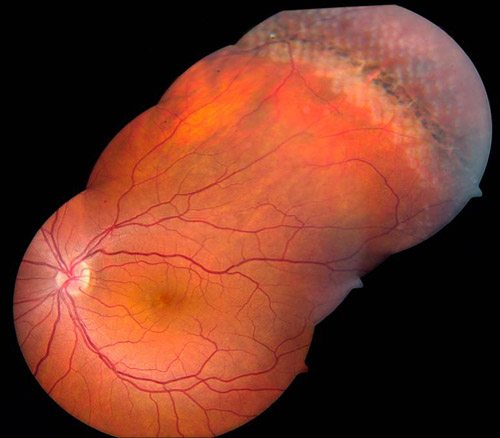
Figure 1. Treatment of lattice degeneration.
Laser Photocoagulation
The goal of laser photocoagulation in the context of the aforementioned retinal breaks warranting prophylactic treatment is to generate regions of firm chorioretinal adhesion completely surrounding a retinal break to both counter vitreoretinal traction and prevent liquefied vitreous from passing into the subretinal space to generate a retinal detachment. To this end, at least 3 near-confluent rows of laser should be applied to closely and completely surround the retinal break. Among the strongest and therefore most pathologic regions of vitreoretinal traction occurs at the vitreous base; thus, treatment of many far-peripheral tears (and all cases of retinal dialysis) necessitate treatment to the ora serrata as the most common reason for treatment failure in these cases is insufficient laser placed around the anterior margin of the retinal break (Figure 2).
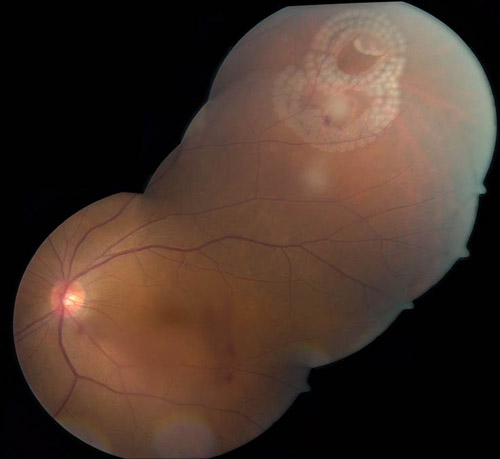
Figure 2. Treatment of clustered peripheral tears using hemi arc patterns.
The 3 pigments of the retina whose absorption of light energy incites photocoagulation are xanthophylls, hemoglobin, and melanin. Melanin is located in the retinal pigment epithelium (RPE) and choroid and has the ability to absorb nearly the entire visible light spectrum as well as infrared light though is a relatively poor absorber of blue light. It is absorption by this pigment to which most of retinal photocoagulation is attributed. Thus fundi, with relatively higher concentrations of melanin in the RPE and choroid, more readily undergo photocoagulation. Hemoglobin absorbs yellow light the best and transmits red light the most. Finally xanthophyll, a pigment in the inner and outer plexiform layers of the retina, maximally absorbs blue light with less absorption of yellow or red wavelengths. For the purposes of laser retinopexy of retinal breaks, green light has emerged as the predominant wavelength owing largely to its excellent absorption by melanin and hemoglobin with relatively poor absorption by xanthophylls thus sparing potential macular damage. Red wavelengths are also poorly absorbed by xanthophyll and well absorbed by melanin but with the added benefit of being better suited for treating retinal tears associated with media opacity such as cataracts or vitreous hemorrhages. Its longer wavelength, however, makes it more prone to deeper, more unpredictable and more heterogeneous absorption by the choroid which can result in pain and even focal damage to Bruch’s membrane.
The power of laser administration is titrated to yield moderately white burns for laser retinopexy. In general, several hundered milliwatts is sufficient for effective photocoagulation but media opacity, the presence of shallow subretinal fluid or a relative paucity of RPE and choroidal melanocytes may necessitate use of higher power. Duration of treatment also affects tissue reactivity. Laser retinopexy is generally performed using a duration of 0.1 to 0.2 seconds with shorter duration treatments requiring more power. In contrast, longer exposure may be more effective in cases with media opacity or shallow subretinal fluid. Spot size also affects photocoagulation. Smaller spot sizes yield a higher power per unit of area than larger spot sizes. Therefore caution should be used to avoid rupture of Bruch’s membrane. Additionally, while spot sizes of 200 to 500 microns are considered the standard for laser retinopexy in the peripheral retina, as discussed below, use of a variety of different contact lenses as well as nonparfocal laser delivery systems can result in a spot size different from that selected on the laser itself.
Laser Indirect Ophthalmoscopy
For the application of laser to peripheral retinal tears another variable is the method by which laser is applied: indirect ophthalmoscopy, slit lamp, or via an endolaser during intraocular surgery. Laser indirect ophthalmoscopy, which was introduced in 1981, is one preferred method for treatment of retinal tears in the far periphery. In addition to providing excellent visualization of these areas, this mode is capable of employing krypton, argon or diode active medium lasers. A disadvantage of this delivery system is the variability in laser spot size, burn intensity, and delivery location––all inherent qualities of a delivery system where the laser, lens, and target are independently mobile and unfixed. Spot size increases as the power of the handheld lens increases and the more hyperopic a patient is. Scleral depression, therefore, will also decrease spot size by bringing the target tissue closer to the laser source and focusing lens. Intraocular fluids also will affect laser spot size with silicone oil-filled eyes, yielding spot size enlargement while intraocular air or gas will decrease spot size. Such variations should be used to titrate laser settings as, again, smaller spot sizes (as well as applications of longer-duration) require less energy than larger spot sizes (and those of shorter-duration). In gas-filled eyes an additional consideration is the effective insulation of retinal tissue by the intraocular gas. This serves to augment laser power by reducing heat dissipation at the treatment site and results in more intense photocoagulation.
Slit-Lamp Laser
Slit-lamp mounted lasers have two different spot size selectors: parfocal and defocal. Parfocal systems provide one spot size for a range of focal planes though magnification may still alter spot size. Defocal systems allow variability in spot size when changing the distance of the focal plane. In practice, most systems have a dual system: providing a parfocal spot size for the lower range of beam sizes and a defocal system above a certain spot size (Figures 3 and 4).
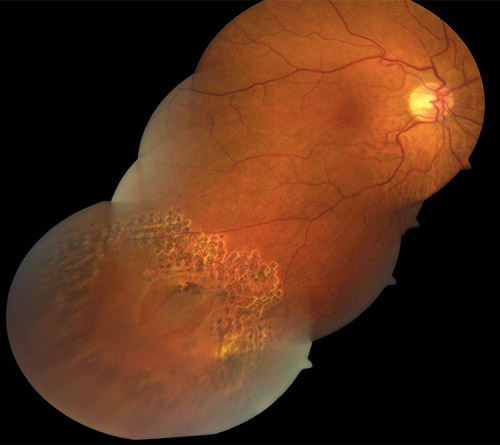
Figure 3. Surround treatment of large horseshoe retinal tear and subclinical retinal detachment with demarcating laser.
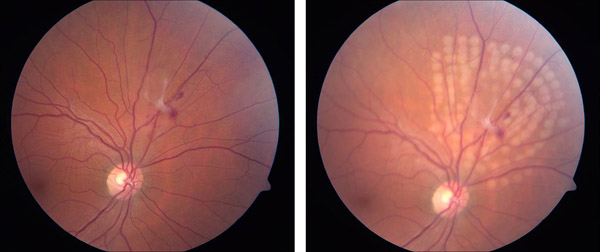
Figure 4. Treatment of posterior retinal tear with subclinical detachment and avulsed vessel.
For patients with midperipheral tears in whom positioning at the slit lamp is preferred, high-power plus contact lenses can provide a view of the area necessitating treatment while also offering stabilization of the patient’s eye. These lenses provide real, inverted images. These lenses often modify the laser spot size and therefore the actual area of photocoagulation on the retina is increased. For tears located further anteriorly, some physicians prefer mirrored negative-power planoconcave lenses, which though providing a better view of the far periphery, do not allow a concurrent view of the macula. Such negative power lenses create virtual, erect images. Patient cooperation can further assist visualization of a region of the retina, with a patient looking away from a given mirror providing a more anterior view (Figure 5).

Figure 5. Laser lenses. Courtesy Harry Flynn, MD.
Postoperative Chorioretinal Adhesion
In addition to the details surrounding laser application, an important further consideration is the time course over which laser retinopexy confers additional chorioretinal adhesion. In vitro studies have determined that after application of argon (blue-green) laser to areas of retina that have never been detached, the adherence of the retina to the RPE actually weakens to 50% of normal at 8 hours, improves to 100% by approximately 18 hours, and then achieves maximal strength (230% of normal) at 5 days after treatment. In regions of retina that have been detached but were reattached at the time of laser application, strengthening was delayed. Normal levels of adherence were achieved only at day 3 after treatment with continued strengthening thereafter. In cases where a small amount of subretinal fluid was present at the time of laser photocoagulation, the time course was even more substantially delayed. Normal levels of chorioretinal adhesion reached baseline levels only several weeks after laser application.
Complications
While generally a safe procedure, complications of laser retinopexy may occur. These include inadvertent laser to the macula, choroidal effusions (particularly in cases where large amounts of laser are used), angle closure glaucoma, epiretinal membrane formation, anterior segment laser burns, hemorrhage (of the retina, vitreous ,or choroid), choroidal neovascular membrane formation, and the formation of new retinal breaks. Follow-up in all cases of patients having laser retinopexy is paramount. Indirect ophthalmoscopy, with or without scleral depression, is recommended at approximately 3 weeks after initial treatment. If chorioretinal scarring is deemed inadequate, particularly with respect to completely surrounding the anterior margins of retinal tears, retreatment is indicated. Re-examination at any interval that shows a retinal break or subretinal fluid extending past the area of previous treatment, or entirely new retinal breaks, (approximately 10% of all patients) should also prompt retreatment, or if necessary, surgical intervention.
Suggested Reading
- Wilkes SR, Beard CM, Kurland LT, Robertson DM, O'Fallon WM. The incidence of retinal detachment in Rochester, Minnesota, 1970-1978. Am J Ophthalmol. 1982;94(5):670-673.
- The Eye Disease Case-Control Study Group. Risk factors for idiopathic rhegmatogenous retinal detachment. Am J Epidemiol. 1993;137(7):749-757.
- Koch DD, Liu JF, Gill EP, Parke DW, 2nd. Axial myopia increases the risk of retinal complications after neodymium-YAG laser posterior capsulotomy. Arch Ophthalmol. 1989;107(7):986-990.
- Olsen G, Olson RJ. Update on a long-term, prospective study of capsulotomy and retinal detachment rates after cataract surgery. J Cataract Refract Surg. 2000;26(7):1017-1021.
- Ranta P, Tommila P, Kivela T. Retinal breaks and detachment after neodymium: YAG laser posterior capsulotomy: five-year incidence in a prospective cohort. J Cataract Refract Surg. 2004;30(1):58-66.
- Cooling RJ. Traumatic retinal detachment--mechanisms and management. Trans Ophthalmol Soc U K. 1986;105 (Pt 5):575-579.
- Byer NE. Rethinking prophylactic therapy of retinal detachment. In: Stirpe M, ed. Advances in Vitreoretinal Surgery. New York, NY: Ophthalmic Communications Society. 1992:399-411.
- Folk JC, Arrindell EL, Klugman MR. The fellow eye of patients with phakic lattice retinal detachment. Ophthalmology. 1989;96(1):72-79.
- Mastropasqua L, Carpineto P, Ciancaglini M, Falconio G, Gallenga PE. Treatment of retinal tears and lattice degenerations in fellow eyes in high risk patients suffering retinal detachment: a prospective study. Br J Ophthalmol. 1999;83(9):1046-1049.
- Cox MS. Retinal breaks caused by blunt nonperforating trauma at the point of impact. Trans Am Ophthalmol Soc. 1980;78:414-466.
- American Academy of Ophthalmology Retina Panel. Preferred Practice Pattern® Guidelines. Posterior Vitreous Detachment RB, and Lattice Degeneration. San Francisco, CA: American Academy of Ophthalmology; 2008.
- Byer NE. What happens to untreated asymptomatic retinal breaks, and are they affected by posterior vitreous detachment? Ophthalmology. 1998;105(6):1045-1049; discussion 1049-1050.
- Davis MD. Natural history of retinal breaks without detachment. Arch Ophthalmol. 1974;92(3):183-194.
- Wilkinson CP. Interventions for asymptomatic retinal breaks and lattice degeneration for preventing retinal detachment. Cochrane Database Syst Rev. 2012;3:CD003170.
- Neumann E, Hyams S. Conservative management of retinal breaks. A follow-up study of subsequent retinal detachment. Br J Ophthalmol. 1972;56(6):482-486.
- Byer NE. Subclinical retinal detachment resulting from asymptomatic retinal breaks: prognosis for progression and regression. Ophthalmology. 2001;108(8):1499-1503; discussion 1503-1494.
- Benson WE, Grand MG, Okun E. Aphakic retinal detachment. Management of the fellow eye. Arch Ophthalmol. 1975;93(4):245-249.
- Coffee RE, Westfall AC, Davis GH, Mieler WF, Holz ER. Symptomatic posterior vitreous detachment and the incidence of delayed retinal breaks: case series and meta-analysis. Am J Ophthalmol. 2007;144(3):409-413.
- Scott IU, Smiddy WE, Merikansky A, Feuer W. Vitreoretinal surgery outcomes. Impact on bilateral visual function. Ophthalmology. 1997;104(6):1041-1048.
- Tani P, Robertson DM, Langworthy A. Rhegmatogenous retinal detachment without macular involvement treated with scleral buckling. Am J Ophthalmol. 1980;90(4):503-508.
- Tasman WS. Posterior vitreous detachment and peripheral retinal breaks. Trans Am Acad Ophthalmol Otolaryngol. 1968;72(2):217-224.
- Dayan MR, Jayamanne DG, Andrews RM, Griffiths PG. Flashes and floaters as predictors of vitreoretinal pathology: is follow-up necessary for posterior vitreous detachment? Eye (Lond). 1996;10(Pt 4):456-458.
- Van Overdam KA, Bettink-Remeijer MW, Mulder PG, van Meurs JC. Symptoms predictive for the later development of retinal breaks. Arch Ophthalmol. 2001;119(10):1483-1486.
- Colyear BH, Jr., Pischel DK. Clinical tears in the retina without detachment. Am J Ophthalmol. 1956;41(5):773-792.
- Colyear BH, Jr., Pischel DK. Preventive treatment of retinal detachment by means of light coagulation. Trans Pac Coast Otoophthalmol Soc Annu Meet. 1960;41:193-217.
- Pollak A, Oliver M. Argon laser photocoagulation of symptomatic flap tears and retinal breaks of fellow eyes. Br J Ophthalmol. 1981;65(7):469-472.
- Robertson DM, Norton EW. Long-term follow-up of treated retinal breaks. Am J Ophthalmol. 1973;75(3):395-404.
- Shea M, Davis MD, Kamel I. Retinal breaks without detachment, treated and untreated. Mod Probl Ophthalmol. 1974;12(0):97-102.
- Smiddy WE, Flynn HW, Jr., Nicholson DH, et al. Results and complications in treated retinal breaks. Am J Ophthalmol. 1991;112(6):623-631.
- Byer NE. Long-term natural history of lattice degeneration of the retina. Ophthalmology. 1989;96(9):1396-1401; discussion 1401-1392.
- Foos RY, Simons KB. Vitreous in lattice degeneration of retina. Ophthalmology. 1984;91(5):452-457.
- Adrean SD, Elliot D. Prophylaxis for retinal detachment. Review of Ophthalmology. 2005;5(6).
- Benson WE, Morse PH, Nantawan P. Late complications following cryotherapy of lattice degeneration. Am J Ophthalmol. 1977;84(4):514-516.
- Delaney WV, Jr. Retinal tear extension through the cryosurgical scar. Br J Ophthalmol. 1971;55(3):205-209.
- Bloom SM, Brucker AJ. Laser Surgery of the Posterior Segment. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1997.
- Folk J, Pulido JS. Laser Photocoagulation of the Retina and Choroid. San Francisco: American Academy of Ophthalmology; 1997.
- Kain HL. Chorioretinal adhesion after argon laser photocoagulation. Arch Ophthalmol. 1984;102(4):612-615.
- Yoon YH, Marmor MF. Rapid enhancement of retinal adhesion by laser photocoagulation. Ophthalmology. 1988;95(10):1385-1388.
- Goldberg RE, Boyer DS. Sequential retinal breaks following a spontaneous initial retinal break. Ophthalmology. 1981;88(1):10-12.