Toxoplasma gondii is an ubiquitous, obligate intracellular protozoan and is considered to be the most common cause of infective retinitis in immunocompetent humans. The course of systemic disease in immunocompetent adults is usually asymptomatic and self-limiting. As soon as infection has occurred, the parasite forms latent cysts in many organs, including the retina. These cysts can reactivate years after the initial infection, giving rise to acute retinochoroiditis and, subsequently, the formation of new retinochoroidal lesions.1 Usually, ocular toxoplasmosis (OT) is clinically diagnosed through recognition of focal retinitis or retinochoroiditis in the setting of an adjacent or nearby retinochoroidal scar.
Serum anti-T. gondii immunoglobulin G (IgG) antibodies support the diagnosis of OT but are not always necessary. In immunocompromised individuals, OT lesions may be extensive or multifocal, complicating the diagnosis.2
Toxplasmosis is endemic throughout most of the world and affects a large proportion of the adult population.3 At least 10 percent of adults in northern temperate countries and more than half of adults in Mediterranean and tropical regions have been infected.4
Pathogenesis
The parasite can be found in the host's tissues and body fluids, such as saliva, milk, semen, urine and peritoneal fluid. The morphology of T. gondii varies depending upon the stage of the life cycle and habitat. It can present in one of three forms: tachyzoite, bradyzoite and sporozoite or oocyst.5
The tachyzoite, also called trophozoite, is the infectious form responsible for the acute phase of the disease. The encysted form, known as the bradyzoite, begins to appear as soon as one week after infection. The cysts are very resistant and can remain dormant in the host for years without reactivation or tissue damage. For reasons that remain unknown, the cyst may rupture, causing reactivation of the disease and intense inflammation.5
Life cycle of T. gondii
Oocysts of T. gondii are found uniquely in the intestinal mucosa of cats. Once they are released, they can be spread to human beings or other animals through a variety of vectors. Although invariably thought to be ingested, the organism may also enter the host through other mucosal surfaces. Humans can also be infected secondarily by meat (pork and lamb, in particular, as well as chicken in endemics areas, but probably not unprocessed beef) contaminated with Toxoplasma cysts. The two forms of the organism that can be found in humans are bradyzoites, or tissue cysts, and tachyzoites. Tissue cysts are up to 200 µm in diameter, contain hundreds to thousands of organisms and have a propensity for cardiac tissue, muscle and neural tissue, including the retina.6
Humans can become infected by the infectious forms of either subcycle, that is, by eating undercooked meat containing tissue cysts or through accidental ingestion of oocyst-contaminated garden vegetables, water or cat litter boxes. Rarely, T. gondii can be transmitted via blood transfusions, solid organ transplants or contaminated water or air. The life cycle of T. gondii is unusual; the organism is capable of indefinite replication using either sexual or asexual subcycles.7 After transmission, actively dividing tachyzoites disseminate via the blood stream and lymphatics.8 Up to 10 percent of infected individuals present with retinal lesions.8,9 These infections account for one-third to one-half of all cases of posterior uveitis.8 Most of the ocular cases occur months to years after initial infection, which is often asymptomatic.
The asexual cycle can occur in virtually any warm-blooded animal, ranging from chickens to sea otters to humans. Transmission occurs when an animal ingests bradyzoite-infected tissue through carnivorism or scavenging. Transmission can also occur accidentally through feed that is contaminated with animal parts. Theoretically, this asexual portion of the organism's life cycle could continue indefinitely via the food chain. The sexual cycle of Toxoplasma gondii occurs only in cats. It includes full gametogenesis and mating within the intestinal epithelium and culminates in the generation of oocysts that are shed in the cat's feces. These oocysts are highly infectious and extremely stable in the environment. Likewise, the asexual cycle can readily flow into the sexual side when a cat eats a mouse or bird infected with tissue cysts.10
Since the 1950s, infections acquired postnatally have been attributed correctly either to ingestion of tissue cysts in raw or undercooked meat or to oocysts on unwashed vegetables that were contaminated with soil containing cat feces. Recent observations have suggested that these are not the only routes of infection. Contaminated drinking water, for example, may be an important source of infection in some situations. Tachyzoites may reach the retina by migration from the brain via the optic nerve, passage from the retinal circulation in infected monocytes or dendritic cells or direct infection of the retinal vascular endothelium by circulating tachyzoites. Different and multiple routes may account for retinal infection in different patients.8,10
Clinical Manifestations
OT manifests predominantly from the second to the fourth decade of life. This is probably because many cases arise up to 10 to 20 years after the infection as either primary OT (isolated retinal lesions not arising from scars; Figure 1A, B and C) or recurrent OT (active retinal lesions associated with old inactive scars; Figure 2). Necrotizing retinitis associated with vitreous and anterior chamber inflammation is the hallmark of OT. The area of a scar that is seen clinically can be smaller than the area of inflamed retina during the active stage of the disease. The degree of pigmentation within and around scars may reflect the extent to which the retinal pigment epithelium is damaged during the active disease stage. In some elderly patients, lesions seem to heal with less severe scarring than would be expected from the extent of infection.11
Click on image to enlarge.
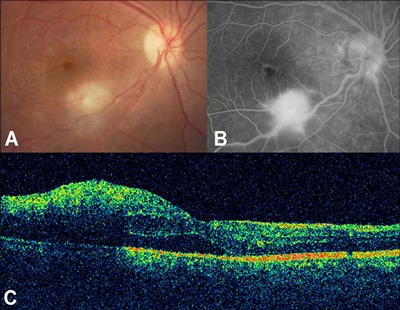
Figure 1. Toxoplasmic retinochoroiditis (TRC) with visual acuity of 20/70. A) Color fundus photograph of the right eye demonstrates an isolated active lesion along the inferotemporal vascular arcade. B) Fluorescein angiography before intravitreal clindamycin and dexamethasone therapy reveals marked hyperfluorescence resulting from leakage of dye from TRC lesion threatening the fovea. C) Horizontal optical coherence tomography scan obtained through the fovea reveals loss of normal foveal contour and diffuse macular thickening with subfoveal serous retinal detachment. Retinal map analysis indicates central macular thickness of 421 µm.
Click on image to enlarge.
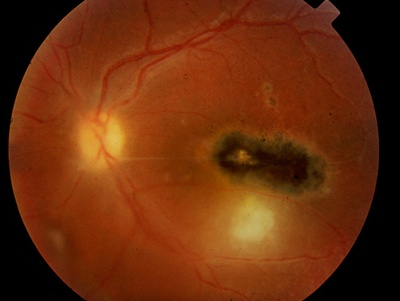
Figure 2. Recurrent ocular toxoplasmosis. Note the active retinal lesion associated with an old inactive scar. (Courtesy of Rubens Belfort Jr., MD, PhD.)
OT can also be divided into congenital and acquired forms. Congenital and acquired presentations can be divided into neonatal and late forms. Every newborn whose mother contracted toxoplasmosis during the pregnancy must receive treatment during the first year of life, independent of the presence of ocular involvement at birth. Congenital toxoplasmosis is most commonly acquired during the last trimester of pregnancy, with infants usually asymptomatic. Acquired toxoplasmosis can be concomitant when it occurs during systemic disease and delayed when there is a variable period (usually five to 10 years) between systemic and ocular disease.12
Toxoplasmic retinochoroiditis is unilateral in 72 to 86 percent of cases.11 The lesions can be solitary, multiple or satellite (adjacent to a cicatricial lesion). T. gondii has a clear preference for the posterior pole, occurring in this location in more than half of cases. Typical congenital retinochoroiditis presents as a macular cicatricial lesion, consisting of radial deposition of pigment around a central necrotic zone. Anterior uveitis is almost always a complication of retinochoroiditis. In fact, the presence of the parasite in the anterior segment without retinal involvement has only been demonstrated in immunocompromised patients.12
Retinal disease recurrences occur with both congenital- and postnatally-acquired infections. Typically these recurrences manifest as "satellite lesions" at the border of a pre-existing retinochoroidal scar (Figures 2 and 3). However, in some patients, new "primary retinal lesions" (those not arising from retinochoroidal scars) can develop far from pre-existing scars in retinal areas that had appeared clinically to be normal. Recurrences are generally assumed to be caused by the release of parasites from tissue cysts in the retina.10
Click on image to enlarge.
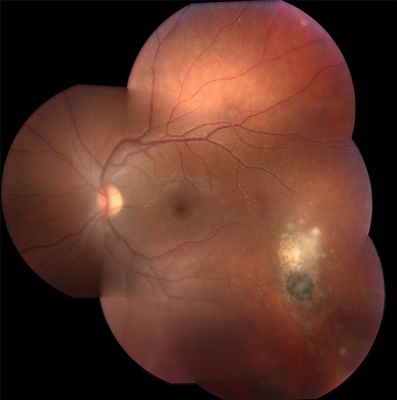
Figure 3. Recurrent satellite lesions of toxoplasmosis. Note the active retinal lesion associated with an old inactive scar. (Courtesy of Emmett T. Cunningham Jr., MD, PhD, MPH.)
Symptoms of OT vary according to the age of the subject. Signs at birth may include fever, maculopapular rash, hepatosplenomegaly, microcephaly, seizures, jaundice, thrombocytopenia and lymphadenopathy. The classic triad of congenital toxoplasmosis (CT) is retinochoroiditis, hydrocephalus and cranial calcifications.12 Children usually present with reduced visual acuity, strabismus, nystagmus and leukocoria. Teenagers and adults complain of decreased vision and floaters. If anterior uveitis is present, photophobia, pain and hyperemia may be present.12
Toxoplasmosis can also affect the optic nerve in many ways. The following atypical presentations of OT have been described: punctate outer retinitis, neuroretinitis, papillitis (Figures 4A and B), pseudo-multiple retinochoroiditis, intraocular inflammation without retinochoroiditis, unilateral pigmentary retinopathy, Fuchs'-like anterior uveitis, scleritis and multifocal or diffuse necrotizing retinitis.
Click on image to enlarge.
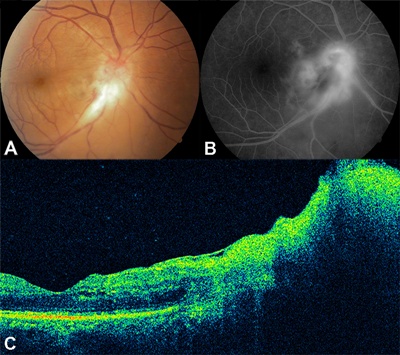
Figure 4. A) Color fundus photograph of the right eye demonstrates an active papillary lesion secondary to toxoplasmic retinochoroiditis (TRC) along the inferotemporal vascular arcade. B) Fluorescein angiography before intravitreal clindamycin and dexamethasone shows remarkable hyperfluorescence resulting from leakage of dye from TRC. C) A horizontal optical coherence tomography scan obtained through the papillomacular bundle demonstrates mild macular thickening with swelling of the optic disc. Retinal map analysis indicates a central macular thickness of 256 µm. The patient's visual acuity was 20/125.
A variety of complications of toxoplasmic retinochoroiditis (TRC) have been described, including rhegmatogenous retinal detachments, glaucoma, vitreous opacification or hemorrhage, retinal hemorrhage, optic atrophy, exudative retinal detachments, retinal vessel occlusions, subretinal and choroidal neovascularization (CNV), epiretinal membrane formation (Figure 5) and macular edema.5,10,13,14 In general, complications occur only in patients with severe ocular disease. Rhegmatogenous retinal detachments, for example, have been related to the severity of inflammation. Macular edema is believed to be uncommon14 for reasons that remain unknown. Prolonged infection, intense inflammation and complications can occasionally lead to phthisis or enucleation.10,15
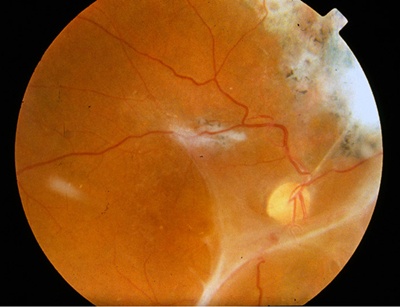
Click on image to enlarge.
Figure 5. Epiretinal membrane in a case of ocular toxoplasmosis.
Visual acuity may range from 20/20 to 20/400 among OT patients, depending upon the extent of macular or optic nerve involvement.15,16 In almost all cases, the diagnosis is based primarily on dilated fundus examination and confirmed by the presence of circulating specific antibodies against T. gondii.17
Immunocompromised patients are at increased risk of developing acute toxoplasmosis, which has a poor prognosis and may be rapidly fatal if left untreated. OT may follow a severe course in patients with AIDS, with atypical features (Figure 6), such as bilateral active lesions, large areas of active retinal necrosis, lesions arising perivascularly and not from old scars, bilateral inflammation and inflammation extending into the orbit and causing cellulitis and panophthalmitis.18
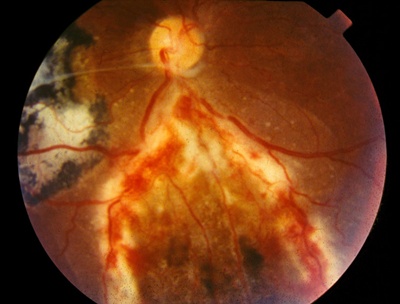
Click on image to enlarge.
Figure 6. Patients with AIDS and OT may have concomitant extensive areas of full-thickness retinal necrosis of cytomegalovirus retinitis. (Courtesy of Rubens Belfort Jr, MD, PhD.)
Diagnosis
The addition of an anti-T. gondii IgA assay to the detection of IgM and IgG increases the sensitivity of identification of cases with recently acquired toxoplasmosis since this class of antibodies disappear quicker than IgM.17 The detection of anti-T. gondii IgM antibodies is not completely reliable for the diagnosis of recent OT infection because IgM antibody titers can be naturally-occurring or occasionally persist for more than two years after disease onset. For the detection of acute congenital disease in fetuses and newborns, IgA antibodies are often used because IgM production is still weak and IgG antibodies can be of maternal origin. Serum IgA antibodies have been found only six to seven months after primary infection. In contrast to IgM antibodies, natural IgA antibodies have not been reported to date, and anti-T. gondii IgA antibodies are not found in chronic toxoplasmosis.17 Most reports of postnatally-acquired OT describe ocular involvement during the acute phase of infection, during which laboratory testing demonstrates a distinct serologic profile, which includes the presence of high IgG levels.19
Demonstration of intraocular antibody production determines the diagnosis of active OT. Antibody titers are measured in aqueous humor and serum, and the Goldmann-Witmer coefficient is calculated. Analysis of IgG, IgM and IgA increases the sensitivity of aqueous humor studies.12
Polymerase chain reaction (PCR) is a diagnostic test with the ability to determine the presence of DNA from an infectious agent and not the response to the infection assessed by antibody response. Therefore, its performance is not altered by the immune response of the patient.
Differential Diagnosis
CT of the newborn must be differentiated from the other infectious diseases of the TORCH group (rubella, cytomegalovirus [CMV], and herpes simplex virus), as well other congenital infectious diseases that may simulate toxoplasmosis, such as syphilis and tuberculosis. Important noninfective ocular entities that may be confused with CT include coloboma, persistent hyperplasic primary vitreous and retinoblastoma.3 Recurrent Toxoplasma lesions adjacent to retinochoroidal scars may resemble serpiginous choroiditis. Other conditions that are important in the differential diagnosis of OT are necrotizing retinitis caused by herpes viruses (CMV, herpes simplex, herpes zoster), fungal retinitis (candidiasis, blastomycosis), septic retinitis, ocular toxocariasis, sarcoidosis, syphilis and tuberculosis.5
Management
Antimicrobial therapy is absolutely required for managing systemic toxoplasmosis in newborns, pregnant women and immunosuppressed patients and in acute symptomatic disease, especially when vision is threatened due to the anatomic location or severe inflammation (Table 1). Patients with chronic toxoplasmosis do not require treatment when the disease is inactive except in special cases in which treatment is used to decrease the chance of recurrence.
Table 1

Click on table to enlarge.
*Adapted and modified from Tabbara KF. Ocular Toxoplasmosis. In: Tasman W, Jaeger EA, eds. Duane's Clinical Ophthalmology. Baltimore, Md: Lippincott Williams & Wilkins; 2008.
**Lasave AF, Díaz-Llopis M, Muccioli C, Belfort R Jr, Arevalo JF. Intravitreal Clindamycin and Dexamethasone for Zone 1 Toxoplasmic Retinochoroiditis at Twenty-four Months. Ophthalmology. 2010;117(9):1831-1838.
In general, TRC resolves spontaneously within six to eight weeks in most patients, although symptoms arising from the accompanying intraocular inflammation (e.g., floaters from the presence of vitreous cells) often take longer. Permanent visual impairment occurs when lesions affect the posterior pole within the macular arcade or adjacent to the optic disc or because of the development of inflammation-related complications (e.g., vitreous opacity, epiretinal membranes, retinal detachment).
The type and duration of OT treatment should be individualized. Treatment should be determined based on several factors, such as the immune status of the patient, severity of the inflammatory response and whether the site of the lesion is close to the macular area or optic nerve head.
Current drug treatment is directed against active lesions but unable to eradicate tissue cysts. Generally, initial antibiotic treatment includes oral pyrimethamine, sulfadiazine and folinic acid. Folinic acid is usually added to decrease the risk of leukopenia and thrombocytopenia associated with pyrimethamine therapy.20 However, the combination of oral trimethoprim-sulfamethoxazole and clindamycin is a well-tolerated therapy that has been used very effectively against OT.21 More recently, other antimicrobials, such as azithromycin and atovaquone, have been used successfully.22-24 There is no controlled evidence showing that one treatment is better than another or that the combination of other drugs with sulfadiazine and pyrimethamine improves results. All of these regimens have potential significant side effects, some of which may be treatment-limiting.
The purpose of treatment is to limit retinal damage by inhibiting parasites from multiplying during the active stage of infection. Systemic corticosteroids can be added to avoid further inflammation-related retinal damage. Classically, the use of intraocular injection of corticosteroids is contraindicated in OT. But injection of short half-life agents, such as dexamethasone, may have a role in selected patients when used with anti-toxoplasmic agents. Oral corticosteroid therapy must be initiated at least 48 hours after antiparasitic drugs. The usual initial dose in adults is 40 mg per day, followed by tapering dependent upon clinical response. Generally, corticosteroid therapy is suspended at least 10 days before use of the specific anti-toxoplasma drugs.5 Topical therapy includes corticosteroids and mydriatic drugs (Table 1).
Pearls
- Acquired toxoplasmosis in childhood may lead to severe blinding OT late in life. Anti-toxoplasma drugs may be indicated when the infection is diagnosed.
- Most cases of OT are acquired not congenital.
- The classic clinical presentation for OT is retinochoroiditis, but rarely only vasculitis or small areas of retinal thickening/whitening can be seen.
- Pregnant woman only rarely transmit the infection to a fetus more than once.
- Cats and meat are not the only source of infection; both air and water sources have been recognized.
- Anti-toxoplasma drugs should be given according to the clinical picture and not just for four to six weeks.
- Prevention of recurrence is possible and should be attempted in high-risk patients.
- Recurrences are not only related to retinal cysts (persistency of infectious agent).
- PCR and modern molecular biologic techniques still play a minor role in diagnosis.
Supported in part by the Arevalo-Coutinho Foundation for Research in Ophthalmology in Caracas, Venezuela.
References
- Stanford MR, See SE, Jones LV, Gilbert RE. Antibiotics for toxoplasmic retinochoroiditis: an evidence-based systematic review. Ophthalmology. 2003;110(5):926-931; quiz 931-932.
- Labalette P, Delhaes L, Margaron F, Fortier B, Rouland JF. Ocular toxoplasmosis after the fifth decade. Am J Ophthalmol. 2002;133(4):506-515.
- Bowie WR, King AS, Werker DH, et al. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350(9072):173-178.
- Fan CK, Hung CC, Su KE, et al. Seroprevalence of Toxoplasma gondii infection among inhabitants in the Democratic Republic of Sao Tome and Principe. Trans R Soc Trop Med Hyg. 2007;101(11):1157-1158.
- Pereira A, Orefice F. Toxoplasmosis. In: Foster CS, Vitale AT, eds. Diagnosis and treatment of uveitis. 1st ed. Philadelphia, Pa: W.B. Saunders Company;2001:385-410.
- Nussenblatt RB. Ocular toxoplasmosis. In: Nussenblatt RB, Whitcup SM, Palestine AG. Uveitis: fundamentals and clinical practice. 2nd ed. St. Louis, Mo: Mosby-Year Book, Inc;1996:211-228.
- Boothroyd JC, Grigg ME. Population biology of toxoplasma gondii and its relevance to human infection: do different strain cause different disease? Curr Opin Microbiol. 2002;5(4):438-442.
- Zamora DO, Rosenbaum JT, Smith JR. Invasion of human retinal vascular endothelial cells by Toxoplasma gondii tachyzoites. Br J Ophthalmol. 2008;92(6):852-855.
- Vallochi AL, Nakamura MV, Schlesinger D, et al. Ocular toxoplasmosis: more than just what meets the eye. Scand J Immunol. 2002;55(4):324-328.
- Holland GN. Ocular toxoplasmosis: a Global Reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol. 2003;136(6):973-988.
- Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol. 2004;137(1):1-17.
- Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol. 2005;20(3):129-141.
- Smith JR, Cunningham ET Jr. Atypical presentations of ocular toxoplasmosis. Curr Opin Ophthalmol. 2002;13(6):387-392.
- Schlaegel TF Jr, Weber JC. The macula in ocular toxoplasmosis. Arch Ophthalmol. 1984;102(5):697-698.
- Mets MB, Chhabra MS. Eye manifestations of intrauterine infections and their impact on childhood blindness. Surv Ophthalmol. 2008;53(2):95-111.
- Tan HK, Schmidt D, Stanford M, et al. Risk of visual impairment in children with congenital toxoplasmic retinochoroiditis. Am J Ophthalmol. 2007;144(5):648-653.
- Ongkosuwito JV, Bosch-Driessen EH, Kijlstra A, Rothova A. Serologic evaluation of patients with primary and recurrent ocular toxoplasmosis for evidence of recent infection. Am J Ophthalmol. 1999;128(4):407-412.
- Kump LI, Androudi SN, Foster CS. Ocular toxoplasmosis in pregnancy. Clin Experiment Ophthalmol. 2005;33(5):455-460.
- Bosch-Driessen EH, Rothova A. Recurrent ocular disease in postnatally acquired toxoplasmosis. Am J Ophthalmol. 1999;128(4):421-425.
- Engstrom RE Jr, Holland GN, Nussenblatt RB, Jabs DA. Current practices in the management of ocular toxoplasmosis. Am J Ophthalmol. 1991;111(5):601-610.
- Opremcak EM, Scales DK, Sharpe MR. Trimethoprim-sulfamethoxazole therapy for ocular toxoplasmosis. Ophthalmology. 1992;99(6):920-925.
- Rothova A, Bosch-Driessen LE, van Loon NH, Treffers WF. Azithromycin for ocular toxoplasmosis. Br J Ophthalmol. 1998;82(11):1306-1308.
- Pearson PA, Piracha AR, Sen HA, Jaffe GJ. Atovaquone for the treatment of toxoplasma retinochoroiditis in immunocompetent patients. Ophthalmology. 1999;106(1):148-153.
- Bosch-Driessen LH, Verbraak FD, Suttorp-Schulten MS, et al. A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am J Ophthalmol. 2002;134(1):34-40.
Author Disclosure
The authors have no financial or proprietary interest in any of the products or techniques mentioned in this article.