Many anterior segment surgeons are embracing the relatively new technology of spectral-domain optical coherence tomography (SD-OCT) and integrating it into their practices. SD-OCT is a painless, safe, fast, easy, reproducible and exact way to look at critical anatomy that correlates well with patient visual perception. As this technology becomes more widespread, it will become increasingly difficult to make critical decisions about patient care without having the information it can provide.
The history of SD-OCT
When time-domain optical coherence tomography (OCT) appeared on the scene a few years ago, the technology was first adopted by retina specialists. It soon became the standard diagnostic tool for following many macular conditions. However, purchasing an OCT device was difficult to justify for most anterior segment surgeons. The high cost of these units was a consideration, as was the technical expertise required to work the equipment, the relatively long exam time and interpretative skills required to understand the images.
But with the advent of SD-OCT, image quality has improved to the point that almost no interpretation is needed because examining the images is like looking at histology; all you need to know is the anatomy of the retina and the pathologic states that affect it. Image acquisition time has improved exponentially, reducing patient chair time. The technical expertise required to obtain high-quality images is now about on par with that required for working an auto refractor or topographer. Although spectral-domain units are more expensive than their time-domain predecessors, since the new units have much more to offer, such as glaucoma diagnostics, many anterior segment surgeons are embracing this technology and integrating it into their practices.
Factors driving SD-OCT demand
One of the key factors driving the need for OCT technology is the increasing use of "premium channel" IOLs. When patients pay a premium for an implant, their expectations are elevated and the need to rule out macular pathology before surgery becomes extremely critical so that patient expectations can be met. With SD-OCT, one can critically evaluate pathology seen on examination, such as epiretinal membranes and macular degeneration, which can assist in IOL selection and patient counseling. You can also see pathology, such as lamellar macula holes, vitreomacular traction and intraretinal and subretinal fluid that is often impossible to visualize with anything other than OCT.
In my practice I am often asked to consult with many patients who have had cataract or refractive surgery and are unhappy with the visual outcome. SD-OCT is a critical evaluative step to gain an understanding of the situation. It is very common for patients to blame their visual dysfunction on their implant when they actually have undiagnosed macular pathology. I also find SD-OCT indispensable in handling our own postoperative patients when their visual function or acuity does not meet their expectations.
The most common pathologies that we see in our practice and diagnose and evaluate with SD-OCT are:
- Epiretinal membrane (ERM)
- Vitreomacula traction syndromes
- Lamellar and full thickness macula holes
- Age-related macular degeneration (AMD)
- Central serous retinopathy
- Cystoid macular edema (CME)
- Submacular nevi (often with occult fluid)
- Optic neuropathy (using nerve fiber layer analysis)
Examples of SD-OCT use
Although many epiretinal membranes can be seen on examination, it is impossible to assess the effect they have on macular architecture without OCT. The following are some examples from my practice.
Cases 1 and 2 demonstrate that the appearance of an ERM on exam does not always correlate reliably with the amount of change it induces to macular anatomy and that an OCT is necessary for evaluation and appropriate surgical planning.
Case 1
This patient is a physician's wife with vision of 20/25 OD and 20/40 OS. She had a history of laser iridotomy for narrow angles, moderate nuclear sclerosis and cortical cataracts and was interested in cataract surgery with implantation of Crystalens (Bausch & Lomb) or AcrySof IQ ReSTOR (Alcon, Inc.). She had mild epiretinal membrane on examination.
The ERM in the left eye (Figure 2) is very mild in appearance, but on OCT we can see that there are marked changes in the macular architecture that would likely be associated with a more complicated postoperative course of macular edema and less-than-perfect vision. I advised the patient to hold off on cataract surgery until the cataract became more severe.
(Click on each image to enlarge.)
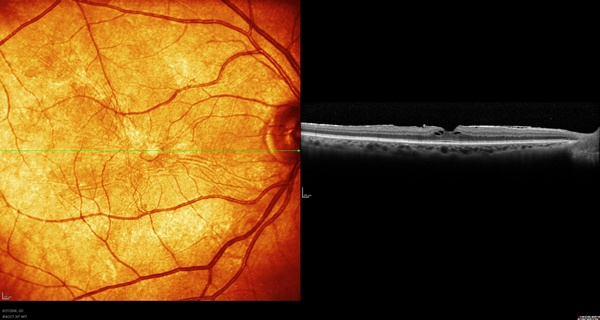
Figure 1. Right eye of case 1.
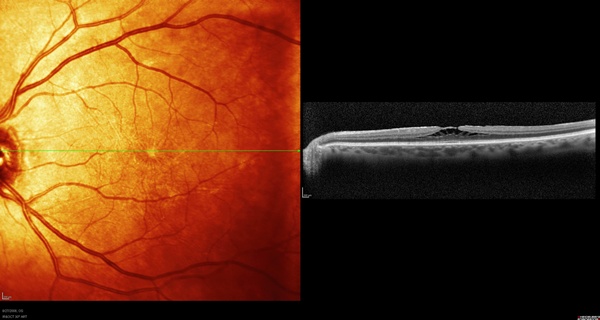
Figure 2. Left eye of case 1.
Case 2
This patient was 52 years old and had a retinal detachment (RD) extending to the temporal edge of the macula of his right eye (Figure 3), which was dominant. It had recently been repaired with a buckle by retina surgeons but had now developed a moderate 20/40 cataract. The patient was interested in Crystalens.
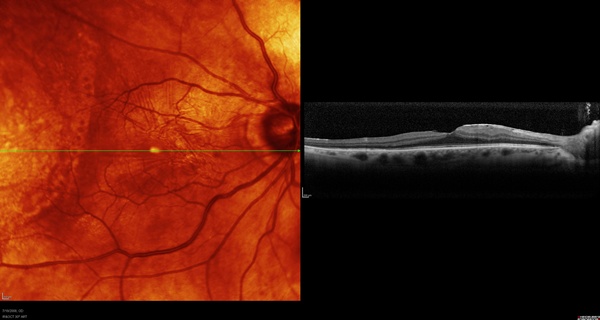
Figure 3. Macula of case 2.
Although this macula appears more abnormal than that of Case 1 due to ERM and other changes from the previous RD, the OCT shows that the anatomy is quite well preserved. We chose to implant a Crystalens HD lens in this patient. Afterward, he did extremely well, obtaining 20/20 distance and J2 near uncorrected.
The following cases are examples of how SD-OCT can be used to help clarify the postoperative situations of patients dissatisfied with their surgery.
Case 3
This patient had undergone cataract surgery recently and was unhappy with her vision in the operative eye. Her best-corrected vision was 20/60. She had mild posterior capsule changes. On examination, the macula looked essentially normal with some very mild pigment changes.
As seen on OCT (Figure 4), the patient has severe vitreomacular traction syndrome that was undetected on clinical exam. She was referred to vitreoretinal surgeons for treatment.
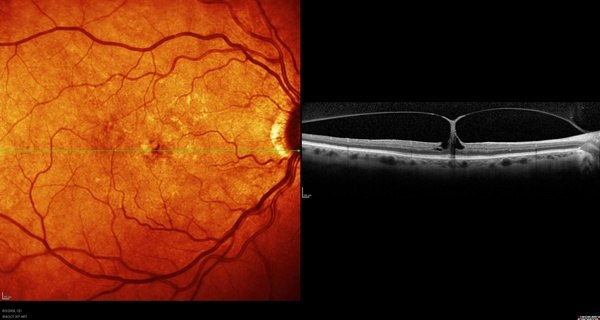
Figure 4. SD-OCT image of Case 3.
Case 4
This patient came into the office unhappy with the results of his left eye toric implant. He was sure that there was something wrong with the lens and that "it wasn't placed properly as it was in the other eye." His right eye, which also had a toric implant, had 20/20 vision, but his left eye had 20/30 and no improvement with refraction. There were very mild posterior capsule changes and some ocular surface issues due to blepharitis and dry eye.
The patient had a submacular choroidal nevus with associated subretinal neovascularization and fluid leakage leading to serous elevation of the macula. This was the cause of his perceived problem relative to the other eye (see Figures 5 and 6).
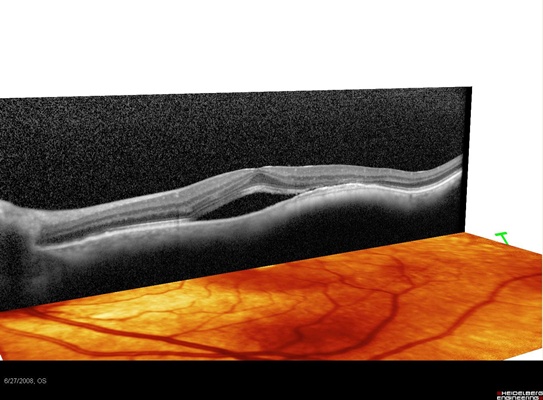
Figure 5. SD-OCT image of Case 4.
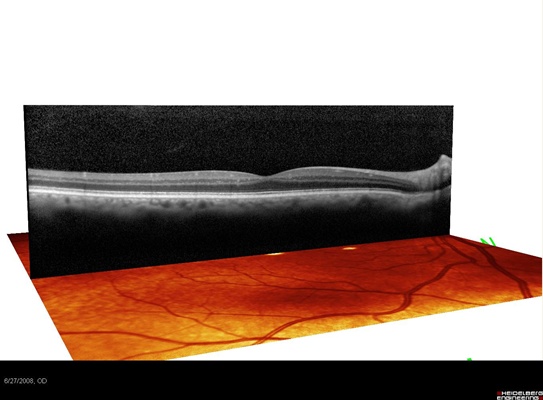
Figure 6. This image of Case 4 shows a submacular nevus associated with occult fluid leakage and serious elevation of the macula.
Case 5
When patients come in for opinions on IOL-related problems, SD-OCT is critical for planning and evaluating the outcome of corrective surgery. This patient illustrates this issue. He had come to my practice for a second opinion three months after cataract surgery, as he was very unhappy with the outcome. His best-corrected vision was 20/80 (with a -1.0 correction), and he was still on topical prednisolone acetate 1% and Ketorolac drops four times a day. On slit-lamp exam, he had a posterior chamber lens with one haptic in the bag and the other inferior haptic in the sulcus. The anterior and posterior capsule were fused behind the lens nasally, and the inferior haptic was seen to be embedded in the ciliary body on gonioscopy, which I felt was causing inflammation. There was also a hole in the posterior capsule at 6 o'clock.
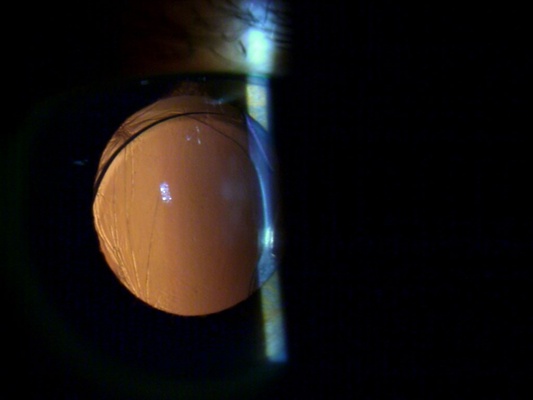
Figures 7A and 7B. Preoperative slit-lamp images of case 5.
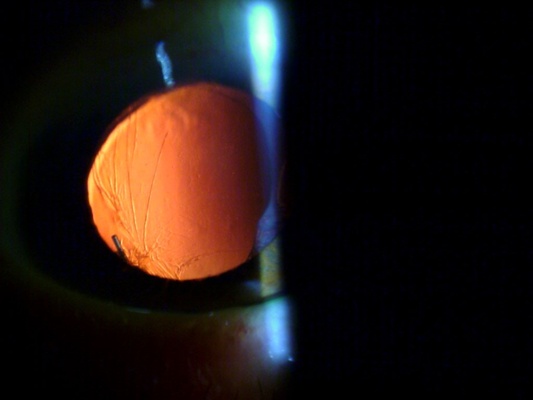

Figure 8. Preoperative gonioscopy of case 5 showing the inferior haptic out of the bag and embedded in the ciliary body.
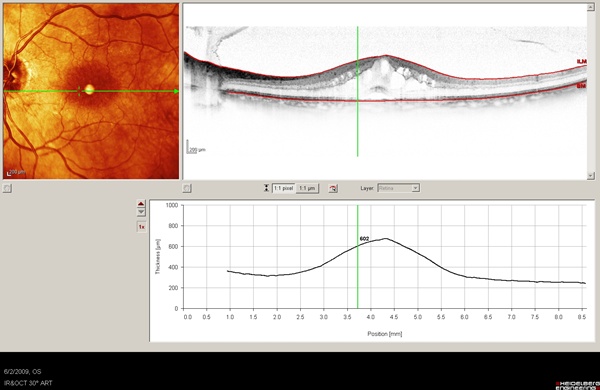
Figure 9. Preoperative assessment of case 5's macula by SD-OCT reveals marked cystoid macula edema.
A decision was made to reposition the implant in the capsular bag by reopening the bag and dialing the lens back into the bag surgically. Below is a postoperative image (see Figure 10) in which the implant can be seen to be completely within the reformed capsular bag. (Note that a YAG capsulotomy was also previously performed).
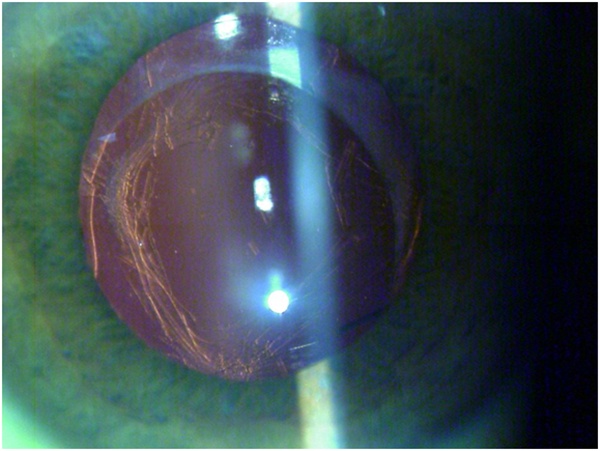
Figure 10. Postoperative image of Case 5.
Below is a follow-up SD-OCT (Figure 11) taken one month after surgery showing complete resolution of CME with the patient on no topical medications. His vision improved to 20/20-2, and his refraction shifted to -0.5 D, as the implant was now more posterior within the capsular bag.
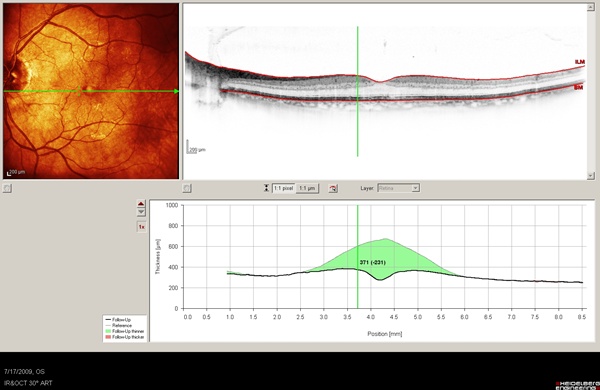
Figure 11. OCT image of case 5 showing the change in macular thickness from baseline in green shadowing.
The SD-OCT information in case 5 was critical in establishing the cause of the patient's poor vision. Once the surgical intervention was performed, we were able to document improvement. By showing the images to the patient, it become very clear to him what was going on and why. Such images are not only helpful for the clinician's understanding of a particular case but also for patient education.
Case 6
Another interesting case is that of a woman who came in for an opinion about positive dysphotopsia. She had been seeing self-described "flashes of light" for two to three years and had been seen by two retina specialists and a neuro-ophthalmologist but had not been given a definitive diagnosis. The OCT image below (Figure 12) demonstrated that she had vitreopapillary traction syndrome.
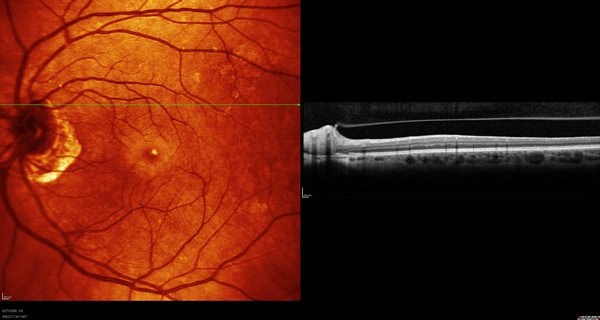
Figure 12. OCT image of case 5 demonstrating the presence of vitreopapillary traction syndrome
We have since seen a few patients with similar presentation. In all cases the point of adhesion between the vitreous and the optic nerve correlates with the portion of visual space from which the dysphotopsias appear to emanate.
Cases 7 and 8
Another area of tremendous utility for SD-OCT in an anterior segment practice is following AMD patients. Now that we have effective treatment for subretinal neovascularization, it is important to know when there is leakage into the retina. With SD-OCT, we can easily determine which of our patients need referral for treatment.
These cases are of patients who presented with one-line drops in vision but minimal changes on exam. SD-OCT (see Figures 13 and 14) enabled the diagnoses, and both patients were treated successfully with anti-VEGF injections.
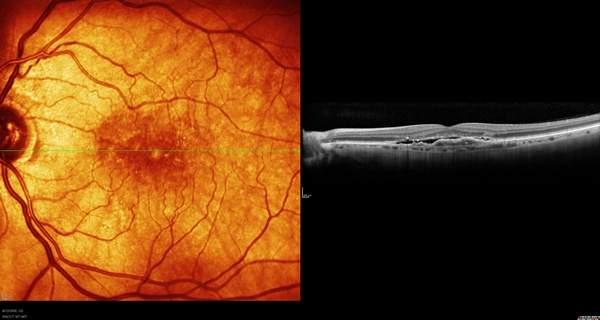
Figure 13. Image of a subretinal membrane associated with occult fluid leakage.
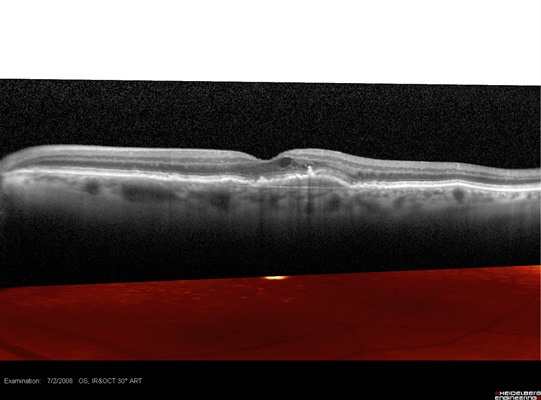
Figure 14. Subretinal neovascularization breaking through Bruch's membrane and causing leakage into the macula.
Author Disclosure
Dr. Safran is a consultant for Bausch and Lomb and Heidelberg Engineering.