Disease Overview
Pediatric dry eye (PeDED) is a neglected and not fully understood disease. Because of the lack of high-evidence epidemiological and normative data, the poor consensus on the diagnostic algorithm, the difficulties in symptom quantification and interpretation, together with attention focused on the hyposecretive form of the disease, PeDED has been considered a rare condition. It has previously mostly been associated with congenital disorders or autoimmune and inflammatory diseases. Recent evolution in dry eye disease understanding and reports on tear film and ocular surface changes in children are calling into question this view of PeDED, which has led to a growing awareness of the importance of this condition.
Definition
The Tear Film and Ocular Surface Dry Eye Workshop II (TFOS DEWS II) recently updated the dry eye disease definition, stating that “Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”1
This definition has shaped the approach to treating the disease in adults, and it may be helpful in understanding PeDED. This is a multifactorial disease involving the whole ocular surface morphofunctional unit, not just a condition or a disturbance involving the tear film. It is a symptomatic disease with significant impact on quality of life,2,3 and specific efforts are needed to elucidate this aspect in children. Moreover, the updated definition suggests 5 potential etiologic mechanisms that need to be thoroughly investigated.
Epidemiology
Prevalence
PeDED is considered a rare condition4 but its diagnosis is often overlooked, affecting the accuracy of our epidemiological data.
The TFOS DEWS II Epidemiology Report5 recently showed that, after the fourth decade of life, the prevalence of dry eye linearly increases. Interestingly, the few data on children included in the analysis showed, in the age range of 15 to 18 years,6,7 the lowest prevalence of clinically diagnosed dry eye (median value < 5%) but an anomalous high prevalence of dry eye symptoms (median value > 20%) and dry eye identified on the basis of the Women's Health Study criteria (median value > 20%).8
The population-based cross-sectional study performed by Uchino and colleagues6 on 3433 Japanese high school students provided major evidence of the discrepancy between clinically diagnosed dry eye (4.3% and 8% in boys and girls, respectively) and severe dry eye symptoms (21% and 24.4% in boys and girls, respectively).
Selected studies investigating PeDED epidemiology are reported in Table 1.
Table 1. Selection of studies investigating the epidemiology of PeDED
|
Authors
|
Country
|
Design of the study
|
Number
|
Age (Mean)
|
Race
|
Specific risk factor
|
Prevalence of DED with Risk factor
|
Prevalence of DED in controls
|
Method
|
Gender DED rate
M:F
|
|
Doughty et al 19979
|
Canada
|
Population based cross-sectional study
|
512
1791
|
9-10
11-20
|
Caucasian
|
N/A
|
N/A
|
9.57%
21.3%
|
Questionnaire
|
N/A
|
|
Uchino et al 20086
|
Japan
|
Population based cross-sectional study
|
2848 M
585 F
|
15-18
|
Asian
|
Hard contact lens (HCL)
Soft contact lens (SCL)
|
M: 8.3% +SCL 9.1% +HCL
F: 13.5% +SCL
7.4% +HCL
(clinically diagnosed)
M: 37.1% +SCL
27.3% +HCL
F: 37.5%+SCL
37% +HCL
(severe symptoms)
|
2.1% M and 3.1% F clinically diagnosed
12.5 % M and 11.3% F severe symptoms
|
Questionnaire data and clinical ophthalmologic testing
|
N/A
|
|
Zang et al 20117
|
China
|
Population based cross-sectional study
|
1885
|
Senior high school students
|
Asian
|
Inadequate refractive correction
Frequent self-administered topical medication
Poor sleep quality
|
40.3% Inadequate refractive correction
24.8% Frequent self-administered topical medication
26.6 % Poor sleep quality
|
23.3%
|
Questionnaire data and clinical ophthalmologic testing
|
51% M, 48.3% F
|
|
Moon et al 201410
|
Korea
|
Population based cross sectional case control
study.
|
260
|
9-11
|
Asian
|
Use of smartphone
|
71%
|
9.7%
|
Questionnaire data and clinical ophthalmologic testing
|
N/A
|
|
Moon et al 201611
|
Korea
|
Population based cross-sectional case control study.
|
916
|
9.90±0.96
|
Asian
|
Use of smartphone
|
96.7
|
6.6% [4% in 7- to 9-year-old children; 9.1% in 10- to 12-year-old children]
|
Questionnaire data and clinical ophthalmologic testing
|
53.3% F
|
|
Chen et al 2016 12
|
China
|
Hospital based prospective case–control study
|
40/40
|
4.76±0.86
|
Asian
|
Seasonal allergic conjunctivitis (SAC) Perennial allergic conjunctivitis
(PAC)
|
97.5% -SAC and PAC
|
27%
|
Tear film breakup time
|
N/A
|
|
Wang et al 2018 13
|
New Zealand
|
Population based cross-sectional observational study
|
70
|
5-18
|
Asian - Caucasian
|
Race
|
No statistically significant differences
|
20% Asian
8% Caucasian
|
Questionnaire data and clinical ophthalmologic testing
|
N/A
|
|
Tityal et al 201914
|
India
|
Hospital-based cross-sectional study
|
1997
|
< 20
|
Indian
|
N/A
|
N/A
|
7.84%
|
Questionnaire data and clinical ophthalmologic testing
|
N/A
|
N/A: not applicable
Risk Factors
Known risk factors for PeDED account for several congenital disorders, autoimmune diseases, ocular allergy, and environmental and iatrogenic factors.
Congenital disorders include familial dysautonomia, Allgrove syndrome, alacrima, ectodermal dysplasia syndromes, multiple endocrine deficiency, cystic fibrosis, and congenital corneal anesthesia.4 These rare conditions mainly affect lacrimal glands and corneal nerve function, leading to hyposecretive dry eye and/or potentially severe damage of ocular surface epithelia.
Autoimmune diseases causing dry eye include Sjögren syndrome (SS),15 graft-vs-host disease (GVHD),16,17 and juvenile idiopathic arthritis (JIA).18,19 As in adults, SS and GVHD induce a hyposecretive and inflammatory dry eye, ranging from mild-moderate to very severe forms requiring topical and systemic immunosuppression. JIA may be associated with milder forms of hyposecretive PeDED.
Although the potential association between ocular allergy and dry eye was hypothesized more than 20 years ago,20 recent evidence sheds light on the pathogenic mechanisms underlying the correlation between the 2 conditions.21 Ocular allergies, mainly severe forms of keratoconjunctivitis (vernal and atopic), are able to decrease tear film stability, impairing both meibomian gland function22 and tear mucin secretion,22,23 to induce ocular surface inflammation and damage,24,25 and to affect corneal innervation.26,27 In general, ocular allergy-related PeDED does not show decreased tear film volume and secretion; reduced tear film break-up time (TBUT) seems to be the hallmark of this condition, interestingly potentially persisting in the quiet phases of severe allergic disease.28
With regard to environmental factors, the use of visual display terminals is the best-known risk factor for PeDED. The prolonged daily use of these devices, especially of smartphones, reduces the blink rate and induces hyperevaporation of the tear fluid.10,11
Dietary vitamin A deficiency is associated with ocular surface squamous metaplasia and xerophthalmia. This condition is typically associated with malnutrition and it represents a major health issue in developing countries. Cases occurring in developed countries may be related to eating disorders,29 vegetarian dietary regimen,30 or various forms of malabsorption syndrome.
Air pollution might be an additional environmental risk factor for dry eye disease. At present, preliminary and conflicting evidence on this issue is mainly focused on adults.31-33 A recent study showing a significant association between air pollution and the rate of pediatric conjunctivitis of unknown origin (not clearly due to infections, allergy, or blepharoconjunctivitis) suggests that the relationship between air pollution and pediatric ocular surface disease needs to be better investigated.34
Iatrogenic risk factors for PeDED mainly include medications and contact lenses. Several drugs can induce or exacerbate dry eye disease, the most relevant in the pediatric population being systemic and topical retinoids for acne vulgaris, systemic and topical anti-allergic drugs, and benzalkonium chloride-preserved eyedrops.4,35
Contact lens use was reported as a risk factor for dry eye in several epidemiological studies on dry eye.35 About PeDED, a few conflicting data are present in literature, with the prevalence of ocular surface symptoms in children wearing contact lens ranging from 7% to 37%.6,36
Clinical and Diagnostic Examination
The current and most widely accepted diagnostic algorithm for dry eye disease requires the presence of symptoms plus at least 1 of 3 objective homeostasis markers (reduced tear film break-up time, increased osmolarity, and ocular surface staining). Given the diagnosis, additional examinations may be aimed to assess tear film volume and meibomian gland (MG) function.37
This diagnostic approach has been developed to be applied to adults. However, its peculiarities, including the secondary role assigned to tear film volume and secretion, seem to make it suitable for PeDED. Major challenges are due to the lack of pediatric normative data, of validated diagnostic cut-offs, and of tests specifically developed for children.
Figure 1 proposes and elaborates on a diagnostic approach, planned to minimize the impact of each test on the following one.
Assessment of symptoms
The assessment and quantification of systems may be a major issue in pediatric ophthalmology.
In preverbal children, information provided by parents can be useful, but their interpretation may be challenging because of disagreement between parents and health professionals and the influence of socioeconomic status.38,39
In children of verbal age, major issues include the need for validated tools for assessment and quantification of symptoms, as well as interpretation of their features and severity. If standardized questionnaires are complex and tailored to adults, visual analogue scales that explore a few key items might be the best approach to pediatric patients. Moreover, interestingly, mild-moderate and chronic DED symptoms might be underreported by children.40
Objective diagnostic tests (assessment of signs)
As mentioned earlier, tear film break-up time, osmolarity, and ocular surface staining are 3 key objective homeostasis markers for DED diagnosis.37
Tear film break-up time is the most frequently employed test of tear film stability and it may be assessed by instilling sodium fluorescein (F-BUT), or noninvasively, without dye (NI-BUT).
F-BUT is low-tech and low-cost, it is supported by a large body of evidence in literature, and it can be performed in a standardized manner in order to limit the variability related to fluorescein instillation and to subjective assessment by the observer.37 However, in children, eyedrop instillation may induce an overreaction, including hyperlacrimation, abnormal and forced blinking, and poor cooperation.
NI-BUT, mainly based on the observation of the specular reflection of an illuminated grid pattern from the tear film, may be automatically assessed with specific software.41 This approach, not requiring eyedrop instillation, may be suitable for tear film stability evaluation in children. The challenges of using this system in children relate to availability of instruments, cooperation (patients have to maintain stable fixation without blinking for up to 15-20 seconds), and lack of normative data in pediatric patients.
Tear film osmolarity, frequently reported as the single best metric to diagnose and classify DED,37 can be measured using a lab-on-a-chip system (TearLab Corp, San Diego, CA). At present, there is scarcity of evidence about this test in children and no data suggesting whether specific diagnostic thresholds in pediatric DED should be adopted. Moreover, recent studies reported conflicting data on the association between osmolarity values and ocular surface health in children.42,43
Ocular surface staining, performed with sodium fluorescein (and lissamine green), is a low-tech and low-cost clinical examination that can provide useful information on epithelial damage. This procedure requires very limited cooperation and it may be helpful in grading the disease severity. Thanks to lessons learned from severe ocular allergies,44,45 quantification and pattern of corneal epitheliopathy can provide indirect information on ocular surface inflammation and visual disturbance.
DED subclassification, aimed to investigate the hyposecretive and the hyperevaporative components of the disease,37 is mainly based on the assessment of tear film secretion/volume and of the morphology and function of the meibomian glands.
The Schirmer test without anesthesia is a very common and well-standardized test to investigate tear film secretion but, because of its relative invasiveness, is not ideal for use in children. Good alternatives might be the phenol red thread test,46 which can be performed in 15 seconds using a thin cotton thread, and tear meniscometry47 performed through optical coherence tomography or other infrared instruments. However, at present, both approaches are limited by the lack of a normative dataset validated in pediatric subjects.
Meibomian gland morphology and function should be assessed combining direct clinical examination, in vivo imaging, and indirect analysis aimed to study the tear film lipid layer.37 Recent advances in meibography have made this the most interesting and promising approach to examining meibomian gland function in children. Infrared systems (including both noncontact and mobile, handheld, and pen-shaped instruments) do not require transillumination, do not dazzle the patient, induce no-to-low discomfort, and allow quick capture and recording of images and videos.48-50 Open issues are related to the availability and validation of software for automated quantitative image analysis and to the clinical interpretation of findings such as meibomian gland atrophy, drop-out, and tortuosity.
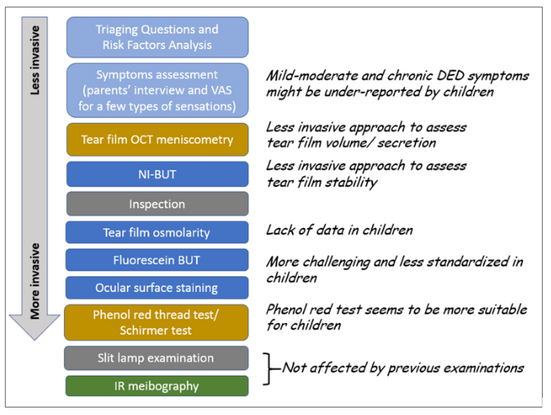
Figure 1. Proposed diagnostic approach for PeDED. Light blue: medical history and symptoms; Blue: key signs for the diagnosis; Gold: volume/secretion assessment for sub-classification; Green: meibomian gland (MG) assessment for subclassification.
BUT= breakup time; IR=infrared; NI-BUT=noninvasive breakup time; OCT=optical coherence tomography; VAS=visual analog scale.
Management and Therapy
The first-line treatment of DED, both in adults and children, includes modification of local environment, education regarding potential dietary modifications, lid hygiene, and warm compresses, and ocular lubricants of various types.4,51 If literature and clinical experience seem to suggest an important role for the complexities of these management options, the individual measures are often poorly supported by Level 1 studies. Several molecules have been studied as potential additional agents for first-line treatment of DED. One of the most promising is N-acetylcysteine, given its antioxidant and mucolytic activity,52 and preliminary studies reported its efficacy (on symptoms more than on objective signs) both in murine models and in patients.53, 54
In 2014, a formulation of chitosan-N-acetylcysteine, a new biopolymer aimed to form a protective glycocalyx-like layer on the ocular surface, was approved in Europe as a class III medical device, and 2 recent retrospective uncontrolled studies reported its efficacy on both signs and symptoms of DED.55, 56
In children, particular attention should be paid to minimize the impact of annoying procedures and of frequent daily eyedrop instillation on everyday activities and quality of life.
Second-line treatments include tea tree oil for Demodex, punctal occlusion, in-office device-assisted therapies (eg, physical heating and expression of the meibomian glands, intense pulsed light), and prescription drugs (mainly intended to control inflammation).51
At present, we have no evidence supporting the efficacy of in-office device-assisted therapies in children. However, if future data supports their use (or the use of modified, less uncomfortable, devices) in PeDED, they might be a precious weapon, potentially able to limit the need for frequent eyedrop instillation.
Prescription drugs for DED include topical corticosteroids, topical secretagogues (not available in US and Europe), topical cyclosporine (limited availability in some countries), topical LFA-1 antagonist (not available outside US), and oral or topical tetracycline/doxycycline or macrolide antibiotics.
Several adults studies have demonstrated the efficacy of topical steroids, used with pulsed or tapered regimens, and clinical experience confirmed their efficacy in PeDED.51,57 However, in treating pediatric patients, even if using topical steroids such as fluorometholone, loteprednol, or hydrocortisone, which have a lower likelihood of having an intraocular effect, special attention should be paid to the risk of inducing ocular hypertension and cataracts.57,58
Use of steroid-sparing drugs in PeDED, indirectly supported by data on children with severe allergic diseases, may be complicated by cost, limited availability, poor tolerability, and lack of specific regimens validated in children.
Oral or topical tetracycline, doxycycline, or macrolide antibiotics are able to decrease bacteria-producing lipolytic exoenzymes, to inhibit lipase production, to decrease the activity of collagenase, phospholipase A2, and several MMPs, and to decrease the production of inflammatory mediators.51 Evidence seems to mostly support the use of oral tetracycline or analogues. However, the use of these drugs should be avoided in prepuberal patients, because it interferes with tooth and bone development.4 Topical antibiotics and oral macrolides are useful alternatives, although supported by less evidence. Topical azithromycin has been shown to improve lipid architecture, TBUT, and patient comfort in a number of studies in adults, and it may also be useful in PeDED.59, 60
Most severe cases of PeDED may require additional therapies including biological tear substitutes (homologous serum from a parent or sibling can be used if venipuncture or blood is impossible or impractical), soft or scleral therapeutic contact lenses, and amniotic membrane grafts.
Summary
PeDED is not yet well known, and is often overlooked. Recent advances of our knowledge of pathophysiology and clinical manifestations of DED in adults might be of crucial importance to begin to better understand, assess, and manage PeDED.
The pediatric tear film and ocular surface have peculiar physiology and pathology, which need to be better understood. Major open issues on PeDED clinical management include the need of specific diagnostic tests to assess both symptoms and signs, the need for normative data, the need for validated diagnostic thresholds, and for a specific therapeutic algorithm that takes into account the specificity of efficacy, safety, and impact on quality of life of treatments in children.
References
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-283.
- Shigeyasu C, Yamada M, Kawashima M, et al. Quality of life measures and health utility values among dry eye subgroups. Health Qual Life Outcomes. 2018;16(1):170.
- Asiedu K, Dzasimatu SK, Kyei S. Impact of dry eye on psychosomatic symptoms and quality of life in a healthy youthful clinical sample. Eye Contact Lens. 2018;44 Suppl 2:S404-S9.
- Alves M, Dias AC, Rocha EM. Dry eye in childhood: epidemiological and clinical aspects. Ocul Surf. 2008;6(1):44-51.
- Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334-365.
- Uchino M, Dogru M, Uchino Y, Fukagawa K, Shimmura S, Takebayashi T, et al. Japan Ministry of Health study on prevalence of dry eye disease among Japanese high school students. Am J Ophthalmol. 2008;146(6):925-929 e2.
- Zhang Y, Chen H, Wu X. Prevalence and risk factors associated with dry eye syndrome among senior high school students in a county of Shandong Province, China. Ophthalmic Epidemiol. 2012;19(4):226-230.
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318-326.
- Doughty MJ, Fonn D, Richter D, Simpson T, Caffery B, Gordon K. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci. 1997;74(8):624-631.
- Moon JH, Lee MY, Moon NJ. Association between video display terminal use and dry eye disease in school children. J Pediatric Ophthalmol Strabismus. 2014;51(2):87-92.
- Moon JH, Kim KW, Moon NJ. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study. BMC Ophthalmol. 2016;16(1):188.
- Chen L, Pi L, Fang J, Chen X, Ke N, Liu Q. High incidence of dry eye in young children with allergic conjunctivitis in Southwest China. Acta Ophthalmol. 2016;94(8):e727-e730.
- Wang MTM, Craig JP. Natural history of dry eye disease: Perspectives from inter-ethnic comparison studies. Ocular Surf. 2019;17(3):424-433.
- Titiyal JS, Falera RC, Kaur M, Sharma V, Sharma N. Prevalence and risk factors of dry eye disease in North India: Ocular surface disease index-based cross-sectional hospital study. Indian J Ophthalmol. 2018;66(2):207-211.
- Cimaz R, Casadei A, Rose C, et al. Primary Sjögren syndrome in the paediatric age: a multicentre survey. Eur J Pediatr. 2003;162(10):661-665.
- Suh DW, Ruttum MS, Stuckenschneider BJ, Mieler WF, Kivlin JD. Ocular findings after bone marrow transplantation in a pediatric population. Ophthalmology. 1999;106(8):1564-1570.
- Hoehn ME, Calderwood J, Gannon E, et al. Ocular complications in a young pediatric population following bone marrow transplantation. J AAPOS. 2018;22(2):102-106 e1.
- Akinci A, Cakar N, Uncu N, Kara N, Acaroglu G. Keratoconjunctivitis sicca in juvenile rheumatoid arthritis. Cornea. 2007;26(8):941-944.
- El-Shazly AA, Mohamed AA. Relation of dry eye to disease activity in juvenile rheumatoid arthritis. Eur J Ophthalmol. 2012;22(3):330-334.
- Toda I, Shimazaki J, Tsubota K. Dry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitis. Ophthalmology. 1995;102(2):302-309.
- Villani E, Rabbiolo G, Nucci P. Ocular allergy as a risk factor for dry eye in adults and children. Curr Opin Allergy Clin Immunol. 2018;18(5):398-403.
- Mizoguchi S, Iwanishi H, Arita R, et al. Ocular surface inflammation impairs structure and function of meibomian gland. Exp Eye Res. 2017;163:78-84.
- Dogru M, Matsumoto Y, Okada N, et al. Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy. 2008;63(10):1324-1334.
- Kumagai N, Yamamoto K, Fukuda K, et al. Active matrix metalloproteinases in the tear fluid of individuals with vernal keratoconjunctivitis. J Allergy Clin Immunol. 2002;110(3):489-491.
- Leonardi A, Brun P, Abatangelo G, Plebani M, Secchi AG. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44(7):3052-3058.
- Leonardi A, Lazzarini D, Bortolotti M, Piliego F, Midena E, Fregona I. Corneal confocal microscopy in patients with vernal keratoconjunctivitis. Ophthalmology. 2012;119(3):509-515.
- Villani E, Mantelli F, Nucci P. In-vivo confocal microscopy of the ocular surface: ocular allergy and dry eye. Curr Opin Allergy Clin Immunol. 2013;13(5):569-576.
- Villani E, Strologo MD, Pichi F, et al. Dry eye in vernal keratoconjunctivitis: a cross-sectional comparative study. Medicine (Baltimore). 2015;94(42):e1648.
- Velasco Cruz AA, Attie-Castro FA, Fernandes SL, et al. Adult blindness secondary to vitamin A deficiency associated with an eating disorder. Nutrition. 2005;21(5):630-633.
- Ramsay A, Sabrosa NA, Pavesio CE. Bitot's spots and vitamin A deficiency in a child from the UK. Br J Ophthalmol. 2001;85(3):372.
- Hwang SH, Choi YH, Paik HJ, Wee WR, Kim MK, Kim DH. Potential importance of ozone in the association between outdoor air pollution and dry eye disease in South Korea. JAMA Ophthalmol. 2016;134(5):503-510.
- Mo Z, Fu Q, Lyu D, et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: A case-crossover study. Environ Pollut. 2019;246:183-189.
- Alves M, Novaes P, Morraye Mde A, Reinach PS, Rocha EM. Is dry eye an environmental disease? Arq Bras Oftalmol. 2014;77(3):193-200.
- Nucci P, Sacchi M, Pichi F, et al. Pediatric conjunctivitis and air pollution exposure: a prospective observational study. Semin Ophthalmol. 2017;32(4):407-411.
- Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511-538.
- Greiner KL, Walline JJ. Dry eye in pediatric contact lens wearers. Eye Contact Lens. 2010;36(6):352-355.
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-574.
- Elias LS, Guinsburg R, Peres CA, Balda RC, Santos AM. Disagreement between parents and health professionals regarding pain intensity in critically ill neonates. J Pediatr (Rio J). 2008;84(1):35-40.
- Shaikh N, Kearney DH, Colborn DK, Balentine T, Feng W, Lin Y, et al. How do parents of preverbal children with acute otitis media determine how much ear pain their child is having? J Pain. 2010;11(12):1291-1294.
- Han SB, Yang HK, Hyon JY, Hwang JM. Children with dry eye type conditions may report less severe symptoms than adult patients. Graefes Archiv Clin Exp Ophthalmol. 2013;251(3):791-796.
- Wang MT, Murphy PJ, Blades KJ, Craig JP. Comparison of non-invasive tear film stability measurement techniques. Clin Exp Optom. 2018;101(1):13-17.
- Gunay M, Celik G, Yildiz E, et al. Ocular surface characteristics in diabetic children. Curr Eye Res. 2016;41(12):1526-1531.
- Ong Tone S, Elbaz U, Silverman E, et al. Evaluation of dry eye disease in children with systemic lupus erythematosus and healthy controls. Cornea. 2019;38(5):581-586.
- Hu Y, Matsumoto Y, Dogru M, et al. The differences of tear function and ocular surface findings in patients with atopic keratoconjunctivitis and vernal keratoconjunctivitis. Allergy. 2007;62(8):917-925.
- Leonardi A, Lazzarini D, La Gloria Valerio A, Scalora T, Fregona I. Corneal staining patterns in vernal keratoconjunctivitis: the new VKC-CLEK scoring scale. Br J Ophthalmol. 2018;102(10):1448-1453.
- Sakamoto R, Bennett ES, Henry VA, et al. The phenol red thread tear test: a cross-cultural study. Invest Ophthalmol Vis Sci. 1993;34(13):3510-3514.
- Baek J, Doh SH, Chung SK. Comparison of tear meniscus height measurements obtained with the keratograph and Fourier domain optical coherence tomography in dry eye. Cornea. 2015;34(10):1209-1213.
- Shirakawa R, Arita R, Amano S. Meibomian gland morphology in Japanese infants, children, and adults observed using a mobile pen-shaped infrared meibography device. Am J Ophthalmol. 2013;155(6):1099-1103 e1.
- Gupta PK, Stevens MN, Kashyap N, Priestley Y. Prevalence of meibomian gland atrophy in a pediatric population. Cornea. 2018;37(4):426-430.
- Al-Hayouti H, Daniel M, Hingorani M, Calder V, Dahlmann-Noor A. Meibography and corneal volume optical coherence tomography to quantify damage to ocular structures in children with blepharokeratoconjunctivitis. Acta Ophthalmol. 2019;97(7):e981-e6.
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017;15(3):575-628.
- Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetylcysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2:38e44.
- Hongyok T, Chae JJ, Shin YJ, Na D, Li L, Chuck RS. Effect of chitosan-N-acetylcysteine conjugate in a mouse model of botulinum toxin B-induced dry eye. Arch Ophthalmol. 2009;127(4):525e32.
- Pokupec R, Petricek I, Sikic J, Bradic M, Popovic-Suic S, Petricek G. Comparison of local acetylcysteine and artificial tears in the management of dry eye syndrome. Acta Med Croat. 2005;59:337e40.
- Messina M, Dua HS. Early results on the use of chitosan-N-acetylcysteine (Lacrimera®) in the management of dry eye disease of varied etiology. Int Ophthalmol. 2019;39(3):693-696.
- Nepp J, Knoetzl W, Prinz A, Hoeller S, Prinz M. Management of moderate-to-severe dry eye disease using chitosan-N-acetylcysteine (Lacrimera®) eye drops: a retrospective case series. Int Ophthalmol. 2020;40(6):1547-1552.
- Kwok AK, Lam DS, Ng JS, Fan DS, Chew SJ, Tso MO. Ocular-hypertensive response to topical steroids in children. Ophthalmology. 1997;104(12):2112-2116.
- Lam DS, Kwok AK, Chew S. Accelerated ocular hypertensive response to topical steroids in children. Br J Ophthalmol. 1997;81(5):422-423.
- Foulks GN, Borchman D, Yappert M, Kim SH, McKay JW. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29(7):781-788.
- Ciloglu E, Özcan AA, Incekalan T, Unal F. The Role of Topical Azithromycin in the Treatment of Meibomian Gland Dysfunction. Cornea. 2020;39(3):321-324.