Authors: Michael S. Berlin, MD, MS; Marc Toeteberg-Harms, MD; Edward Kim, BA; Iris Vuong, BS: Ulrich Giers, MD
Introduction
Elevated intraocular pressure (IOP) in most open-angle glaucoma is due to an obstruction of aqueous outflow considered predominantly localized at the juxtacanalicular trabecular meshwork (TM) and the inner wall of Schlemm's canal (SC). There have been surgical attempts to directly treat this pathology by creating channels to connect the anterior chamber to SC. Iterations have included mechanical drilling techniques, thermal laser and alternative thermal cautery techniques, and a variety of tube implants with the common goal of bypassing this increased outflow resistance.
However the majority of these techniques concurrently or subsequently create sufficient adjacent tissue disruption to result in inflammatory healing responses that outweigh the benefits of the procedures, leading to short- and long-term failures. To overcome these known causes of failure, technologies and techniques were sought to create channels without causing scar-tissue formation. Requirements included minimal tissue disruption during the process of creating the openings and channels, which are of adequate size and number to enable outflow but not so large as to alter the aqueous composition.
The excimer laser trabeculostomy (ELT) concept uses a 308‑nm xenon chloride excimer laser to provide a precise and essentially nonthermal approach to improve outflow in a manner that does not provoke a healing response. ELT is performed via a clear corneal incision, sparing conjunctiva, in which direct visualization of the target tissue via a goniolens or an endoscope provides immediate feedback to the surgeon. When compared to the more invasive glaucoma surgical procedures such as trabeculectomy, ELT is almost as effective in both lowering IOP and decreasing use of pressure lowering medication and is far less traumatic.
The sparing of conjunctiva is a major advantage of this technique because success rates of a subsequent trabeculectomy would not be compromised. This microinvasive glaucoma surgery (MIGS) procedure has a high safety profile, rapid stabilization, clinical verification of long-term efficacy with minimal impact on quality of life and may also become a replacement option for topical glaucoma medicinal therapies eliminating their associated cost, compliance issues, and side effects in their long-term use.
What Are MIGS Procedures and Where Do They Fit in Glaucoma Treatment?
The invasive procedure and lifestyle-altering sequelae of trabeculectomy limit the use of this procedure to later stage and recalcitrant patients with glaucoma. Issues regarding medication costs, compliance, and toxicity of preservatives suggest these therapeutic options also have limitations. There remains an unmet patient need for treatments that could effectively treat mild to moderate glaucoma. In recent years, several MIGS procedures have been developed to address these limitations of traditional treatment options. These procedures have advantages and disadvantages, but no current technique fulfills all 5 requirements, which are
- Ab interno microincision: MIGS procedures use an ab interno microincisional approach through a clear corneal incision of less than 2 mm. This spares the conjunctiva and thus prevents significant external scarring and allows future unaffected conjunctival surgery, if needed, and direct visualization of anatomic landmarks to optimize placement of a device or incision within the angle. The procedure is easily combined with cataract surgery. A microincision also facilitates the intraoperative maintenance of the anterior chamber and retention of normal ocular anatomy, minimizes changes in refractive outcome, and adds to procedural safety.
- Minimal trauma: MIGS procedures are minimally traumatic to the target tissue, with negligible disruption of normal anatomy and physiology. Devices are biocompatible and ideally enhance physiologic outflow pathways.
- Efficacy: MIGS procedures must be effective in both lowering IOP and reducing medication use.
- High safety profile: MIGS procedures are characterized by an extremely high safety profile. Many MIGS procedures are less efficacious when compared to more invasive glaucoma surgeries, such as trabeculectomy with antimetabolites or glaucoma drainage devices. This compromise in efficacy is balanced by their low-risk profile. MIGS procedures are associated with a decreased incidence of serious complications often associated with other glaucoma surgeries including hypotony, choroidal effusions, suprachoroidal hemorrhage, anterior chamber shallowing, corneal decompensation, cataract formation, diplopia, and bleb-related complications such as bleb dysesthesia and endophthalmitis.
- Rapid recovery: MIGS procedures have a rapid recovery with minimal impact on the patient's quality of life.
Previous Meshwork Surgeries and Their disadvantages
Current MIGS procedures can successfully bypass the TM to create direct flow from the anterior chamber into SC, unlike earlier attempts to address this anatomic pathology, which often failed. Procedures using mechanical devices and lasers to perforate the TM have been shown to adequately bypass the outflow obstruction for the short-term, but have been unsuccessful in the long-term due to the amount of adjacent tissue damage related to the nature of the technique or device. The adjacent tissue damage evokes a healing response which eventually closes the channels. As laser technology evolved, each new laser was applied for this purpose, but none enabled long-term patent openings.
Krasnov et al. showed moderate success using a ruby laser (943 nm) to perform "trabeculopuncture." Other laser trabeculopuncture attempts, including Hager's use of an argon laser (488 + 514 nm) and Fankhauser's use of a Nd:YAG laser (continuous wave 1064 nm), have been unsuccessful due to scarring. These and other laser trabeculopuncture attempts limit prospective options for subsequent procedures should they become necessary as they induce destruction of local tissue and scar formation of adjacent tissue, often involving large regional areas. In addition, with large openings and markedly increased outflow, the compositional alterations of the aqueous would supplement the tissue destructive healing responses.
Following these initial attempts using lasers ab interno to perforate TM or to create full thickness sclerostomies, Wise et al. determined that a continuous wave, essentially long pulsed argon laser (488 + 514 nm) could successfully modify the TM to increase outflow without perforation. Their argon laser trabeculoplasty (ALT) procedure effectively lowered IOP, but it functioned by creating thermal damage to the target tissue, causing coagulative necrosis of the TM. In contrast to ALT, laser trabeculoplasty (LTP) is now more commonly performed with solid-state (532, frequency doubled Nd:YAG) and diode (810 nm) lasers. Studies comparing efficacy of these thermal lasers demonstrate minimal differences in efficacy, longevity, or repeatability.
The efficacy of LTP in lowering IOP is well documented. Long-term studies, however, have shown that the IOP lowering efficacy of LTP decreases over time, from 77% success rate at 1 year, to 49% at 5 years, and to 32% at 10 years. Because of the significant TM scarring caused by ALT, repeat treatments are not recommended and have not proven successful clinically. LTP causes thermal coagulative damage to the uveoscleral meshwork, disruption of the trabecular beams, and heat damage to the surrounding collagen fibers. This thermal damage is in part responsible for the inflammatory response, scarring of the TM tissue, peripheral anterior synechiae, and IOP spikes sometimes observed in eyes that have undergone LTP.
In contrast to the diffuse thermal effects of argon, solid-state, and diode laser with relatively long pulse durations in the range of 0.1 second, recent advances in LTP use lasers with shorter pulse durations of 3–10 ns. Selective laser trabeculoplasty (SLT), based on the principle of selective photothermolysis, relies on selective absorption of short laser pulses to generate and spatially confine heat to pigmented targets within TM cells. SLT uses a Q-switched, frequency-doubled 532‑nm neodymium:yttrium-aluminum-garnet (Nd:YAG) laser. Q-switching of the laser allows for an extremely brief and thus high-powered light pulse to be delivered to the target tissues. The short duration of the pulse is critical in preventing collateral damage to the surrounding tissues. The mechanisms of the pressure effects following ALT, LTP, and SLT, which alter but do not perforate TM, are yet undetermined.
Preserving the TM may become more important in the near future, as surgical techniques are developed to operate directly on SC or the juxtacanalicular TM, the region considered responsible for most of the outflow obstruction that causes open-angle glaucoma. Thermal LTP would preclude the use of the newer MIGS procedures in these patients, as their TM and adjacent tissues, including SC would be likely to have been damaged. Once methods of preoperative evaluations of the patency of the required outflow pathways are clinically useful, such patients can be better evaluated as candidates for MIGS procedures.
The Concept of ELT
Ultraviolet excimer laser photoablation enables the precise removal of targeted tissue with local and adjacent temperature control to prevent thermal damage to surrounding tissues, exemplified by the use of 193‑nm excimer lasers for corneal surface ablation, enabling successful refractive surgery. The 193‑nm wavelength, although precise and nontissue damaging, is not readily transmissible via fiberoptics and cannot be used for intracameral procedures. In contrast, 308‑nm excimer laser generated light is amenable to fiberoptic transmission and after extensive preclinical experimentation became the wavelength of choice for nonthermal, precisely targeted ab interno fistulizing procedures.
The applications of this nonthermal, ab interno, fiberoptic-delivered energy evolved initially from full thickness sclerostomy, creating a bleb via an ab interno approach, subsequently to trabeculostomy, once the parameters of the target tissue anatomy, localization of SC and ablation rates for this wavelength in this tissue were determined and idealized to enable a successful procedure to bypass TM and juxtacanalicular TM obstructions allowing and improving physiological outflow directly into SC.
Target tissue anatomic considerations to minimize healing responses include avoiding trauma to the outer wall of SC, as the outer wall endothelium contains fibroblasts whereas the inner wall endothelium does not. Avoiding trauma to the outer wall is paramount to the successful maintenance of outflow. In addition, as the space between the inner and outer walls of SC can be less than 20 microns, the accuracy of the tool to enter the inner wall and not disturb the outer wall had to be of this same scale. The ablation precision of 308 nm on this tissue, unlike lasers and devices used in earlier attempts, enable the nonthermal and accurate tissue removal, which resulted in the ELT procedure.
Initial in vitro experiments led to animal trials. In a study of the effects of 308‑nm excimer laser energy applied ab interno to the limbal sclera of rabbit eyes, long-term decreases in IOP were achievable. The use of this 308‑nm wavelength, unlike that of earlier trials with thermal lasers, enables laser-tissue interactions with significant advantages. This excimer laser is less likely to evoke a cicatricle response in the TM or sclera than those seen in trials using visible or infrared lasers, which cause thermal tissue damage and subsequent healing responses. There is minimal exposure of adjacent tissue to radiation enabled by direct contact of the laser energy to the target tissue via the fiberoptic delivery system.
With evidence that TM and scleral tissue could be successfully removed without adjacent tissue damage, without subsequent scar formation or hole closure, and with ablation accuracy that would enable precise targeting of TM, juxtacanalicular TM, and the inner wall of SC without perforating the outer wall of SC, these studies formed the basis for the development of the current ELT technology and techniques.
ELT Technique
ELT, first used clinically in 1997 by Vogel and Lauritzen, treats the pathology responsible for most open-angle glaucoma by decreasing the outflow resistance at the juxtacanalicular TM and the inner wall of SC. ELT is performed with a short-pulsed (80 ns) 308‑nm xenon-chloride (XeCl) excimer laser, which delivers photoablative energy to precisely remove the tissue that obstructs aqueous outflow with minimal thermal damage to adjacent tissue. The surgery is performed as an outpatient procedure under topical, peribulbar, or retrobulbar local anesthesia.
- Following a paracentesis and stabilization of the anterior chamber with viscoelastic, the surgeon introduces a fiberoptic probe, which is advanced across the anterior chamber to contact the TM (Figure 1).
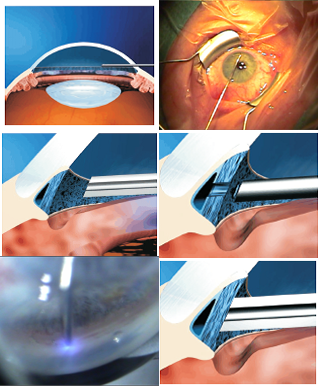
Figure 1. Drawings and photos of the excimer laser trabeculostomy procedure.
- Probe placement is controlled by direct observation using either a goniolens (Figure 2) or an endoscope (Figure 3).
Figure 2. Gonioscope view/IOP high = SC not visible.
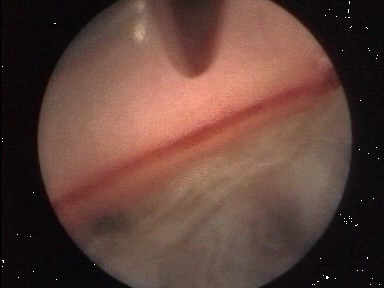
Figure 3. Endoscopic view/IOP low = SC visible, blood filled.
- Four to ten channels are created into SC.
- The probe is then removed, the viscoelastic is replaced by balanced salt solution, and the patient is monitored postoperatively.
Most commonly the probe insertion is superotemporal and the laser channels inferonasal. Variations include spacing the channels over both inferior quadrants, creating temporary hypotony following removal of the probe to enable enumeration of the number of patent channels into SC by observing an induced blood reflux followed by repressurization. The blood reflux is a common but inconsequential occurrence. No studies have shown benefit from treatment in more than one quadrant. As no single protocol has been established, each surgeon tends to create their own technique. In spite of these numerous variations, the outcomes are remarkably similar.
By means of the essentially nonthermal photoablation using the specified laser parameters, ELT evaporates human tissue, denaturing the organic structures without producing undesirable marginal necrosis. ELT excises the TM, the juxtacanalicular TM, and the inner wall of SC without damaging the outer wall of SC or the collector channels. No filtering fistula or bleb is created.
Technical Aspects of Performing an ELT Procedure
The current ELT procedure is performed with an AIDA XeCl laser (TUI-Laser AG, Germering, Germany) in the following manner:
- The laser is automated to internally calibrate and control the output fluence in accordance with the manufacturer's specifications. Unlike solid-state lasers, this XeCl gas laser requires a short "warm up" time during which the output energy is stabilized before use.
- The sterile fiberoptic delivery system is coupled to the laser. The output beam is then adjusted at the fiber tip to ensure suprathreshold fluence for tissue ablation at the fiber tip. The console includes a power meter to enable this calibration, which is performed prior to each procedure, similar to the tuning of a phaco handpiece before use.
- Topical, peribulbar, or retrobulbar local anesthesia is administered. In ELT alone, topical Pilocarpine 2% is used preoperatively to constrict the pupil. In patients with phakic intraocular lenses, Pilocarpine 2% may also assist in protecting the lens. Alternatively, intracameral Miochol (Acetylcholine 10 mg/ml) may be used.
- A 1‑mm paracentesis is created in the superotemporal perilimbal cornea 2 o'clock for left eyes and 10 o'clock for right eyes. In combined cataract and ELT procedures, the previously created phaco-tunnel may be used in a similar fashion (Figure 4).
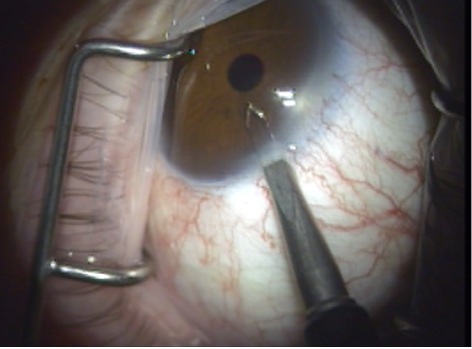
Figure 4. Paracentesis in the superotemporal perilimbal cornea.
- A viscoelastic agent (Healon) fills the anterior chamber through the paracentesis. Depending on the surgeon's preference, the IOP may be unchanged or increased by this injection, enabling or precluding visualization of SC by blood reflux (Figure 5).
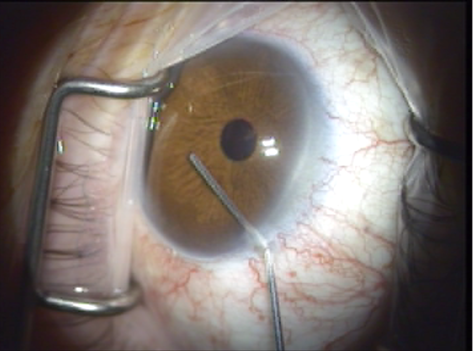
Figure 5. Viscoelastic agent is introduced.
- The laser probe is inserted into the anterior chamber through the paracentesis (Figure 6) and is advanced to the opposite chamber angle via gonioscopic (using the surgeon's preferred goniolens) or endoscopic visualization (Figure 7). The ELT fiber may be attached coaxial with an endoscope or a second paracentesis endoscopic view may be used.
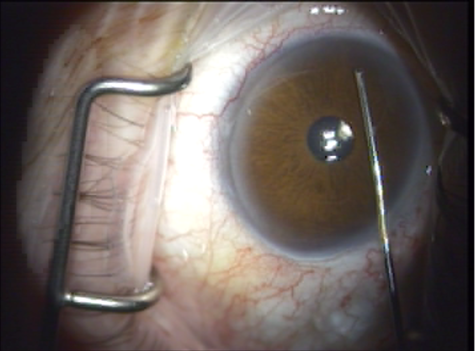
Figure 6. Laser probe is inserted through the paracentesis to cross the anterior chamber.
A 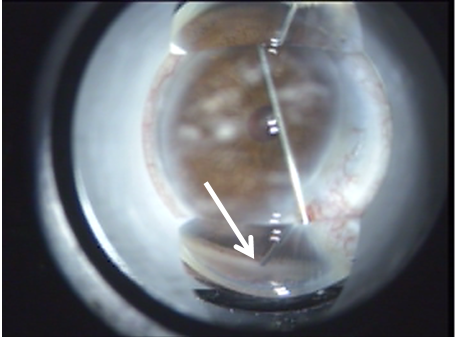
B 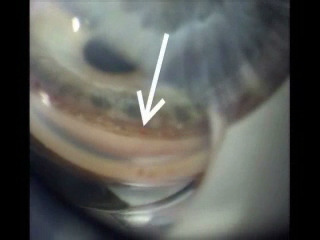
C 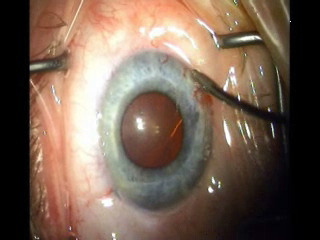
Figure 7. (A) ELT probe in contact with TM, gonioscopic view. (B) Blood in SC verifies appropriate targeting and depth, gonioscopic view. (C) Induced blood reflux verifies success and enables documentation of number of successful channels into SC.
- The fiber tip is centered on the pigmented TM and advanced to be in contact with the TM. SC is targeted whenever visible, or surgeons are advised to alternate placement of the fiber to anterior, mid, and posterior TM regions to assure some of the channels will enter into SC. The number of pulses, which controls the penetration depth, is fixed, similar to the penetration depth control in LASIK. Perforation of the inner wall of SC into SC depends on its distance from the fiber tip, which can be variable depending on the angle of placement and the amount of pressure on the fiber. The calculation of this distance was determined in numerous preclinical experiments. The current protocol consists of 10 probe placement sites on TM, a percentage of which is likely to enter SC.
- The laser is activated, delivering laser pulses at 20 Hz per treatment site. Each pulse converts the tissue at the fiber tip into gas. This gas may be seen exiting around the fiber tip during each ablation (Figure 8).
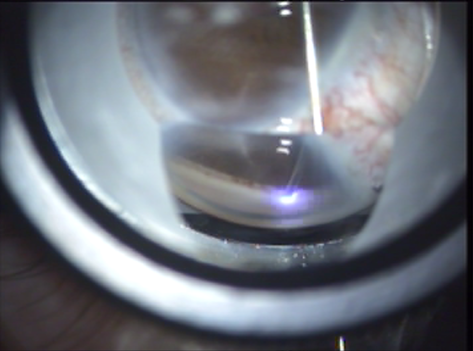
Figure 8. Laser pulse.
- As the channel is created, pulse-by-pulse, an opening is created from the anterior chamber into SC through photoablation of the TM, the juxtacanalicular TM, and the inner wall of SC. As soon as the outflow obstruction at the fiber tip is removed and SC is entered, the pathway for the gas, previously retrograde around the fiber, becomes anterograde, entering SC. This product-of-ablation gas is then assumed to dilate SC, pushing the outer wall away and concurrently dilating the adjacent surrounding collector channels. This concept of the product-of-ablation gas dilation of SC and collector channels is termed "pneumatic canaloplasty." Once adequate imaging devices are developed to visualize this process in real time, this theory can be validated.
- The probe tip is then repositioned such that ten channels are created.
- The probe is removed from the anterior chamber.
- The viscoelastic is exchanged for BSS with irrigation/aspiration, coaxial or bimanual (Figure 9).
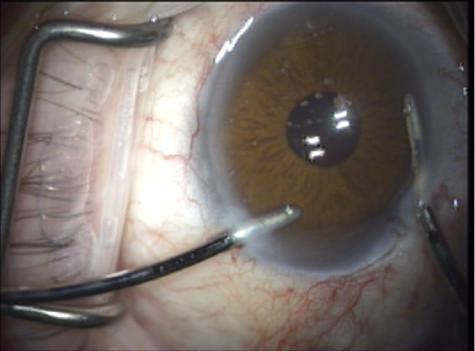
Figure 9. Viscoelastic is exchanged for BSS.
- The anterior chamber can be monitored for the number and location of patent trabeculostomy sites by blood reflux from SC during a period of iatrogenic temporarily hyopotony during the viscoelastic/fluid exchange.
Postoperative topical ophthalmic medication regimens are individualized by surgeon, most include
- A combination of steroid and antibiotic eye drops or ointment is administered immediately at the end of the procedure.
- The operated eye is shielded or patched, and the patient is released once stable, similar to after phacoemulsification.
- The operated eye is usually treated with a topical fixed combination of steroid (dexamethasone 0.1%) and antibiotic eye drops qid for one week and then tapered over 3 weeks.
- In the rare case of an anterior chamber inflammatory reaction, mydriatic eye drops may be added, and the topical steroid can be intensified.
Most surgeons discontinue all anti-glaucoma medications (AGM) after the procedure. Preoperative AGM may be continued and later reduced dependent on the postoperative IOP. When medications are reduced, IOP should be monitored carefully on a regular basis.
After ELT
Ideally, patients are monitored postoperatively with gonioscopy, in addition to IOP, and the findings documented. Channels are documented to remain patent years after the ELT procedure and in some of these patients goniolens "pumping" can induce blood to appear at the channels, further confirming the patency of these channels into SC (Figures 10 and 11).
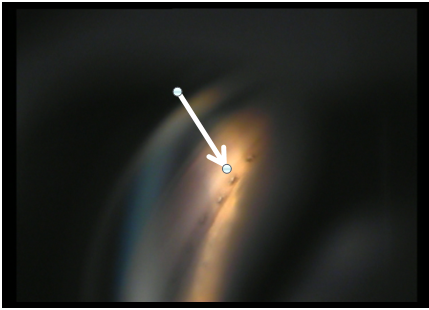
Figure 10: Patent trabeculostomy channels into SC (arrow) noted 2.5 years after ELT.
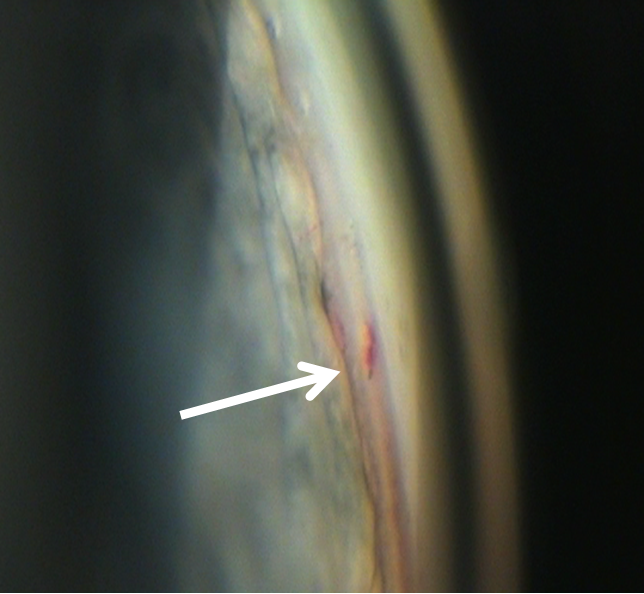
Figure 11: Blood reflux from SC induced by "pumping" the goniolens 3 years after ELT.
ELT Enables Pneumatic Canaloplasty
Another potential benefit of ELT is that it enables pneumatic canaloplasty. Both endoscopic and gonioscopic views of ELT have revealed gas bubble formation in the tissue and the anterior chamber as a result of photoablation of the TM tissue. When the ablation penetrates the outflow obstruction, gas is able to enter SC through the newly formed openings in the TM. The pressure of this gas is thought to dilate SC and collector channels to improve aqueous outflow, thereby lowering IOP. Observing gas bubbles exiting the adjacent openings confirms continuity of flow from SC.
This hypothesis has yet to be confirmed via real-time imaging or histologic studies. Real-time imaging and postmortem histologic studies, in addition to histologic studies after ELT to evaluate the long-term changes that occur subsequent to this procedure, will enable better understanding of the effects and effectiveness of the current procedure and enable suggestions for modifications to potentially further improve the outcomes (Figure 12)
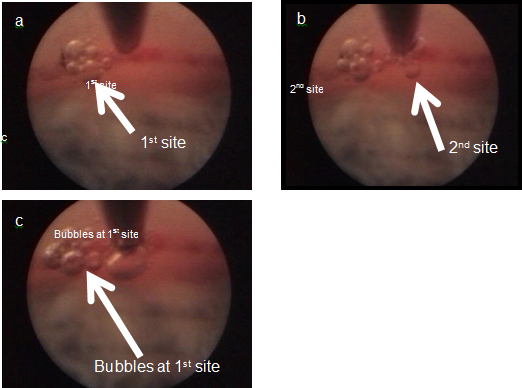
Figure 12: Photos of the ELT procedure demonstrating pneumatic canaloplasty. (A)Coaxial endoscopic view. (B) As the second channel is created into SC, bubble expansion is observed at the first ELT site (C), confirming channel patency into SC at both sites.
ELT as a MIGS Procedure
The study with the current largest sample size was published in 2006 by Pache et al.Included were 135 eyes with open-angle glaucoma or ocular hypertension after ELT alone or ELT combined with phacoemulsification. Follow up was 1 year. Separately 2 subgroups (subgroup 1 with IOP > 22 mm Hg at baseline and subgroup 2 with IOP ≤ 21 mm Hg at baseline) were analyzed.
Success (IOP ≤ 21 mm Hg, 20% reduction from baseline, AGM ≤ baseline, and no subsequent IOP-lowering surgery) was for
ELT alone in SG1 57% (baseline IOP: 27.9 ± 3.9 mm Hg; IOP at 1‑year follow-up: 19.3 ± 5.5 mm Hg)
and in SG2 41% (baseline IOP: 20.2 ± 1.1 mm Hg; IOP at 1‑year follow-up: 17.6 ± 3.3 mm Hg)
and for ELT combined with phacoemulsification,
in SG1 91% (baseline IOP: 26.4 ± 2.8 mm Hg; IOP at 1‑year follow-up: 16.7 ± 2.8 mm Hg)
and in SG2 52% (baseline IOP: 19.6 ± 1.1 mm Hg; IOP at 1‑year follow-up: 16.3 ± 2.2 mm Hg).
IOP reduction by ELT appears to be effective in both groups, but much more effective in eyes with higher baseline IOP.
Wilmsmeyer et al. investigated outcome after ELT alone (70 eyes) vs. ELT combined with phacoemulsification (60 eyes) after a follow up of 2 years in patients with open-angle glaucoma or ocular hypertension. They found a higher reduction of IOP after the combined procedure. IOP reduced from 24.1 ± 0.7 mm Hg to 16.8 ± 1.0 mm Hg at 2 years after ELT alone versus reduced from 22.4 ± 0.6 mm Hg to 12.6 ± 1.5 at 2 years after combined ELT. AGM use remained more or less unchanged.
Babighian et al. found comparable results in an ELT study with 2 years' follow-up of 21 eyes with open-angle glaucoma. Success (IOP decrease 20% with no additional medication or IOP-lowering surgery) rates were 54% and IOP was reduced from 24.8 ± 2.0 mm Hg at baseline by 7.9 ± 0.1 mm Hg at 2 years.
Töteberg-Harms et al. were the first to show simultaneous IOP reduction and reduction of AGM after combined ELT (IOP changed from 25, 33 ± 2, 85 at baseline to 16, 54 ± 4, 95 at 1 year and medications were reduced from 2, 25 ± 1, 26 at baseline to 1, 46 ± 1, 38 at 1 year). Complete success (IOP < 21 mm Hg, IOP reduction ≥ 20%, without AGM, and no subsequent IOP-lowering surgery) in their study population was 21.4% and qualified success (same as complete success but additional AGM were not excluded) 64.3% at 1 year.
Berlin et al.investigated 46 eyes after combined ELT with a follow up of 5 years. They showed that IOP was lowered (from 25.5 ± 6.3 at baseline to 15.9 ± 3.0 at 5 years) and AGM were reduced (from 1.93 ± 0.87 at baseline to 0.93±1.12 at 5 years) and that this effect remained stable over the follow-up of 5 years.
In conclusion, currently published data demonstrates that ELT can reduce IOP and lower AGM simultaneously for up to 5 years. The combined procedure, ELT plus phacoemulsification, appears to be more effective than ELT alone. The amount of IOP lowering seems to be dependent on baseline IOP and is more effective in eyes with higher baseline IOP. ELT has shown favorable outcomes in comparison to other outflow procedures (Figure 13).
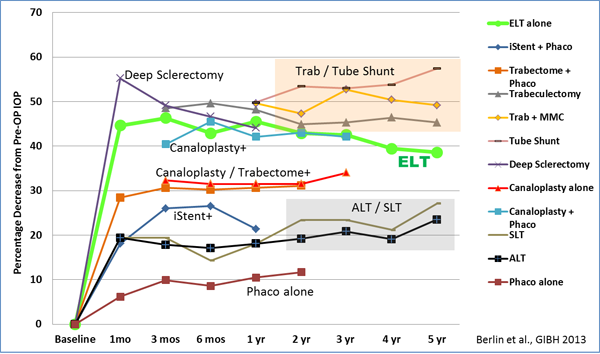
Figure 13. Comparison of various outflow procedures.
Although ELT is not comparable in laser/tissue interaction effects, as a laser treatment for glaucoma, it is likely to be considered with other glaucoma laser treatments. When compared to office laser treatments, patients undergoing SLT achieved up to a 27% postoperative decrease in IOP but a negligible decrease in medications. Patients undergoing ALT had similar results, with a maximum of 23.5% postoperative decrease in IOP and negligible decreases in medications.
ELT as an invasive surgical procedure has also shown favorable outcomes when compared to other MIGS procedures. Patients undergoing clear cornea phacoemulsification followed by ab interno gonioscopically guided implantation of one iStent, achieved an IOP decrease of 21.4% and a medication decrease of 75% one year postoperatively. Patients undergoing phacoemulsification cataract extraction combined with trabectome, achieved IOP decreases of up to 31.1% and medication decreases of 41.7%.
Most relevant, ELT has shown comparable long term IOP lowering results (decrease of 38.6% after 5 years) to significantly more invasive and risk inherent traditional operating room surgeries, trabeculectomy, and tube shunt procedures. Though trabeculectomy has demonstrated IOP decreases of 57.1% and medication decreases of up to 90%, when comparing the data obtained in the Collaborative Initial Glaucoma Treatment Study (CIGTS) on trabeculectomy patients, the 5‑year postoperative ELT IOP measurements at all time points averaged 1 mm higher than the 300 patients documented in CIGTS who underwent trabeculectomy. The intraoperative complication rate of trabeculectomy was 12%, and the one-month postoperative complication rate was 50% versus ELT intraoperative and postoperative complication rates of 0%.
Limitations and Next Generation
Feedback from leading surgeons who have performed over 2,000 ELT procedures has identified several limitations of the current ELT technique. The next generation of devices, called Guided ELT, is currently under development and is aimed to address the feedback from reporting surgeons. New sensors are being produced to better enable the surgeon to locate SC and TM and to automate the control of the laser. Tactile contact now provides feedback as to the amount of pressure applied to the meshwork. New system controls help guide the surgeon as to the depth of tissue removal and number of laser pulses required to penetrate the inner wall of SC with the addition of automated detectors and with imaging, especially real-time imaging. The ongoing development of spectral-domain optical coherence tomography shows promising abilities to meet these needs.
Conclusion
- ELT is a safe and effective method to reduce both IOP and medication use in most patients with open angle glaucoma.
- ELT is less invasive relative to current methodologies and reduces many postoperative issues, including patient discomfort, number of postoperative visits required to assure adequacy and stability, and the long-term risks of filtering procedures.
- By essentially eliminating the damaging and healing response-inducing thermal effects and tissue trauma seen with other laser and device procedures, ELT enables the lowering of IOP on a long-term basis.
- Due to the minimal tissue trauma associated with UV tissue photoablation, only a few, small channels into SC have proven adequate to control the IOP.
- Unlike trabeculectomy, ELT preserves the integrity of the meshwork and SC, which restores the natural outflow without the use of blebs or invasive foreign body implants.
- ELT is an important adjunct to cataract surgery, allowing physicians to address 2 pathologies in 1 surgical intervention without cutting the conjunctiva.
- ELT has been approved for use in the European Union and Switzerland since 1998. Thousands of ELT procedures have been successful in lowering and maintaining lower IOP for years. Clinical studies are pending in both Canada and the United States.
Suggested Reading
- Krasnov MM. Laseropuncture of anterior chamber angle in glaucoma. Am J Ophthamol. 1973;75(4):674-678.
- van der Zypen E, Fankhauser F, Bebie H, Marshall J. Changes in the ultrastructure of the iris after irradiation with intense light. A study of long-term effects after irradiation with argon ion, Nd:YAG and Q-switched ruby laser. Adv Ophthalmol. 1979;39:59-180.
- Beckman H, Kinoshita A, Rota AN, Sugar HS. Transscleral ruby laser irridation of the ciliary body in the treatment of intractable glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1973;46(2):423-436.
- Wise JB. Long-term control of adult open angle glaucoma by argon laser treatment. Ophthamology. 1981;8(3):197-202.
- Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT) and glaucoma laser trials follow-up study: 7. Results. Am Journal Ophthalmol. 1995;120(6):718-731.
- Odberg T, Sandvik L. The medium and long-term efficacy of primary argon laser trabeculoplasty in avoiding topical medication in open angle glaucoma. Act Ophthalmol Scand. 1999;77(2):176-181.
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of IOP and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268-1279.
- Damji KF, Shah KC, Rock WJ, Bains HS, Hodge WG. Selective laser trabeculoplasty v argon laser trabeculoplasty: a prospective randomised clinical trial. Br J Ophthalmol. 1999;83(6):718-722.
- Jacobi PC, Dietlein TS, Krieglstein GK. Prospective study of ab externo erbium:YAG laser sclerostomy in humans. Am J Ophthalmol. 1997;123(4):478-486.
- Iwach AG, Hoskins HD Jr, Mora JS, et al. Update on the subconjunctival THC:YAG (holmium) laser sclerostomy ab externo clinical trial: a 4-year report. Ophthalmic Surg Lasers. 1996;27(10):823-831.
- Latina MA, Park C. Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW interactions. Exp Eye Res. 1995;60(4):359-371.
- Tripathy RC. Ultrastructure of schlemm's canal in relation to aqueous outflow. Exp Eye Res. 1968;7(3):335-341.
- Berlin MS, Rajacich G, Duffy M, Grundfest W, Goldenberg T. Excimer laser photoablation in glaucoma filtering surgery. Am J Ophthalmol. 1987;103(5):713-714.
- El Sayyad F, Helal M, El-Kholify H, Khalil M, El-Maghraby A. Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology. 2000;107(9):1671-1674.
- Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: Randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407-412.
- Matlach J, Freiberg FJ, Leippi S, Grehn F, Klink T. Comparison of phacotrabeculectomy versus phacocanaloplasty in the treatment of patients with concomitant cataract and glaucoma. BMC Ophthalmol. 2013(29);13:1.
- Spiegel D, Wetzel W, Neuhann T, et al. Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery. Eur J Ophthalmol. 2009;19(3):393-399.
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL; Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789-803.
- Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: Three-year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open-angle glaucoma. J Cataract Refract Surg. 2011;37(4):692-690.
- Lichter P, Musch D, Gillespie B, et al., Collaborative Initial Glaucoma Treatment Study Group. Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943-1953.
- Jampel HD, Musch DC, Gillespie BW, et al.; Collaborative Initial Glaucoma Treatment Study Group. Perioperative complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2005;140(1):16-22.