Authors: Lingmin (Lisa) He, MD; Mark S. Blumenkranz, MD
Retinal vein occlusion (RVO) is one of the most common causes of retinal vascular disease, with an estimated prevalence of 16 million worldwide. There can be significant associated vision loss related to the development of macular edema or neovascularization as a response to retinal ischemia. Lasers have successfully been used since the 1970s to treat these sequelae of vein occlusions. Although anti-VEGF agents are increasingly being used as first-line agents for the treatment of vision loss in this disorder, lasers continue to play an important role in the management of these conditions. Branch and central retinal vein occlusions have different pathophysiologic mechanisms and so will be addressed separately.
Branch Retinal Vein Occlusion (BRVO)
The mechanism of this disease is thought to be thickening of the arterial wall causing compression of the vein, turbulent flow, damage to the vascular endothelium, and eventually thrombosis of the vein. The build up of hydrostatic venous pressure then contributes to areas of capillary nonperfusion and intraretinal hemorrhages. Risk factors for this condition are systemic hypertension, cardiovascular disease, increased body mass index and history of glaucoma. Vision loss can be caused by macular edema, foveal ischemia, or neovascularization and secondarily vitreous hemorrhage, alone or in concert with one another. Other causes of vision loss include epiretinal membrane formation, subretinal fibrosis, retinal arteriolar macroaneurysms and tractional retinal detachments. The major prognostic factor for visual outcomes is the extent of initial ischemia and whether or not the areas of nonperfusion involve the fovea.
The Branch Vein Occlusion Study (BVOS) in the 1980s established laser treatment as the standard of care for the treatment of vision loss associated with macular edema and for the prevention of vitreous hemorrhage in this disease, and it remains an effective treatment option up to the present.
Grid Photocoagulation
The BVOS showed that patients with edema secondary to BRVO and vision reduced to 20/40 or worse had significant improvements in vision––on average almost twice the number of patients gained 2 lines or more of vision ––if treated with grid photocoagulation compared to untreated controls. Generally, the patient was observed over a period of 3 months prior to attempted treatment, as natural history studies of the disease demonstrate that the edema may frequently resolve without intervention. If the edema is refractory, then a light grid is applied over the areas that on fluorescein angiogram appear ischemic and/or demonstrate vascular leakage. The laser should avoid the foveal avascular zone. The treatment parameters in the study were with an argon green laser of 514 nm. Current semiconductor green 532 nm or yellow 577 nm lasers with spot size of 50-100 µm, exposure of 20-100 ms are employed and the power is titrated until mild whitening is visible.
While laser photocoagulation is generally very safe, there are complications than can result from its use. Heat transmission to the photoreceptors can lead to a scotoma and inadvertent foveal burns can cause significant central visual loss. Care must be taken to always be aware of the foveal center and some clinicians/patients prefer to have peribulbar anesthesia to avoid this. Laser burns have been shown to expand over years and this atrophic creep can compromise central vision if treatment is performed too close to the fovea. When high levels of laser energy are applied over a small area of the retina, Bruch’s membrane ruptures can occur that can lead to subretinal hemorrhage and the development of choroidal neovascularization. A fibrous reaction can occur in response to the photocoagulation and lead to retinal tears or striae. Treatment over blood vessels can lead to rupture and preretinal or vitreous hemorrhage.
One innovation to reduce complications has been use of shorter duration pulses of 10-20ms to more precisely deliver energy to the retinal pigment epithelium. As thermal energy does not spread as far, there is less collateral damage. Lesions created with shorter pulses have been shown to contract rather than expand over time with remodeling of the surrounding photoreceptors. A trial of these shorter duration pulsed treatments, in addition to the application of the laser in an automated pattern, has been shown to be effective in treating the macular edema associated with BRVOs.
The optimal wavelength of the laser has been under investigation because of tissue absorption properties. Green wavelengths (514 nm argon and 532 nm Nd:YAG) are absorbed well by melanin and hemoglobin, yellow (570 nm) is similar but may have less xanthophyll absorption. Red (647 nm) and infrared lasers may penetrate dense cataracts and hazy media better but may spread more deeply into the choroid and cause more pain. The trials to treat macular edema generally have used the green to yellow wavelengths.
There is also investigation into the use of subthreshold micropulse 810-nm laser––with short bursts of energy on the order of 0.1 ms delivered within the pulse length set––to treat macular edema secondary to BRVO. Preliminary work has shown that it may be as effective as conventional therapy without visible burns, although this has yet to be tested in a head-to-head randomized comparison to conventional treatment in this condition. Because there is no visible laser burn, it is difficult to titrate treatment, although software programs allow for the creation of visible corners in grid treatments.
There has also been interest in the development of pharmacotherapeutics, given that complications can arise from laser treatments. Because of their anti-inflammatory effects and their ability to inhibit the breakdown of the blood-retinal barrier, corticosteroids were investigated to treat macular edema in vein occlusions. The BRVO arm of the Standard Care versus Corticosteroid for Retinal Vein Occlusion (SCORE) study compared intravitreal steroid injections with standard of care (laser treatment). It showed that grid laser had a beneficial effect over the first year and a more durable response over 3 years than intravitreal injections of triamcinolone 1 mg and 4 mg, and it also had fewer side effects, such as cataract, endophthalmitis, and elevated intraocular pressures. Additional investigations into the use of steroids as an adjunct to grid laser photocoagulation showed a small additional reduction in macular edema that quickly wore off.
Physicians have started to use intravitreal injections of vascular endothelial growth factor (VEGF) inhibitors such as ranibizumab, bevacizumab, and aflibercept. The Branch Retinal Vein Occlusion study (BRAVO) compared injections of 0.3 or 0.5 mg ranibizumab to sham injections for treating patients with macular edema secondary to BRVOs and showed that visual acuity and central macular thickness measurements were significantly better for patients receiving drug injections after 6 months and 1 year. Laser, however, was used in all groups if patients had vision worse than 20/40 and unresolved macular edema or less than 5 letters of improvement in vision, so no direct comparison was made between laser and the drug treatment. Other studies investigating the efficacy of grid laser photocoagulation in conjunction with intravitreal bevacizumab have shown that a combined treatment may reduce the number of injections.
Our practice is currently to offer patients an intravitreal anti-VEGF agent injection if macular edema is present at the time of diagnosis. If the edema resolves with only injections, then patients will usually continue on a monthly regimen with extension on an individualized basis. However, if the edema is refractory, then they are offered grid laser treatment.
Scatter Panretinal Photocoagulation
Generally, scatter panretinal or sectoral photocoagulation should not be applied unless patients develop neovascularization (either retinal, optic nerve, or iris). This more commonly occurs in central retinal vein occlusions (CRVOs) as the ischemia is more widespread, but occurs in 40% of patients with BRVOs who have more than 5 disc diameters of retinal ischemia. As in diabetes, panretinal photocoagulation can induce the regression of neovascularization and reduce the development of vitreous hemorrhage by 50%.
Traditionally, laser treatment parameters have been with argon green, 200-500 um spots, 50-200 ms duration, and power titrated up from 200 mW to the endpoint of a visible white retinal lesion. Significant complications––similar to those seen after photocoagulation for diabetic retinopathy––can develop after treatment, including loss of night vision, inadvertent foveal burn, scotomas, loss of peripheral vision, loss of accommodation, and anterior segment ischemia if treatment is applied over the long posterior ciliary arteries and nerves. Breaks in Bruch’s membrane and subsequent choroidal neovascularization can also develop, along with worsening of macular edema.
Automated pattern scanning lasers with shorter pulse durations as low as 20 ms and the ability to deliver multiple spots with regular spacing have been recently developed (PASCAL, Topcon Medical Laser Systems and Mc-500 Vixi, Nidek Co.). While the efficacy of these shorter pulsed treatments have been investigated in diabetic retinopathy, they have not been compared directed to conventional treatment for vein occlusions (Figure 1).
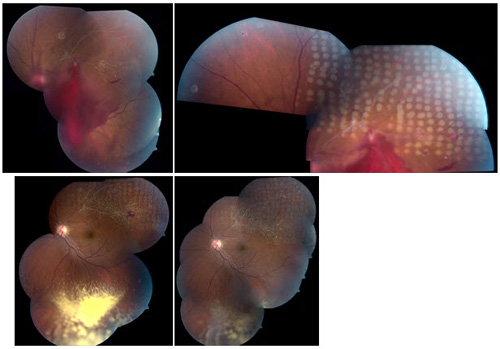
Figure 1. Treatment of neovascularization associated with vitreous hemorrhage in ischemic BRVO with sectoral scatter photocoagulation.
Central retinal vein occlusion (CRVO)
CRVOs can take on a mild, nonischemic form or a severe, ischemic form with a much worse visual prognosis. The best predictor of final visual acuity is the vision at presentation with 80% of patients presenting with worse than 20/200 vision unable to achieve improvement. The etiology of this condition is thought to be thrombosis of the central retinal vein posterior to the lamina cribosa. Risk factors are hypertension, diabetes, and open angle glaucoma. Occasionally, patients with clotting disorders, dysproteinemias, and vasculitis can develop CRVOs. Subsequent to the inciting event, approximately 16% of patients developed neovascularization of the iris and/or angle which can cause difficult to manage neovascular glaucoma. The main risk factor in the development of neovascularization is the extent of ischemia (Figure 2).
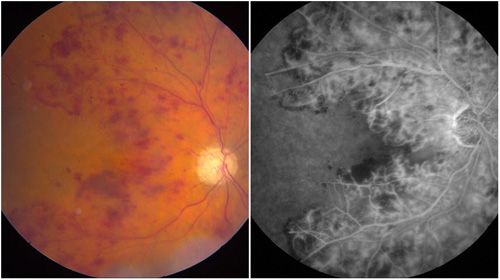
Figure 2. Ischemic CRVO with severe nonperfusion and iris neovascularization.
Grid Photocoagulation
Unlike in BRVOs, there is a very limited roll for grid photocoagulation to treat the macular edema that develops after CRVOs. The Central Vein Occlusion Study Group (CVOS) showed that while macular edema was reduced, there was no improvement in visual acuity after grid treatment and no difference in final visual outcome compared to control eyes. Therefore, observation became the standard of care for the treatment of macular edema following central retinal vein occlusions.
As laser photocoagulation did not improve visual outcomes, the focus for the treatment of CRVOs has been on pharmacologic agents. Steroids and anti-VEGF agents are thought to reduce vascular permeability and therefore reverse macular edema that can lead to vision loss. The SCORE-CRVO trial showed that patients treated with triamcinolone 1 mg had equal gains in visual acuity but fewer steroid-related side effects than those treated with 4 mg of the drug, and both groups had better visual acuity than patients who were in the observation group. The CRUISE study showed that monthly injections of ranibizumab 0.3 mg and 0.5 mg were able to significantly improve the proportion of patients gaining more than 15 ETDRS letters of best corrected visual acuity with very few side effects over 1 year of follow up. The HORIZON extension study showed that the vision became worse in patients who were followed at 3-month intervals over the second year. The COPERNICUS and GALILEO trials have shown visual improvement when patients are treated with aflibercept on an initial monthly basis. Ongoing investigation in the RELATE trial seeks to determine if there is an additive benefit from laser treatment.
Scatter Panretinal Photocoagulation
The CVOS randomized patients with ischemic CRVOS to prophylactic scatter photocoagulation or laser after neovascularization developed and showed that there was no significant decrease in the development of neovascularization when patients were treated early prior to the onset of neovascularization. Given that complications, such as loss of peripheral vision, can arise from laser treatment and that late treatment was associated with a 4-fold higher chance of not developing further neovascularization, the recommendation is that treatment is delayed until there is visible evidence of iris neovascularization––at least 2 clock hours by gonioscopy. Even in the age of anti-VEGF therapies, laser is still a mainstay of therapy when neovascular glaucoma develops. The common practice is to give the injection to quickly induce regression of the vessels and then scatter laser is applied to permanently reduce the production of VEGF (Figure 2). Laser settings are similar to those used for PRP diabetic retinopathy and for neovascularization after branch retinal vein occlusions (Figure 3).
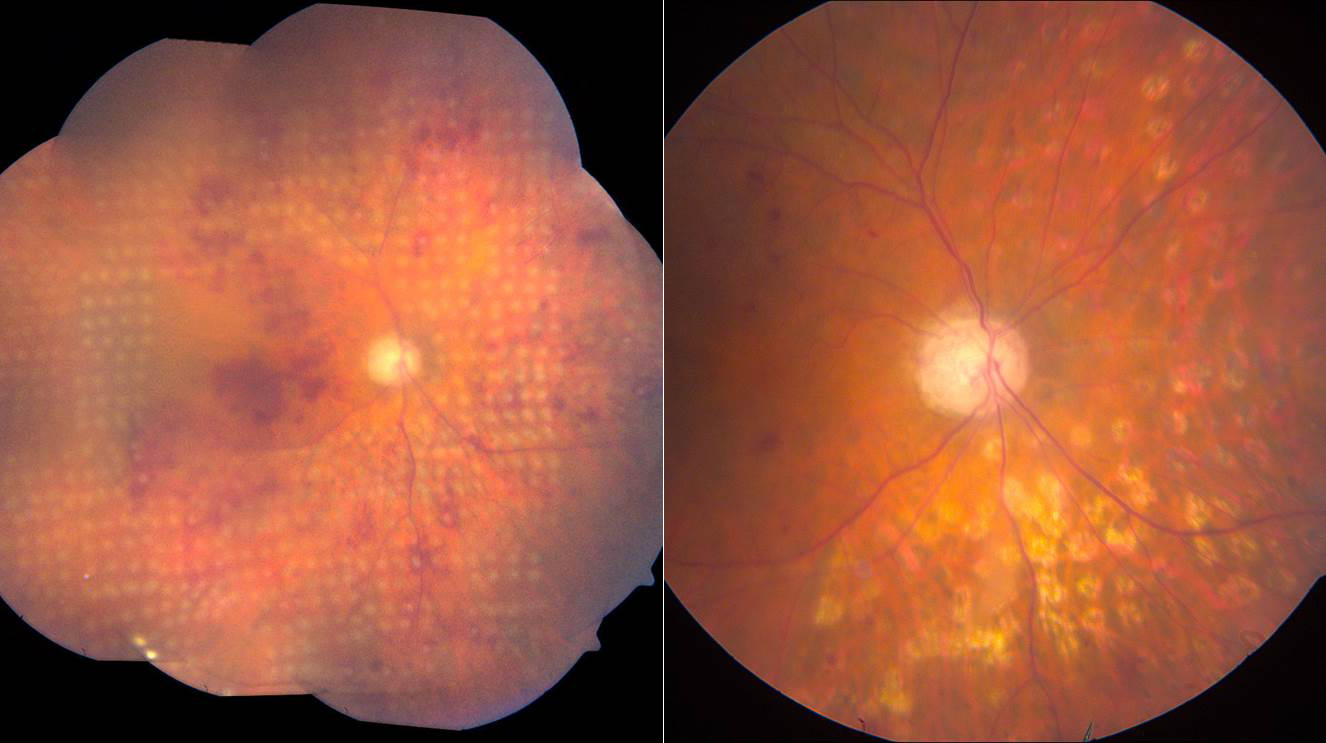
Figure 3. Ischemic CRVO immediately and 3 months following PRP.
Experimental Applications
Lasers have been used to purposefully create an anastomosis between a retinal vein and the choroidal circulation in order to allow the drainage of the obstructed vein and therefore preventing further endothelial damage, reducing venous hydrostatic pressure, and reducing ischemia. These laser-induced chorioretinal venous anastomosis can be complicated by the development of distal venous segment closure and additional ischemia leading to choroidal, subretinal, and intraretinal neovascular membranes, vitreous hemorrhage, and even worsening vision loss. Other groups have shown fewer complications when the wall of the vein is not ruptured. The Central Retinal Vein Bypass Study is the first randomized controlled study investigating the efficacy of this treatment. One hundred thirteen patients who had between 3- and 12-months onset of nonischemic CRVO were randomized to laser-induced chorioretinal venous anastomosis or sham treatment. They used a custom-built argon green laser (or Nd:YAG laser if the argon was unsucessful) with a spot size of 50 µm, 100 ms duration, and power from 3.5 to 6.0W to apply spots to rupture Bruch’s membrane and a superior and an inferior retinal vein within 2-5 disc diameters of the optic nerve. After 18 months, treated eyes had significantly better visual acuity than controls, but 18.2% developed neovascularization at the anastomosis site and required treatment with additional photocoagulation.
Suggested Reading
- Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313-319.
- The Eye Disease Case-control Study Group. Risk factors for branch retinal vein occlusion. Am J Ophthalmol. 1993;116(3):286-296.
- The Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98(3):271-282.
- Battaglia Parodi M, Saviano S, Bergamini L, Ravalico G. Grid laser treatment of macular edema in macular branch retinal vein occlusion. Doc Ophthalmol. 1999;97(3-4):427-431.
- McIntosh RL, Mohamed Q, Saw SM, Wong TY. Interventions for branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114(5):835-854.
- Morgan CM, Schatz H. Atrophic creep of the retinal pigment epithelium after focal macular photocoagulation. Ophthalmology. 1989;96(1):96-103.
- Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109(11):1549-1551.
- Blumenkranz MS, Yellachich D, Andersen DE, et al. Semiautomated patterned scanning laser for retinal photocoagulation. Retina. 2006;26(3):370-376.
- Palanker DV, Blumenkranz MS, Marmor MF. Fifty years of ophthalmic laser therapy. Arch Ophthalmol. 2011;129(12):1613-1619.
- Paulus YM, Jain A, Gariano RF, et al. Healing of retinal photocoagulation lesions. Invest Ophthalmol Vis Sci. 2008;49(12):5540-5545.
- Jain A, Blumenkranz MS, Paulus Y, et al. Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Arch Ophthalmol. 2008;126(1):78-85.
- Pitcher JD, 3rd, Liu T, Prasad PS, Schwartz SD, Hubschman JP. Short-duration focal pattern grid photocoagulation for macular edema secondary to branch retinal vein occlusion. Semin Ophthalmol. 2012;27(3-4):69-72.
- James C Folk JSP. Laser Photocoagulation of the Retina and Choroid. San Francisco: American Academy of Ophthalmology. 1997.
- Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: evolution and clinical applications. Surv Ophthalmol. 2010;55(6):516-530.
- Luttrull JK, Dorin G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Curr Diabetes Rev. 2012;8(4):274-284.
- Parodi MB, Spasse S, Iacono P, Di Stefano G, Canziani T, Ravalico G. Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology. 2006;113(12):2237-2242.
- Moorman CM, Hamilton AM. Clinical applications of the MicroPulse diode laser. Eye (Lond). 1999;13(Pt 2):145-150.
- Friberg TR, Karatza EC. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology. 1997;104(12):2030-2038.
- Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115-1128.
- Cakir M, Dogan M, Bayraktar Z, et al. Efficacy of intravitreal triamcinolone for the treatment of macular edema secondary to branch retinal vein occlusion in eyes with or without grid laser photocoagulation. Retina. 2008;28(3):465-472.
- Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 117(6):1102-1112.
- Donati S, Barosi P, Bianchi M, Al Oum M, Azzolini C. Combined intravitreal bevacizumab and grid laser photocoagulation for macular edema secondary to branch retinal vein occlusion. Eur J Ophthalmol. 2012;22():607-614.
- Diabetic Retinopathy. In: Preferred Practice Pattern® Guidelines. San Francisco, CA: American Academy of Ophthalmology; 2008.
- The Central Vein Occlusion Study Group. Baseline and early natural history report. Arch Ophthalmol. 1993;111(8):1087-1095.
- The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115(4):486-491.
- Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Trans Am Ophthalmol Soc. 1981;79:371-422.
- The Eye Disease Case-Control Study Group. Risk factors for central retinal vein occlusion. Arch Ophthalmol. 1996;114(5):545-554.
- Hayreh SS, Rojas P, Podhajsky P, Montague P, Woolson RF. Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90(5):488-506.
- The Central Vein Occlusion Study Group M report. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology. 1995;102(10):1425-1433.
- Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127(9):1101-1114.
- Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 117(6):1124-1133.
- Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119(4):802-809.
- Holz FG, Roider J, Ogura Y, et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. British J Ophthalmology. 2013;97(3):278-284.
- Brown DM, Heier JS, Clark WL, et al. Intravitreal Aflibercept Injection for Macular Edema Secondary to Central Retinal Vein Occlusion: 1-Year Results From the Phase 3 COPERNICUS Study. Am J Ophthalmol. 2012;155(3):429-437.
- Aref AA, Scott IU. Management of macular edema secondary to central retinal vein occlusion: an evidence-based update. Adv Ther. 2011;28(1):40-50.
- The Central Vein Occlusion Study Group N report. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. Ophthalmology. 1995;102(10):1434-1444.
- Shah AM, Bressler NM, Jampol LM. Does laser still have a role in the management of retinal vascular and neovascular diseases? Am J Ophthalmol. 2011;152(3):332-339.
- McAllister IL, Constable IJ. Laser-induced chorioretinal venous anastomosis for treatment of nonischemic central retinal vein occlusion. Arch Ophthalmol. 1995;113(4):456-462.
- McAllister IL, Douglas JP, Constable IJ, Yu DY. Laser-induced chorioretinal venous anastomosis for nonischemic central retinal vein occlusion: evaluation of the complications and their risk factors. Am J Ophthalmol. 1998;126(2):219-229.
- Leonard BC, Coupland SG, Kertes PJ, Bate R. Long-term follow-up of a modified technique for laser-induced chorioretinal venous anastomosis in nonischemic central retinal vein occlusion. Ophthalmology. 2003;110(5):948-954.
- McAllister IL, Gillies ME, Smithies LA, et al. The Central Retinal Vein Bypass Study: a trial of laser-induced chorioretinal venous anastomosis for central retinal vein occlusion. Ophthalmology. 2010;117(5):954-965.