Authors: Roger F. Steinert, MD, and Ann Z. McColgin, MD
Figures and portions of the text were previously published in Tasman W, Jaeger EA. Duane's Ophthalmology. Philadelphia, PA: Lippincott Williams & Wilkins. 2009.
Introduction
The development of excimer laser ablation represented a breakthrough in the correction of ametropia. Photorefractive keratectomy (PRK) employs a 193-nm argon fluoride excimer laser to ablate the anterior corneal stroma to a new radius of curvature to decrease refractive error. In the early 1990s, PRK became a common technique worldwide for treating low to moderate myopia because it offered a wider treatment range and more predictable and stable results than incisional keratotomy. PRK has been the subject of extensive investigations, with ongoing improvements in hardware and technique. Laser in situ keratomileusis (LASIK) uses PRK technology but performs the procedure under a lamellar flap formed with a microkeratome or femtosecond laser.
The background, preoperative evaluation, surgical technique, clinical results, and complications of PRK, and its more recent variants, LASEK, Epi-LASIK, and Epi-LASEK are reviewed. The current techniques for these surface treatments are sometimes collectively termed advanced surface ablation (ASA).
Background
Trokel et al. and Srinivasan demonstrated a new form of laser–tissue interaction, termed photoablation, in 1983. Srinivasan, an IBM engineer, was studying the far-ultraviolet (UV; 193 nm) argon fluoride excimer (a contraction of "excited dimer," the photochemical process that emits the laser photons) laser for photoetching of computer chips when Trokel, an ophthalmologist, postulated and proved that a similar process could remove corneal tissue discretely and precisely with minimal damage to the adjacent cornea. The original observation of the effect of ultrashort UV light on the cornea is attributed to a civilian contractor, John Taboada, who was studying potential laser toxicity for the military. Trokel recognized the potential of the excimer laser for refractive and therapeutic corneal surgery.
Photoablation occurs because the cornea has an extremely high absorption coefficient at 193 nm. A single 193 nm photon has sufficient energy to break the carbon–carbon and carbon–nitrogen bonds that form the peptide backbone of the corneal collagen molecules. Excimer laser radiation ruptures the collagen polymer into small fragments, and a discrete volume of corneal tissue is expelled from the surface with each laser pulse.
Initially, investigators studied whether the excimer laser could be used as a "laser scalpel" for corneal surgery in procedures such asl astigmatic and radial keratotomy (Figure 1). The excimer laser is a poor replacement for a cutting scalpel because the laser removes tissue, creating a "kerf"—it does not incise the tissue.
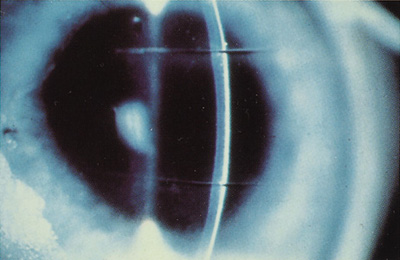
Figure 1. Two linear deep incisions created by the excimer laser in an ex vivo human cornea.
The more successful application of the excimer laser for correcting ametropia is the sculpting or reshaping of the outer de-epithelialized surface of the cornea to alter its refractive power. This surgical procedure, termed photorefractive keratectomy (PRK) by Marshall and Trokel, was the focus of extensive preclinical investigations before being applied to sighted human eyes. Early animal studies showed normal wound healing in laser-ablated corneas, and as the laser and delivery system technology matured, confidence grew that sighted ametropic human eyes could successfully undergo PRK.
McDonald et al. treated the first sighted human eye in 1988. PRK's popularity rapidly faded when LASIK was popularized in the late 1990s, primarily because LASIK offered a faster visual recovery and less postoperative discomfort. Although LASIK procedures outnumber PRK and its variants, surface ablation has returned as an attractive alternative in specific indications such as very low corrections and thin corneas.
LASIK flaps were thought to be associated with increased postoperative higher-order optical aberrations than PRK, and postoperative quality of vision was considered better with wavefront-guided PRK than with LASIK. Consequently, some surgeons advocated a complete return to surface ablation and that LASIK should be avoided altogether for standard corneal refractive surgery.
Studies have shown an increase in aberration levels after LASIK. Recent data, however, suggests that both techniques are effective with equal vision and equal higher order aberrations at a 6 month postoperative time point. Both PRK and LASIK can provide successful refractive outcomes.
Preoperative Evaluation
When considering refractive surgery, several issues must be evaluated preoperatively. Evaluation of pre-existing ocular or systemic conditions that could interfere with healing or the predictability of the procedure; Evaluation of the patient's refractive status, including stability, degree of refractive error, and astigmatism; and Evaluation of the patient's goals and expectations.
Obtain the patient's full medical history, review the systems, and perform a complete ocular examination to rule out the presence of any conditions in which refractive surgery is contraindicated.
A history of collagen-vascular and autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosis, and Sjögren's syndrome, are considered contraindications to PRK due to unpredictable corneal wound healing with the potential for corneal melting. A history of keloid scar formation and prior herpes simplex keratitis are relative contraindications. Systemic antiviral prophylaxis begun preoperatively and continued for several months postoperatively may make PRK safe in patients with prior herpes simplex keratitis. Diabetes must be well controlled preoperatively because it involves instability of refraction and potentially poor wound healing. Dense amblyopia is a relative contraindication because the refractive procedure puts the patient's better eye at risk.
Patients should wear protective spectacles rather than undergo surgery to reduce spectacle use.
A full ocular examination is mandatory. An external examination should consider the orbital anatomy. A deep-set globe, high brow, and/or a narrow palpebral fissure may favor PRK and LASEK over LASIK, Epi-LASIK, or Epi-LASEK, because with the latter techniques, it may be difficult or impossible to access the microkeratome and apply the suction ring or patient interface.
Examination of extraocular movements is important because the presence of a phoria or tropia could result in postoperative diplopia.
Pupil size should be measured because a large pupil may contribute to glare and haloes postoperatively, although improved understanding of dim light vision symptoms suggests that corneal aberrations (not the pupil size itself) are the principal issue. A commercial pupillometer can accurately assess pupil size under mesopic or scotopic conditions.
Eye dominance is tested if the patient desires monovision (in monovision corrections, typically the dominant eye is corrected for distance vision). Eyelids and tear film should be evaluated for blepharitis or dry eyes that should be treated preoperatively. Cyclosporin-A has been found to be effective in treating dry eye resulting from refractive surgery.
Examination of the cornea should reveal corneal scars, vessels, or previous inflammation. Patients with epithelial basement membrane dystrophy (EBMD, often termed map-dot-fingerprint dystrophy) are better candidates for PRK as PRK may be therapeutic, by enhancing epithelial adhesion, whereas the microkeratome friction in LASIK may cause a frank epithelial defect.
Preoperative corneal pachymetry is mandatory for all patients. Central corneal thickness is determined by ultrasonic pachymetry, or with a Scheimpflug, or scanning slit topographer. Corneal pachymetry can be a screening test for preexisting keratoconus or other types of corneal ectasia (see below), and can determine the amount of corneal tissue available for excimer tissue ablation without risk of weakening the cornea where postoperative ectasia and irregular astigmatism become significant risks. The cornea must have at least 250 µ or 50% of the original thickness (whichever is greater) of the residual stromal bed under a LASIK flap. If the flap thickness plus tissue ablation depth are calculated to leave inadequate residual stromal thickness, LASIK is contraindicated and surface ablation is preferable.
Examine the lens to rule out an early cataract. Perform a dilated-fundus examination to rule out preexisting peripheral retinal degeneration or peripheral holes that should be treated preoperatively.
Computed corneal topography is vital in the preoperative evaluation of refractive surgery patients. Corneal topography is useful in the evaluation of irregular astigmatism. Corneal warpage can occur with the use of contact lenses, and its presence will interfere with the accuracy of preoperative refractive measurements. Soft contacts should not be worn for a minimum of 3 days, and hard contacts should be avoided for at least 2 weeks prior to the preoperative evaluation. If corneal warpage is suspected, the patient should not wear contact lenses until the topography is stabilized (Figure 2).
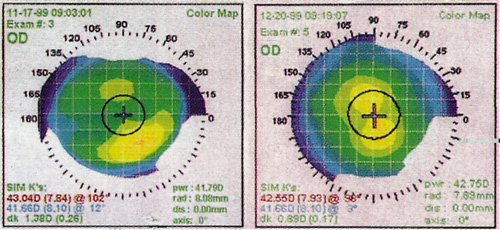
Figure 2. Left: Corneal topography of a patient with corneal warpage from hard contact lens wear. Right: One month after the contact lenses were discontinued, the warpage has resolved.
Corneal topography can detect frank or subclinical (forme fruste) keratoconus (Figure 3). Successful PRK in keratoconus suspects has been reported, however, presence of keratoconus is generally considered a contraindication to PRK because the removal of additional tissue from an already ectatic cornea may cause further ectasia and unpredictable results.
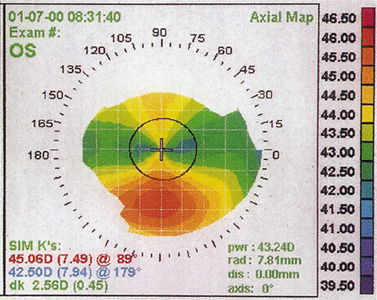
Figure 3. Corneal topography of a forme fruste keratoconus. Note the inferior corneal steepening.
When keratoconus is suspected on topography but is not visible on examination, 2 tests are helpful. The topography should be repeated with the patient looking slightly superiorly (one or two rings on the placido disc). If the patient has true keratoconus, the abnormality of eccentric inferior steepening will persist. If the patient has a slightly inferiorly shifted corneal apex rather than a true keratoconus, the pattern will become symmetric with a slight upgaze.
The second test is corneal pachymetry. In true keratoconus, the central cornea will be thinner than average and will thin slightly more when the pachymetry is repeated 1–2 mm inferior to the corneal center.
Alternatively, anterior segment optical coherence tomography allows for a pachymetry map of the entire cornea, which can be useful in evaluating relative corneal thickness. Current topography devices often include a readout of the posterior corneal topography. An abnormal amount of anterior curvature of the posterior cornea may indicate corneal ectasia. A retinoscope should be used to determine whether a "scissoring" light reflex is present, indicating an increased likelihood of keratoconus.
Careful evaluation of the patient's refractive status is critical. Patients should be 18 years of age or older, and the refraction should be stable to within +/- 0.5 diopter (D) over the previous year. Visual acuity should be checked without correction, as well as with the patient's current spectacles.
Manifest refraction and cycloplegic refraction must be performed. Corneal topography is a useful in evaluating astigmatism. Clinically significant disparities between the manifest and cycloplegic refractions must be evaluated. Most patients will have a cycloplegic refraction between 0.25 and 1.0 D shifted in the hyperopic direction. Because this disparity can be attributed to the optics of the peripheral cornea and a small posterior shift of the crystalline lens with cycloplegia, laser input may be planned based on the manifest refraction if it has been performed with a careful defogging technique.
A disparity between the manifest and cycloplegic refractions of more than 1 D of sphere warrants re-evaluation, often including a postcycloplegic manifest refraction "pushing plus" to overcome accommodative spasm. Accommodative spasm is common in myopes who are "over-minused" in their contact lenses or spectacles (extra minus causes a slight minification of the image, which causes a perception of higher contrast) and in hyperopes who have habitual accommodation to improve distance vision.
A cycloplegic refraction is mandatory in middle-aged patients because surprising amounts of accommodative spasm may remain. Tropicamide 1% is usually strong enough to relax accommodation in patients over 40, but cyclogyl 1% may be advisable in younger patients, especially hyperopes.
Several clinical tests are helpful for resolving disparities between the manifest and cycloplegic refractions. In addition to a "defogging" technique that "pushes plus," a time-honored technique is the duochrome chart (one half of the chart is green, and the other half is red). Because of chromatic aberration, the longer red wavelengths are focused slightly behind the shorter green wavelengths. An over-minused refraction will cause the focal point to be behind the retina. Red wavelengths will be further retro-focused, and letters on the chart's red half will appear more blurred than the letters on the green half of the chart.
Other clinical strategies include placing a 4-mm aperture in the trial frame during the cycloplegic refraction to restrict vision to the more central cornea, and comparing the refraction to the readings from an auto-refractor with a defogging device system or a wavefront analyzer.
If a discrepancy exists between the patient's refractive and corneal cylinders, the refraction should be re-evaluated. It is desirable to perform a wavefront analysis to identify patients with a high level of preoperative aberration that may become more symptomatic postoperatively, as well as to guide a customized ablation, if indicated.
Proper patient education is the key to a successful refractive surgery procedure. The goal of refractive surgery is to decrease the patient's dependence on glasses and contact lenses. Achieving this goal depends on the patient's daily visual needs. The patient must understand that the technique is still not as accurate or as predictable as correcting myopia with contact lenses or spectacles, which has a virtually 100% chance of achieving 20/20 or better vision in a healthy eye. Ensuring patients understand the goal of the procedure will increase postoperative satisfaction.
Informed consent should include a discussion of surgical and nonsurgical options. Likely outcomes, the potential need for enhancements, and potential complications must be discussed. Presbyopia should be explained, and monovision (in which one eye is intentionally left mildly myopic for near vision) should be offered as an option to middle-aged patients. A monovision simulation with contact lenses could be performed prior to the procedure.
Surgical Technique
Laser Calibration
The day of surgery, the laser should be checked for an adequate homogenous beam profile, alignment, and power output, following the manufacturer's guidelines.
Preoperative Planning
Many surgeons employ a nomogram adjustment of the correction to be programmed into the laser. It is preferable to perform these calculations in advance of the moment of surgery to avoid time pressure or distractions that may lead to calculation errors. Most nomograms are individualized to the surgeon, laser, and specific technique used, as well as amount of correction, patient age, gender, and other patient variables. These factors may influence the outcome of the ablation.
Often the manifest and cycloplegic refractions differ, or the amount and axis of the topographic and refractive astigmatism differ. Consequently it may be unclear which refraction should be entered into the laser. If the refractive cylinder differs from the topographic cylinder, it is assumed that lenticular astigmatism or posterior corneal curvature is the cause. In this case, the laser is usually still programmed with the axis and amount of cylinder noted on refraction. The consistency of the axis on the refraction and topography should be checked with the value programmed into the laser. This is a common source of error, particularly when there is a conversion between plus and minus cylinder formats.
In many laser models the size of the optic zone must be entered, and determination made whether or not a blend of the ablation zone should be performed. If there is sufficient corneal tissue, an ablation zone larger than the scotopic pupil size is usually selected. A "blend zone" is an area of peripheral asphericity designed to reduce the possibly undesirable effects of an abrupt transition from the optical zone to an untreated cornea. A common approach to creating a "blend zone" is to have, for example, a -6 D correction consist of a -5 D correction at a 6-mm optical zone, and a -1 D correction at an 8-mm optical zone. The larger the treatment area, the deeper the ablation.
In wavefront-guided "custom" and wavefront-optimized ablations, parameters are usually fixed by the wavefront optical measurement and the laser model. The surgeon must calculate whether an adequate stromal bed will remain. This is rarely an issue for surface ablation, but in thinner corneas and higher dioptric treatments, there may be inadequate tissue for LASIK, prompting the surgeon to select surface ablation.
In simple spherical corrections of myopia, the approximate depth of ablation can be calculated by the Munnerlyn formula, t = S2 D/3, where t is the thickness of the tissue ablated in microns, S is the diameter of the optical zone in millimeters, and D is the dioptric correction. In a 6-mm optical zone, the formula is simply 12 × D. A 4 D myopic correction would remove about 48 µ centrally.
Note that aspheric peripheral blend zones and wavefront corrections remove more tissue than the spherical correction in the Munnerlyn formula, and the surgeon is advised to have the laser software calculate the ablation depth. The intended total correction value must be input, not the nomogram-reduced value, in order to obtain the true amount of tissue removal.
Patient Preparation
Before the laser treatment is performed, the patient should be instructed about the sounds and smell of the laser. Patients may receive an oral sedative, such as diazepam, and the operative eye (in unilateral treatments) should be indicated with an adhesive label or some other temporary mark on the forehead.
The patient's skin is typically prepped with povidone iodine (Betadine) or alcohol wipes. A sterile drape may be used over the skin and lashes. Topical tetracaine and/or proparacaine anesthetic drops are placed in the eye. A lid speculum is placed in the operative eye and a patch is placed over the fellow eye to avoid cross-fixation. A gauze pad may be taped over the temple between the operative eye and the ear to absorb fluid run-off. The amount of desired correction, accounting for the vertex distance, is entered into the laser and checked by the surgeon. The patient is asked to fixate on the laser centration light while the surgeon focuses and centers the laser. For most patients, line-of-sight fixation by the patient during PRK produces more accurate centration than globe immobilization by the surgeon.
Surgical Technique
In most original techniques and in U.S. phase III investigations, the epithelium is removed by a sharp blade or a blunt spatula (Figure 4). In this technique, the surgeon defines the outer limit of de-epithelialization with an optical zone marker and then debrides the periphery followed by the center. To avoid dessication and resultant variability in ablation rates, the surgeon must perform this procedure efficiently. Skill is required to avoid nicking Bowman's layer. An ophthalmic surgical cellulose sponge lightly moistened with an artificial tear lubricant, such as carboxymethylcellulose 0.5%, is brushed over the surface of the cornea to remove any residual epithelium and provide a smooth surface (Figure 5).
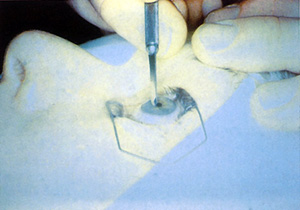
Figure 4. De-epithelialization with a blade before PRK.
Figure 5. Final cleaning of the surface before beginning the laser exposure.
Alternative methods for removing the epithelium include the use of a rotating corneal brush, application of diluted absolute alcohol (typically around 20% concentration) to the corneal surface to loosen the epithelium, and transepithelial ablation by the excimer laser itself. The epithelium should be removed efficiently and consistently to prevent hydration changes in the stroma, because the rate of excimer laser ablation may be increased by excessive corneal stromal dehydration, resulting in an overcorrection.
In the LASEK variant of PRK, which was first described by Camellin, the goal is to preserve the patient's epithelium. Instead of debriding and discarding the epithelium, or ablating the epithelium with the excimer laser, the surgeon removes an intact sheet of epithelium. A radial mark of gentian violet ink is used to assist in realignment of the epithelial flap.
A solution of approximately 20% diluted absolute alcohol is applied for 20 to 30 seconds. The alcohol is restricted to the area to be de-epithelialized with a ring (usually an optical zone marker) pressed onto the corneal surface, or by means of a round surgical sponge soaked with the alcohol solution. After the desired exposure time has passed, the alcohol is removed from the "well" of the optical zone marker by absorption into a microsurgical spear sponge. After the alcohol is fully absorbed and the optical zone marker is removed, the ocular surface is copiously irrigated with balanced salt solution (BSS) to minimize toxicity to the limbal germinal epithelium.
An instrument, which often has a hoe or spatula configuration, is used to separate a flap of full-thickness epithelium from the underlying Bowman's layer. The epithelium is delicately folded back on itself until all of the epithelium has been removed except for a small "hinge," which is usually located superiorly. Figure 6 shows the key steps involved in performing LASEK.

Figure 6. Technique for LASEK. (A) After the gentian violet radial orientation marks are placed, the epithelial flap is delineated with an optical zone marker or similar instrument. (B) Alcohol to loosen the epithelial flap is carefully contained within a trephine-shaped well pressed on the cornea. (C) The separation of the flap edge is begun with a "microhoe." (D) The separation of the epithelial flap continues as a "micro hockey stick" strokes the epithelium back, with care taken to not perforate the epithelium. (E) The epithelium is fully retracted off the area to be ablated by the laser, leaving a "hinge" of epithelium for about one clock hour in the superior periphery. (G) After the laser exposure is completed, the epithelial sheet is gently replaced by floating it and stroking it with a BSS cannula. (H) The epithelial flap is realigned using the radial marks. (I) The epithelial sheet is briefly dried with filtered air through a microtip to begin adhesion to the stroma. (J) After a BSCL is placed, the procedure is complete. Image courtesy Daniel Durrie, MD.
The goal of LASEK is to reduce postoperative pain and achieve a speedier return of vision compared to PRK, and possibly reduce postoperative haze formation. A meta-analysis comparing LASEK and PRK showed comparable refractive efficacy and accuracy between the two groups at 1 and 12 months. Compared with PRK, LASEK did not relieve discomfort on day 1 post-treatment or reduce corneal haze intensity at 6 and 12 months posttreatment.
Efforts have been made to develop a variant of the microkeratome used in LASIK. The microkeratome is modified with a dull blade and thin applanation plate to mechanically remove the epithelial flap without the use of toxic agents (eg, alcohol) that are used in LASEK. The goal is to create an epithelial flap that remains viable and successfully re-adheres postoperatively. These techniques are most commonly termed Epi-LASIK.
Hondur et al. compared Epi-LASIK to LASEK and found similar results at 1 year. Epi-LASIK initially had a slightly longer mean time to epithelial healing, but a similar amount of haze clinically and by confocal microscopy grey scale quantification at all time points.
Due to incomplete epithelial flaps and occasional stromal incursions with some of the epikeratomes, the volume of epi-LASIK procedures has decreased. A newer variation of advanced surface ablation is Epi-LASEK. Epi-LASEK is similar to Epi-LASIK except that alcohol is added with the goal of facilitating creation of the epithelial flap. Camellin and Wyler showed that the addition of alcohol allowed for a better flap and hinge creation without the addition of pain or haze when comparing epi-LASEK to epi-LASIK.
Lastly, the excimer laser itself may be used to remove the epithelium in transepithelial photorefractive keratectomy (T-PRK). In this variant, the epithelium is removed with the excimer laser by monitoring the disappearance of blue fluorescence during the ablation. Ghadhfan et al. reported T-PRK visual outcomes to be equivalent to traditional PRK and superior to LASIK or LASEK for low to moderate myopia, and T-PRK visual outcomes to be superior to LASIK, LASEK, and traditional PRK for high myopia.
Laser Ablation
After the epithelium is out of the treatment zone and the stroma has been exposed with one of these techniques, the laser is centered and focused according to the manufacturer's recommendations. Improved PRK centration occurs when the aiming beams or reticule are centered on the entrance pupil instead of the corneal apical light reflex. The patient is instructed to maintain good fixation during the stromal ablation. Small microsaccades should not adversely affect the outcome of the procedure. If the patient begins to lose fixation, the surgeon should immediately stop the treatment until adequate refixation is achieved. If the laser includes a tracking mechanism, it is still important for the surgeon to monitor for excessive eye roll, which can result in decentration despite the tracking device.Some current excimer lasers offer iris registration to improve toric accuracy. If the treatment involves a large amount of astigmatism correction, this should be employed. If iris registration is not present, the eye should be marked at 3 and 9 o'clock (prior to removal of the epithelium) to account for any cyclotorsion.
To correct myopia, the surgeon flattens the cornea by placing the largest number of laser pulses centrally, and progressively fewer pulses toward the periphery of the optical zone (Figure 7). To correct hyperopia, the cornea must be steepened. No pulses are placed in the precise center, the maximum number of pulses is placed in the peripheral optical zone, and then a blend zone creates a smooth transition from the edge of the optical zone back to the peripheral corneal surface (Figure 8).
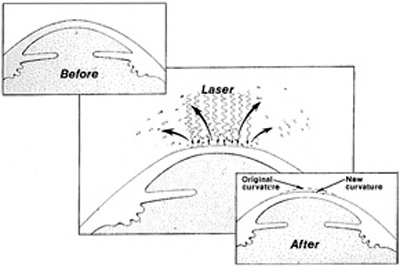
Figure 7. Schematic representation of the excimer laser flattening the central cornea for the treatment of myopia.
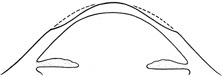
Figure 8. Schematic representation of the pattern of corneal ablation necessary to correct hyperopia with the excimer laser. The greatest amount of tissue is removed from the periphery of the optical zone with a gradual transition toward the anterior corneal surface.
Although the excimer laser beam at 193 nm is invisible to the human eye, a faint fluorescence of deep blue light is sometimes visible during stromal ablation. The sound of the laser firing is the main feedback signal to the surgeon, along with an alteration in the light reflex, as the stromal ablation progresses.
If topical mitomycin C (MMC), usually 0.2 mg/ml, is employed (usually in cases of high myopic correction) when the PRK is applied to a LASIK flap, or to enhance a previous PRK hazy scar, it may be applied as a soaked pledget placed on the ablated surface for 1 minute or less at the end of the laser exposure. Studies have shown MMC to be a safe adjunct in PRK. In highly myopic eyes (> -8 D), MMC 0.2mg/ml for 45 seconds did not delay epithelialization nor cause any adverse effects at 12 months. It did, however, induce an approximately 6% overcorretion. MMC has been shown to effectively prevent primary or recurrent haze in primary or retreatment PRK or PTK eyes at 6 months. Confocal microscopy has shown no significant side effects on keratocytes after PTK with MMC compared to PTK alone.
Krueger's group studied the effect of low dose MMC (0.02mg/ml) on PRK and found that the traditional dose of 0.2 mg/ml was more effective for myopia > or = -6 D and deeper ablation depths of > or = 75 microns. For lower amounts of myopia or shallow ablations the lower dose of MMC (0.02mg/ml ) appears to be as effective as the traditional dose (0.2mg/ml).
Despite the reported safety of MMC, vision-threatening complications, including glaucoma and corneal perforation, have been reported. The search continues for the ideal topical wound-healing modulator that has a high specificity for collagen synthesis without toxic side effects.
Immediate Postoperative Treatment
After the procedure is completed, antibiotic, steroid, and nonsteroidal anti-inflammatory (NSAID) drops are usually placed in the eye, followed by a bandage soft contact lens (BSCL). If the LASEK, Epi-LASIK, or Epi-LASEK variants have been performed, the surgeon carefully floats and repositions the epithelial sheet back into position with BSS before applying the medications and the BSCL. Chilled BSS may be applied before and/or after the PRK procedure, with the belief that cooling reduces pain and haze formation, although this has not been substantiated. If the patient cannot tolerate a BSCL, a pressure patch may be used.
Postoperative Care
During the first 24 hours, the patient may experience a large amount of pain, which may be relieved by an oral narcotic pain medication. Topical NSAID drops have been shown to significantly reduce postoperative pain, although they may slow the rate of re-epithelialization and promote sterile infiltrates.
A reduction in pain with the use of topical anesthetic drops has been demonstrated. Topical anesthetic drops must be used cautiously because they may cause severe corneal complications when used excessively over a prolonged period. Topical tetracaine used ad libitum in conjunction with a BSCL for 1 or 2 days after PRK does not appear to delay re-epithelialization or cause keratopathy. Oral analgesics including oral NSAIDs, oral prednisone, gabapentin or pregabalin, and opiate pain medications, may be necessary.
The patient should be followed closely until the epithelium is intact, usually within 72 hours. At this point, the BSCL, antibiotic, and NSAID drops (if used) may be discontinued. If they have been used at all, the topical anesthetic drops must be confiscated from the patient to prevent prolonged use.
Following re-epithelialization, a decision is made to continue or discontinue the topical steroid drops. The administration of topical steroids to modulate postoperative wound healing and reduce anterior stromal haze and regression of the refractive effect remains a controversial subject. Some studies have demonstrated that corticosteroids have no significant long-term effect on corneal haze or visual outcome after PRK. Other studies have demonstrated that steroids were effective in limiting haze and myopic regression after PRK, particularly after higher myopic corrections.
Surgeons who advocate the use of topical steroids after removal of the BSCL generally believe that only patients with higher levels of myopia (eg, greater than about 4.0 D) require long-term topical steroids. Steroid drops are usually tapered over a 3- to 4-month period, depending on the patient's corneal haze and refractive outcome. Keratocyte healing activity is maximal at 1 to 2 months. Abrupt termination of steroid drops at this time may trigger an excess healing reaction with haze formation and regression of the correction.
Clinical Results
Impact of Evolving Technology
The effectiveness of PRK improved markedly during the pre–market-approval clinical trials conducted in the United States from 1990 to 1994, because the broad-beam excimer laser systems were updated several times and surgeons became more familiar with the PRK technique. The size of the ablation zone increased as experience revealed that small ablation zones, which were originally believed to be desirable to limit the depth of tissue removal, produced more haze, and regression, as well as subjective glare and haloes. Larger treatment zones, including larger true optical zones and aspheric peripheral blend zones, are commonly employed to improve both optical quality and refractive stability in both myopic and hyperopic treatments.
Subsequent improvements in beam quality and the development of scanning slit and spot lasers have been associated with better outcomes, including less postoperative haze and less irregularities (especially fewer "central island" elevations).
Many surgeons employ outcome analysis programs that refine the success rates.
Scanning spot laser technology necessitated the development of tracking technology to ensure proper registration of the shot pattern. The potential for pupil tracking technology to improve centration led all of the manufacturers of such devices to introduce pupil tracker technology.
The most common types of tracker technology employ one of two strategies. In "closed-loop trackers," high-speed oscillating infrared beams scan across the edge of a fixed dilated pupil. These beams detect the abrupt change in reflected light at the edge of the pupil. This signal then directs rapidly responding mirrors to create a space-stabilized image, and the laser treatment is located on the cornea based on that image.
The second type of tracker is based on an infrared image of the pupil and uses video technology to monitor the location of the pupil image and shift the laser beam in an "open-loop system."
Improvements in excimer laser technology include tracking positionally induced cyclotorsion, either by detection of limbal marks (placed by the surgeon) or iris registration. Iris registration maps the iris and creates "landmarks" during wavefront acquisition when the patient is seated, and detects these "landmarks" when the patient is supine. This allows for accurate tracking of cyclotorsion. This technology has not translated into improved outcomes in wavefront-guided treatments.
A significant improvement in refractive surgery has been the development and implementation of wavefront-guided and wavefront-optimized treatments. Conventional treatments are spherocylindrical only. A wavefront-guided excimer laser uses an evaluation unique to each eye to determine the best laser treatment plan for an individual patient. Wavefront-guided treatments are based on aberrometry measurements and are designed to treat both spherocylinder and higher order aberrations.
Wavefront-guided lasers approved in the US include the Alcon LADARVision CustomCornea, AMO VISX S4 CustomVue, and the Bausch & Lomb Technolas 217z Zyoptix. Wavefront-optimized laser uses theoretical wavefront data to determine the best overall tissue ablation plan rather than patient-specific measurements. Wavefront optimized treatments are designed to treat spherocylinder errors while reducing induced spherical aberration. The Alcon Allegretto Wave Light offers both wavefront-optimized and wavefront-guided treatments.
Trials of wavefront analysis-guided and optimized treatments generally report improvements in visual acuity and quality of vision compared to conventional excimer treatments. Recently, topography-guided treatments are being evaluated. Topography-guided treatments may be beneficial in patients with highly aberrated corneal topographies and irregular astigmatism. Topography-guided treatments evaluate many more data points compared to wavefront-guided treatments.
Clinical Outcomes
In a study of the first laser to gain FDA approval in the United States (the Summit Phase III PRK study), 701 eyes with myopia ranging between -1.5 to -6.0 D were enrolled, with 2-year follow-up data available on 612 of the eyes. In that trial, 92.5% of the eyes had an uncorrected visual acuity (UCVA) of 20/40 or better, and 66.5% had a UCVA of 20/20 or better. Additionally, 77.8% of the eyes had a spherical equivalent manifest refraction within 1.0 D from emmetropia, and 6.9% had lost two lines of best-corrected visual acuity (BCVA).
In the VISX Phase III Study for low myopia, 691 eyes with myopia ranging from -1.0 to -6.0 D were followed for 24 months. In that study, 85% of the eyes had a UCVA of 20/40 or better, with 79% of the eyes within 1.0 D of emmetropia, and 1% of the eyes lost two lines of BCVA.
The trend has been for improved outcomes compared to the initial PRK studies. The trend toward reduced accuracy with higher myopic corrections persists, however. The long-term safety and stability of PRK were confirmed by a 12-year follow-up of patients in one of the initial PRK clinical trials. Refractive outcome was stable after the first year, and haze diminished slightly.
Other studies have further validated the safety and efficacy of myopic PRK. A 14-year follow up study of eyes ranging from -1.50D to -13.00 D showed stability of refraction, and no abnormalities in endothelial cell count or morphology, astigmatism, or ectasia. Alió et al. reported 10-year follow-up of eyes > -6.00 D with 58% of eyes within ± 1.00 D and 78% within ± 2.00 D. Mean haze was less at the 10-year time point compared to 3 months post-treatment.
Most studies indicate that when higher degrees of myopia are treated, there is an increased severity of haze and loss of BCVA. Greater amounts of attempted correction are associated with decreased predictability, increased regression, and less likelihood of obtaining 20/40 or better UCVA. No consensus about the upper limit of PRK has not been achieved, but most agree the results are unacceptable for myopia of more than -10.0 D, because of the combination of an increased risk of haze and scar formation, less accuracy, and degradation of the optical performance when the cornea is excessively flattened. Progress in the successful treatment of higher levels of myopia with PRK may depend on pharmacologic mediators of wound healing, including but not limited to intra-operative topical MMC.
Technical improvements in the excimer laser, particularly the adoption of a peripheral transition zone, appear to result in better predictability. In one study of myopic eyes between -7 and -17 D, 25 of 47 eyes (53%) were within 1 D of attempted correction at the 2-year follow-up. Another study of myopic eyes showed aspheric profile PRK induced a smaller increment of total wavefront error, related to a smaller increase in spherical aberration, and better maintained the physiology of the corneal surface than conventional treatment. Wavefront-guided treatments appear safe and effective for the treatment of myopia and result in decreased wavefront abnormalities when compared to conventional treatments. This is especially true in eyes with higher root-mean-square of higher order aberrations.
Toric PRK
To correct astigmatism, the excimer laser flattens the steep meridian of the cornea to match the flatter meridian. Generally, toric PRK was not as predictable as spherical PRK, and tended to undercorrect the cylinder. The trend toward undercorrection of the cylinder in earlier studies is probably attributable to conservative nomograms rather than axis error, since the postoperative axis was usually within 10 to 15 degrees of preoperative values.
Scanning lasers allow more direct recontouring of the toric corneal surface into a sphere, in conjunction with simultaneous correction of hyperopia or myopia. Less tissue is usually removed during toric PRK with a scanning laser as compared to a broad beam laser. A report of PRK using wavefront-guided ablation patterns in 23 eyes with myopia less than -8 D and cylinder less than -3.5 D, UCVA of 20/16 or better was achieved in 25 eyes (83%) at 1 year, with no loss of high-contrast conventional BCVA in any of the eyes, and no loss of BCVA with glare in 25 (83%) of the eyes. Similar reports show effective treatments with wavefront-guided toric ablations.
Hyperopic PRK
In contrast to myopic PRK, in which the central cornea is flattened (Figure 6), in hyperopic PRK more tissue is removed from the midperiphery than the central cornea, which results in an effective steepening (Figure 7). A sharp transition between treated and untreated cornea in the periphery can cause significant haze and regression. For this reason, additional pulses are applied to blend the maximally ablated midperipheral area with the untreated peripheral cornea. Hyperopic PRK therefore requires a large ablation area in order to maintain an adequate size of central hyperopic treatment.
Initial studies using hyperopic treatment zones of 4.0 mm combined with a total blended area out to 7.0 mm revealed an unacceptable regression effect. A significant number of eyes lost best corrected vision, largely secondary to mild decentration combined with the small optical zones. Later studies used larger hyperopic treatment zones with transition zones out to 9.5 mm. These studies generally demonstrated that with at least 12 months of follow-up after treatment for low hyperopia (up to +4.0 to +5.0 D) 65% to 92% of the eyes were within 1.0 D of the intended correction. The period from surgery to postoperative stabilization for the same quantity of correction is longer for hyperopic than myopic corrections. Treatment of higher degrees of hyperopia results in poorer predictability and stability.
Wavefront-guided hyperopic PRK has shown success with trends of initial myopic overcorrection with hyperopic regression over a 24 month follow-up period. O'Brart et al. reported in hyperopic PRK refractive stability achieved at 1 year was maintained up to 7.5 years with no evidence of hyperopic shift, diurnal fluctuation, or late regression. Peripheral corneal haze decreased with time but was still evident in a number of eyes at the last follow-up visit. Hyperopic PRK has been used for the treatment of accommodative esotropia in young adults with promising results.
Hyperopic Astigmatic PRK
Most studies have reported that approximately 50% of patients with hyperopic astigmatism achieve 20/20 UCVA at 6 months, and 95% have 20/40 or better UCVA at 1 year. LASIK is employed much more often than PRK for this indication.
Enhancements
After the UCVA and refraction are stabilized (typically about 3 to 6 months after the PRK), reoperation or "enhancement" can be performed to treat the residual refractive error. Enhancements for a patient with unrealistic expectations should be avoided. The patient who wants enhancement to improve the UCVA of 20/20 in his "bad eye" to the UCVA of "20/15" that his "good eye" has achieved should be re-educated about the realistic results. The goal of refractive surgery is improvement in the UCVA, not perfect vision.
When enhancement of myopic PRK is contemplated, the surgeon must be aware that the epithelium is often thickened (hyperplastic) centrally as a reaction to the flattened corneal profile created by myopic ablation. Initial undercorrection of the laser ablation must be differentiated from regression of the initial treatment. Regression may be due to stromal collagen healing, epithelial hyperplasia, or a combination of the two. A PRK enhancement may result in overcorrection if the regression due to epithelial hyperplasia is included in the enhancement and an equal amount of epithelial hyperplasia does not reoccur.
When debriding the epithelium for PRK enhancement, the surgeon will usually recognizes that the exposed previously ablated stroma is rougher than the normal smooth appearance of Bowman's layer.
Comparisons of ASA Techniques
Recent investigations have shifted away from reports of basic outcomes to comparisons of competitive techniques. After the initial wave of enthusiasm for LASEK as a method to reduce pain and speed visual recovery compared to PRK subsided, objective studies showed less benefit from LASEK. Pirouzian et al. randomized both eyes of 30 military patients between LASEK and PRK. No difference in pain between the two techniques was demonstrated, and epithelial defects were smaller on day 1 but larger on day 3 with LASEK. Hashemi et al. found no difference in pain and vision recovery, or in re-epithelialization between PRK and LASEK. A comparison of LASEK and LASIK for low myopia revealed visual improvement is significantly slower after LASEK, however, visual outcomes at 3 months were comparable.
In comparison with earlier experience with PRK and LASIK for low myopia, in which the outcomes did not differ in the long term, Kaya et al. found similar outcomes at 6 months for LASIK and LASEK for myopia under -6.0 D. LASEK has not been proven to induce fewer high-order optical aberrations than LASIK.
In one series of 470 eyes with greater than -6 D of myopia, the overall results for UCVA and predictability with LASIK were superior to those obtained with LASEK.
A Cochrane review of PRK versus LASIK for myopia showed that visual recovery is faster following LASIK than PRK, the final uncorrected visual acuity is comparable, there is no difference in accuracy, however, there is some evidence suggesting that LASIK may result in fewer eyes losing two or more lines of visual acuity than PRK. A Cochrane review of PRK versus LASIK for hyperopia did not find any randomized controlled trials to evaluate. Based on nonrandomized small studies, the review found similar results between the two modalities.
When comparing the various methods of advanced surface ablation, surgeons tend to favor techniques with which they are most comfortable.
Complications
Because refractive surgery performed with the excimer laser is elective, and the patient's preoperative BCVA is excellent, PRK must provide a very high standard of safety. Although severe complications after PRK are rare, patients need to be informed regarding the potential of such complications.
Central Islands
Central islands are disclosed by computerized videokeratography as an area of central corneal elevation, surrounded by an area of flattening corresponding to the myopic treatment zone in the paracentral region (Figure 9). Different studies employ varying thresholds to define a central island; at a minimum, an elevation of at least 1 D with a diameter of >1 mm compared to the paracentral flattened area should be present. Islands generally occur with the use of wide-beam laser systems, rather than scanning delivery systems.
With scanning spot and slit lasers, instead of the original broad-beam laser systems, the incidence of central islands is virtually eliminated. Traditionally, they have been reported more frequently in ablations larger than 5.0 mm in diameter and with greater attempted corrections. Central topographic islands may be associated with decreased visual acuity, monocular diplopia and multiplopia, ghost images, and decreased contrast sensitivity. Reports have indicated that they may be present in 29% to 71% of patients treated with broad-beam lasers in the early postoperative period.
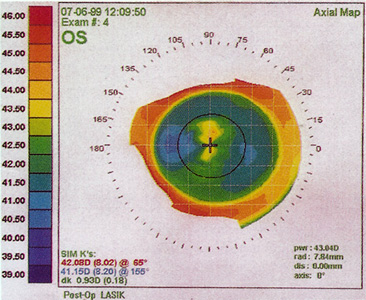
Figure 9. Corneal topography of a central island 1 month after PRK. Note the central steepening within the flattened ablation zone. The patient complained of mild "ghosting" of images.
The etiology of central islands is unknown, but several theories about their origin have been developed. The plume theorystates that a plume of gaseous and particulate debris is emitted from the corneal surface whenever the energy delivered by the laser strikes the cornea (Figure 10). The gas or debris tends to block the laser energy from reaching the central cornea during succeeding pulses. Initially, the VISX system used a flow of nitrogen gas across the corneal surface to remove this plume of material. This gas flow was subsequently discontinued because it led to corneal drying and surface irregularities. After the gas flow was discontinued, a markedly greater incidence of central island formation occurred.

Figure 10. Sequential images of the ablation plume obtained at various times after a 7-mm circular zone was ablated, demonstrating central vortex currents. Reprinted from Schipper I, Senn P, Wienecke L, Oyo-Szerenyi KD: Photoastigmatic refractive keratectomy for primary treatment and revision of myopic astigmatism. J Cataract Refract Surg. 23:1465–1471, 1997.
A variant of the plume concept is the vortex theory. During the ejection of corneal particles from the surface, a pressure gradient creates a vortex that may deposit ablation debris in the center of the ablation zone. This debris acts as a masking agent, reducing the central tissue removal with each successive laser pulse.
Another theory relates to the lack of homogeneity of the laser beam. Poor homogeneity may lead to less laser energy being delivered to the central cornea. Certain laser systems may have a beam profile that causes less energy to be delivered in the center, thus contributing to the formation of central islands. A decrease in the beam homogeneity may result from degradation of beam optics over time within the laser delivery system. This theory appears less plausible, since most surgeons find that the frequency of central islands does not correlate with the maintenance cycle of the laser.
Another potential cause of central island formation is uneven corneal surface hydration. One proposed explanation for differential corneal hydration is the "shock wave" theory, which postulates that as laser energy strikes the cornea during ablation, the laser pressure wave may force water from the underlying stroma to the center of the cornea. This fluid then acts as a masking agent, reducing the central corneal ablation rate. With the nitrogen gas flow, a uniform, dry, "frosted" appearance of the cornea is observed that is no longer present after the gas flow is discontinued. Lin proposed another mechanism in addition to the differential corneal hydration theory: the cornea acts like a "sponge" and is continuously hydrated from the endothelial surface. Since the cornea is thinner in the center compared to the periphery, the central cornea may become moist more quickly than the periphery as the ablation proceeds. This added moisture centrally may limit the amount of laser energy that reaches the corneal surface.
A final theory implicates the wound-healing properties of the cornea after PRK, rather than intraoperative problems, in the formation of central islands. This theory proposes that a differential amount of epithelial hyperplasia occurs after PRK, with more epithelium being formed in the central cornea than in the peripheral cornea. This results in a steeper area being present centrally on the topography map. Retreatment of persistent central islands generally discloses hypoplastic, thin epithelium over the elevated subepithelial substance.
Applying extra laser pulses (usually calculated as a fixed percentage of the total number of treatment pulses) to the central cornea reduces the incidence of central islands in broad-beam lasers. Multizone and multipass techniques have been proposed to result in a smoother ablation. If the islands do not resolve spontaneously by 6–12 months, they may be retreated directly with the excimer laser. Treatment of central islands with topography-guided excimer treatments and use of customized ablation algorithms have shown positive results.
Optical Aberrations
Optical aberrations, including glare, ghost images, and haloes after PRK have been reported. These symptoms are most prevalent after treatment with smaller ablation zones and after a higher attempted correction. These complaints seem to be exacerbated at night, and are most prevalent in young, myopic patients with large pupillary diameters due to an optical zone that is smaller than the entrance pupil under conditions of dim illumination. A larger, more uniform, and well-centered optical zone will generally provide a better quality of vision, especially at night.
Analyses of the wavefront optics of the eye indicate that night vision complaints are often attributable to spherical aberration, although other higher-order aberrations may contribute to distortions. Spherical aberration generally increases as a function of the amount of induced change in corneal curvature, particularly in the midperipheral cornea, as well as the amount of total spherical aberration present in the cornea and lens prior to laser treatment. Larger pupil size correlates with the frequency of complaints about aberrations, because spherical aberrations increase when the midperipheral corneal optics contribute to the light energy that passes to the retina.
Wavefront-guided customized corneal treatment patterns are designed to reduce both existing aberrations and the creation of increased aberrations, and thus promise a better quality of vision after laser ablation. This has been validated in several studies. In a study of 126 myopic and myopic-astigmatic eyes the data supported the safety and effectiveness of the wavefront-guided PRK for correction of low to moderate refractive errors, with resultant reduction in the number of higher order aberrations induced by excimer laser surgery and improved uncorrected and spectacle-corrected visual acuity when compared to conventional PRK. Carones et al. demonstrated favorable use of wavefront-guided ablations to treat eyes with clinically significant visual symptoms related to the presence of higher order aberrations in eyes that had previously had keratorefractive surgery. Randleman et al. demonstrated that wavefront-optimized ablations did not significantly increase higher order aberrations in 100 consecutive PRK treated eyes.
Decentered Ablation
It is important to achieve accurate centration during the PRK procedure to optimize a patient's visual potential. Centration is more critical for hyperopic than myopic treatments. A decentered stromal ablation may occur if the patient's eye slowly begins to drift and loses fixation, or if the surgeon improperly positions the patient's head (Figure 11). Decentrations are associated with greater attempted correction, probably secondary to an increased duration of fixation time required for higher refractive corrections. A greater number of decentrations are seen with inexperienced surgeons.
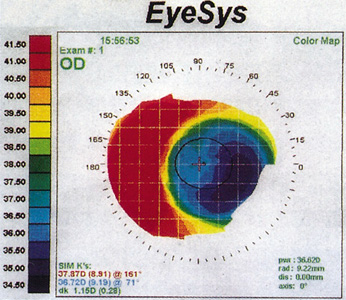
Figure 11. Corneal topography of a horizontally decentered ablation. The patient has a best spectacle-corrected vision of 20/30 associated with glare and haloes.
Decentration may result in glare, haloes, and decreased visual acuity. More symptoms may be experienced if the decentration is more than 1 mm in severity, but may not necessarily show any symptoms if less than 0.5 mm. Patients with larger pupils may experience symptoms with smaller amounts of decentration, because induced aberration (principally coma) worsens in the periphery.
Decentrations may be prevented through proper stabilization of the patient's head and alertness by the surgeon to stop the ablation if the patient begins to lose fixation. Pupil tracking technology has led to decreased amounts of decentration and therefore improvements in overall outcomes. Eye tracking is more important for eyes with higher levels of correction. Qazi et al. compared outcomes with and without automated infrared pupil tracking and found that the use of infrared pupil tracking improved uncorrected visual acuity, produced more predictive refractive outcomes, and fewer large centration outliers.
If the patient is symptomatic from a decentration, wavefront-guided enhancement may reduce the aberrations induced by the decentration. Wavefront treatments have been approved for primary treatments, not therapeutic retreatments, and experience with wavefront-guided enhancements is preliminary and "off-label" in the United States.
Corneal Haze
Subepithelial corneal haze typically appears several weeks after PRK, peaks in intensity at 1–2 months, and gradually disappears during the next 6–12 months (Figure 12). Histologic animal studies have shown that the subepithelial corneal haze resulting from PRK is most likely due to abnormal glycosaminoglycans and/or nonlamellar collagen deposited in the anterior stroma as a consequence of epithelial-stromal wound healing. Histologic specimens obtained from patients who have undergone PRK reveal a continuous acellular collagen layer underlying the epithelium. Human studies indicate that the subepithelial haze is composed primarily of glycosaminoglycans. Histologic studies from animals and humans treated with PRK have demonstrated an increase in the number and activity of stromal keratocytes, which suggests that increased keratocyte activity may be the source of the extracellular deposits.
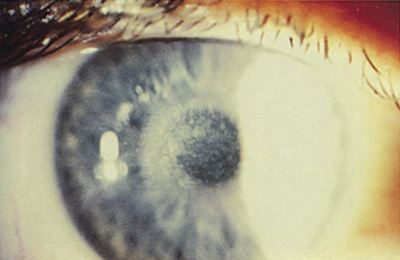
Figure 12. Fine reticular corneal haze 2 months after PRK. The patient is asymptomatic.
Confocal microscopy has been used to demonstrate the occurrence and time course of the corneal haze. This technique allows thin, optical sections of tissue to be viewed under high magnification in vivo. Stromal keratocyte numbers and size were found to peak at 1 month postoperatively and return to almost normal levels by 6 months. The time course of the keratocyte activity corresponded to the early phase of the subepithelial haze. Confocal microscopy demonstrated a bright subepithelial deposit that appeared by 2 weeks in the majority of patients. The level of subepithelial deposit peaked between 1 and 3 months, and then gradually declined.
Subepithelial deposits may contribute to the loss of BCVA seen during the first 3 months following PRK. Using confocal microscopy, one study found that keratocyte disturbance within the first 1 to 2 months after PRK was associated with marked loss of contrast sensitivity. Between 2 and 6 months, the subepithelial deposition of new tissue was correlated with significant loss of low-frequency contrast sensitivity and visual dysfunction. Most studies have found that the corneal transparency improves after 3 months, and haze that is graded 2 or higher in the ranking scale is rare at 6 months.
Persistent severe haze is usually associated with greater amounts of myopic correction. Seiler et al. found the presence of scar tissue in 1% of eyes that did not exceed -6.00 D, and in 17% of eyes with myopia that exceeded -6.00 D. Using a scatterometer, which is an objective means of measuring of corneal light scattering, Braunstein et al. found that ablations with depths greater than 80 µm produced higher levels of haze with decreased visual acuity than ablations less than 80 µm. Corneal subepithelial haze is more severe in patients who have been treated with smaller-diameter ablation.
Animal studies demonstrated that UV-B exposure after PRK prolongs the stromal healing process and results in augmentation of subepithelial haze. Clinical case reports of haze after high UV exposure, such as at high altitudes, corroborate these studies. A late-onset corneal haze has been described that occurs several months postoperatively after a period of relatively clear cornea.
Haze sometimes responds to an increase in the frequency and intensity of corticosteroids. If clinically unacceptable haze persists, treatment with topical MMC alone, a superficial keratectomy, or phototherapeutic keratectomy (PTK) with or without MMC, may be performed. Re-ablation should therefore be delayed for at least 6 months. The refraction is often inaccurate and overestimates the amount of myopia in the presence of haze.
A study demonstrated that in eyes with mild to moderate haze after PRK, the deterioration in low-contrast visual acuity is mainly attributable to increases in the wavefront aberration rather than the corneal haze.
Several proposed measures that have been advocated to decrease haze. Iintra-operative MMC is commonly used. Patients undergoing PRK are advised to use ultraviolet protecting eyewear during the year following treatment. Stojanovic et al. evaluated the use of systemic ascobate (vitamin C) on the development of haze after PRK in a non-randomized fashion. Administration of systemic ascorbate was associated with a decreased prevalence of corneal haze. Use of chilled balanced salt solution to reduce corneal haze is advocated by some.
Overcorrection
Overcorrections may occur if substantial stromal dehydration develops before the laser treatment is initiated, because more stromal tissue will be ablated per pulse. Overcorrection tends to occur more often in older individuals because they do not have as strong a wound-healing response. Loewenstein et al. demonstrated that older patients (35 to 54 years old) with moderate to high myopia displayed a greater response to the same amount of dioptric correction compared to younger patients. Overcorrections may be induced by the use of MMC.
Overcorrections may be treated by inducing a myopic regression through wound healing by abruptly discontinuing corticosteroids. If the overcorrection persists, the wound-healing response may be stimulated by epithelial scraping. The re-steepening of the cornea is accomplished by the laying down of tissue through the stimulation of subepithelial haze. In a study by Cherry, 12 of 19 eyes (63%) responded to scraping, with an improvement of over 1.00 D. No significant change in the refraction occurred in the first month post-scraping, but the hyperopia was much improved at 3 months and remained stable after that point.
Experience indicates that the wound-healing response sometimes may be stimulated, or the epithelial regularity enhanced, by placement of an extended-wear contact lens for 1 or more months. If regression does not occur within 1 month, we further stimulate regression by having the patient use a topical nonsteroidal agent four times a day for 1 to 2 months, while at the same time we monitor the refractive status and perform corneal examinations.
Undercorrection
Undercorrections occur after PRK most often when a significant myopic regression occurs. The incidence and amount of myopic regression depend on the degree of correction that is attempted. Regression is markedly increased with optical zones less than 6.0 mm in diameter. Individuals who experience regression after treatment of their first eye have an increased likelihood of regression in their second eye. Sometimes the regression may be reversed with aggressive use of topical corticosteroids. Alternatively, the patient may undergo a retreatment after the refraction has remained stable for 3 to 6 months.
Re-treatments are less predictable than primary procedures, but appear to effectively reduce residual myopia that is present after the initial procedure. Patients who have significant corneal haze associated with regression are at a higher risk for further regression after retreatment, as well as for redevelopment of significant haze with loss of BCVA. MMC administered at the time of retreatment can be used to modulate the response. It is recommended that the patient wait at least 6 or even 12 months for the haze to improve before the PRK is repeated.
Dry EYE
Dry eye conditions may occur after PRK as a result of denervation. This is similar to conditions after LASIK, but generally with PRK the results are less severe and of shorter duration. Treatment of post-refractive dry eye must be aggressive. Options include artificial tears, punctal occlusion, management of lid hygiene/meibomian gland dysfunction, omega-3 fatty acids, topical anti-inflammatories, and topical cyclosporine.
Epithelial Defects and Infiltrates
The epithelial defect created during PRK usually heals within 3 to 4 days with the aid of a BSCL or pressure patch. A frequent cause of delayed re-epithelialization is keratoconjunctivitis sicca, which may be treated with increased lubrication and temporary punctal occlusion. Patients with undiagnosed autoimmune connective tissue disease or diabetes mellitus may have poor epithelial healing. Patients must be closely monitored until re-epithelialization occurs.
Infiltrates
In the early postoperative period, subepithelial infiltrates may occur. In a survey by Teal et al., the incidence of these infiltrates was found to be approximately one in 300 cases. These infiltrates were first noted when surgeons began to switch to a combination of NSAID drops with BSCLs. These infiltrates are usually sterile and secondary to an immune reaction. None of the cultures of these infiltrates revealed a bacterial etiology, and the final visual outcome was the same irrespective of antibiotic therapy. Rarely, cases of infectious corneal infiltrates may occur that must be treated with appropriate topical antibiotics.
Elevated IOP
Patients may experience an increase in intraocular pressure after PRK. Most cases of elevated pressure occur secondary to prolonged therapy with corticosteroids. The incidence of increased pressure may range from 11% 9 to 25%. Occasionally, the elevated pressure may be quite high. In one study, 2% of the patients had a pressure greater than 40 mm Hg. Elevated pressures are usually controlled with topical intraocular pressure-lowering medications, and typically normalize after the steroids are decreased or discontinued. The intraocular pressure measured by applanation tonometry may be artifactually less than the actual pressure by several mm Hg after PRK.
Endothelial Effects
Unlike radial keratotomy, no significant change occurs in central endothelial cell density after PRK. The polymegethism associated with contact lens use typically improves after PRK, as demonstrated by the significant decrease in the peripheral coefficient of variation of cell size 2 years postoperatively.
Conclusion
PRK, LASEK, Epi-LASIK, and Epi-LASEK, are reasonably safe, effective, and predictable techniques for correcting low to moderate myopia, astigmatism, and low hyperopia. The primary disadvantages of advanced surface ablation are the degree of postoperative discomfort, length of time required for visual recovery, and increased corneal haze with treatment of higher refractive errors. Wound-modulating agents, such as MMC, are expanding the range of treatable refractive errors. Surface ablation may be preferable to LASIK in patients with epithelial basement membrane disease and thin corneas. Advances in wavefront-guide and wavefront-optimized ablations have made these modalities the mainstays of current treatment. Advancements in topography-guided treatments will likely offer the refractive surgeon numerous options in the treatment of normal and highly aberrated eyes.
Suggested Reading
- Salz JJ. Radial keratotomy versus photorefractive keratectomy. In: Thompson FB, McDonnell PJ, eds. Color Atlas/Text of Excimer Laser Surgery: The Cornea. New York: Igako-Shoin; 1993:63–75.
- Srinivasan R, Sutcliffe E. Dynamics of the ultraviolet laser ablation of corneal tissue. Am J Ophthalmol. 1987;103(3 Pt 2):470–471.
- Liu JC, McDonald MB, Vernell R, et al. Myopic excimer laser photorefractive keratectomy: An analysis of clinical correlations. Refract Corneal Surg. 1990;6(5):321–328.
- Hanna KD, Chastang JC, Asfar L, et al. Scanning slit delivery system. J Cataract Refract Surg. 1989;15(4):390–396.
- Medieros FW, Stapleton WM, Hammel J, et al. Wavefront analysis comparision of LASIK outcomes versus femtosecond laser and mechanical microkeratomes. J Refract Surg. 2007;23(9):880–887.
- Quinto GC, Camacho W, Behrens A. Postrefractive surgery dry eye. Curr Opin Ophthalmol. 2008;19(4):335–341.
- Shah S, Doyle SJ, Chatterjee A, et al. Comparison of 18% ethanol and mechanical debridement for epithelial removal before photorefractive keratectomy. J Refract Surg. 1998;14(2 Suppl):S212–S214.
- Cui M, Chen XM, Lu P. Comparison of laser epithelial keratomileusis and photorefractive keratectomy for the correction of myopia: a meta-analysis. Chin Med J (Engl). 2008;121(22):2331–2335.
- Cavanaugh TB, Durrie DS, Riedel SM, et al. Topographical analysis of the centration of excimer laser photorefractive keratectomy. J Cataract Refract Surg. 1993;19 Suppl:136–143.
- Arshinoff S, D'Addario D, Sadler C, et al. Use of topical nonsteroidal anti-inflammatory drugs in excimer laser photorefractive keratectomy. J Cataract Refract Surg. 1994;20 Suppl:216–222.
- Ar Ozdamar A, Aktunc R, Ercikan C. The effects of topical steroids on refractive outcome and corneal haze, thickness, and curvature after photorefractive keratectomy with a 6.0-mm ablation diameter. Ophthalmic Surg Lasers. 1998;29(8):621–627.
- Moshirfar M, Chen MC, Espandar L, et al. Effect of iris registration on outcomes of LASIK for myopia with the VISX CustomVue platform. J Refract Surg. 2009;25(6):493–502.
- Rajan MS, Jaycock P, O'Brart D, et al. A long-term study of photorefractive keratectomy 12-year follow-up. Ophthalmology. 2004;111(10):1799–1800.
- Mastropasqua L, Toto L, Zuppardi E, et al. Zyoptix wavefront-guided versus standard photorefractive keratectomy (PRK) in low and moderate myopia: randomized controlled 6-month study. Eur J Ophthalmol. 2006;16(2):219–228.
- Anshutz T. Laser correction of hyperopia and presbyopia. Int Ophthalmol Clin. 1994;34(4):107–137.
- Pacella E, Abdolrahimzadeh S, Mollo R, et al. Photorefractive keratectomy in the management of refractive accommodative esotropia in young adult patients. J Cataract Refract Surg. 2009;35(11):1873–1877.
- Lee HK, Lee KS, Kim JK, et al. Epithelial healing and clinical outcomes in excimer laser photorefractive surgery following three epithelial removal techniques: mechanical, alcohol, and excimer laser. Am J Ophthalmol. 2005;139(1):56–63.
- Kreuger RR, Krasinski JS, Radzewicz C, et al. Photography of shock waves during excimer laser ablation of the cornea: Effect of helium gas on propagation velocity. Cornea. 1993;12(4):330–334.
- Cheng AC, Lam DS. Central island treatment using Technolas 217 based on Orbscan II assessment. J Refract Surg. 2005;21(3):294–296.
- O'Brart DP, Gartry DS, Lohmann CP, et al. Excimer laser photorefractive keratectomy for myopia: Comparison of 4.00- and 5.00-millimeter ablation zones. J Refract Corneal Surg. 1994;10(2):87–94.
- Cantera E, Cantera I, Olivieri L. Corneal topographic analysis of photorefractive keratectomy in 175 myopic eyes. Refract Corneal Surg. 1993;9(2 Suppl):S19–S22.
- L'Esperance FA, Taylor DM, Warner JW. Human excimer laser keratectomy. Short-term histopathology. Bull N Y Acad Med. 1989;65(5):557–573.
- Gartry DS, Ker Muir MG, Marshall J. Photorefractive keratectomy with an argon fluoride excimer laser: A clinical study. Refract Corneal Surg. 1991;7(6):420–435.
- Meyer JC, Stulting RD, Thompson KP, Durrie DS. Late onset of corneal scar after excimer laser photorefractive keratectomy. Am J Ophthalmol. 1996;121(5):529–539.
- Dutt S, Steinert RF, Raizman MB, et al. One-year results of excimer laser photorefractive keratectomy for low to moderate myopia. Arch Ophthalmol. 1994;112(11):1427–1436.
- Taylor HR, McCarty CA, Aldred GF; Melbourne Excimer Laser Group. Predictability of excimer laser treatment of myopia. Arch Ophthalmol. 1996;114(3):248–251.
- Epstein D, Tengroth B, Fagerholm P, Hamberg-Nystrom H. Excimer retreatment of regression after photorefractive keratectomy. Am J Ophthalmol. 1994;117(4):456–461.
- Rozsival P, Feuermannova A. Retreatment after photorefractive keratectomy for low myopia. Ophthalmology. 1998;105(7):1189–1193.
- Sampath R, Ridgway AE, Leatherbarrow B. Bacterial keratitis following excimer laser photorefractive keratectomy: A case report. Eye. 1994;8(Pt 4):481–482.
- Carones F, Brancato R, Venturi E, Morico A. The corneal endothelium after myopic excimer laser photorefractive keratectomy. Arch Ophthalmol. 1994;112(7):920–924.