EPIDEMIOLOGY
Global Information
- Cataracts are estimated to be present in approximately 1 to 15/10,000 children worldwide, accounting for 5%–20% of childhood blindness (Foster et al, 1997).
- Globally, about 200,000 children are blind from bilateral cataracts worldwide (Foster et al, 1997; Medsinge et al, 2015).
- About 20,000 to 40,000 new cases of bilateral congenital cataracts are diagnosed each year (Apple et al, 2000).
Regional Information (MIDDLE EAST)
- The minimum prevalence of childhood blindness was estimated to be 0.32/1000 children in the Gaza Strip population. Of this group, roughly 7% of blindness was attributable to cataract. This is relatively less than the figure reported from other geographic regions. The greatest proportion of childhood blindness was attributable to heritable retinal conditions due to the high degree of consanguinity (Elder et al, 1993).
- In a population-based study in Saudi Arabia, the prevalence of cataracts among children age 5–18 was 14.7/10,000 (Khan et al, 1989). This in fact is the highest estimate worldwide.
- Mostafa et al studied inheritance patterns of congenital cataract in Egypt, where one-third of marriages are consanguineous. The study found that 6 of 7 pedigrees with inherited infantile cataracts exhibited autosomal recessive inheritance (Mostafa et al, 1981).
Regional Information (AFRICA)
- Cataract is the main cause of blindness among children in Africa, replacing vitamin A deficiency and measles. (See Table 1.)
- In a study conducted in rural Malawi, 151 blind children were identified using the community-based key informant method. Cataract was the leading cause of blindness, affected 27 (35%) children (Kalua et al, 2008).
- A study of children receiving surgery for congenital/developmental cataract in the 2 Child Eye Health Tertiary Facilities in Tanzania in 2004–2006 estimated that the childhood cataract surgical rate in Tanzania was 9.9/1,000,000 people.
- The rate varied by region, from 5.4 in regions not adjacent to the eye centers to 32.3 in regions where the eye centers are located (Courtright et al, 2008).
| Table 1. Children Blind from Cataract in Schools for the Blind in Africa |
| Country |
Number of Blind Children in Survey (% Lens Involvement) |
| Malawi, Kenya, Tanzania, Uganda (2009) |
701 (13.1%) |
| Nigeria (2009, 2011) |
45 (13.3%)
30 (26.7%) |
| Ghana (2008) |
201 (23%) |
| Cameroon (2010) |
56 (19.6%) |
Source: Courtright P. Childhood cataract in sub-Saharan Africa. Saudi J Ophthalmol. 2012;26(1):3–6.
DIFFERENTIAL DIAGNOSIS
PATHOPHYSIOLOGY/DEFINITION
Cataract refers to opacity within the lens of the eye. The term pediatric cataract encompasses a wide range of childhood lenticular opacifications, as described in Table 2. Considering the onset, pediatric cataract covers congenital, infantile, and developmental cataracts and is equal with the less popular terminology of childhood cataract. Pediatric cataracts may be congenital if present within the first year of life, developmental if present after infancy, or traumatic (Medsinge et al, 2015). The lenticular change can be visually significant or not associated with any abnormality of visual development. It is helpful to consider the morphological classification of pediatric cataracts to understand the age of onset, etiology, and visual prognosis.
| Table 2. Morphological Classification of Pediatric Cataract1 |
| Morphological Classification |
Characterization
| Etiology and Associations
| |
| Cortical cataract |
Cortical material opacification that does not involve the lens nucleus
Three morphologic subtypes: blue dot opacities, pulverulent/cerulean and coronary
|
With punctate changes association with female carrier of Lowe syndrome |
| Congenital nuclear cataract (Figure 12) |
Lenticular opacity occurring between the anterior and posterior sutures |
Often associated with galactosemia |
| Anterior lenticonus |
Classically an oil-droplet reflex on retinoscopy; cataract may develop later |
Presents bilaterally
Seen in association with Alport, Fechtner, and Waardenburg syndromes
|
| Anterior polar cataract |
A small, usually central opacity of the front part of the lens capsule that generally does not grow during childhood and is typically not visually significant; often managed without surgery |
Derives from incomplete or abnormal separation of the lens from the surface ectoderm during embryonic development.
Often seen with persistent pupillary membrane or iris strands connecting to the cataract
|
Anterior pyramidal cataract2
| A white conical cataract measuring 2.0-2.5 mm at the base that protrudes into the anterior chamber like a pyramid |
Associated with aniridia |
|
| Anterior subcapsular cataract |
A area of opacity immediately beneath the anterior capsule in the anterior lens cortex |
Can be associated with galactosemia or Wilson disease
Associated with other anterior lens abnormalities, trauma, or atopic dermatitis
|
| Posterior polar cataract |
Focal opacity of the posterior capsule without other lenticular opacity |
Can be an early finding in Lowe syndrome |
| Lamellar cataract |
Opacity occurring between the nuclear and cortical layers of the lens |
Maternal hypoparathyroidism or rubella infection in the third trimester can result in lamellar congenital cataracts
Seen in the genetic disorders Bardet-Biedl, Rothmund-Thomson, and McKusick-Kaufman syndromes
|
| Total/complete cataract |
Presence of both nuclear and cortical opacity; many times appears as a white cataract |
Associated with trauma in children
Can be a late finding in Lowe syndrome
|
| Posterior lentiglobus |
Posterior bowing of the posterior capsule; can occur in the presence or absence of a posterior capsule defect |
- |
| Posterior lenticonus |
A localized, cone-shaped change in the posterior lens surface; more common than anterior lenticonus |
Unilateral and axial in location
Cataract can develop rapidly with spontaneous rupture of posterior capsule
|
| Persistent fetal vasculature |
Presence of a retrolental membrane with or without posterior involvement. |
Not exactly a cataract, rather an opacity behind the posterior capsule of the natural lens |
| Posterior subcapsular cataract |
A thin layer of opacity affecting the posterior lens cortex; the most common form of secondary cataract |
Can be associated with galactosemia, Bardet-Biedl syndrome, Fabry disease, Alström syndrome, Refsum disease, and neurofibromatosis type II |
| Sutural/stellate cataract |
Opacities involving the Y-sutures of the lens |
Usually visually insignificant |
| Membranous |
Natural cortical material absorbed (eg, following a trauma) |
End-stage cataract; associated with trauma, intrauterine infection (TORCH), PHPV, or any long-standing congenital cataract. |
1 There is no single standard classification for pediatric cataract.
2 Anterior pyramidal cataract is a variation of anterior polar/capsular cataract.
The etiology, and therefore the basic pathophysiology, of childhood cataract varies widely; in many children, the etiology may not be determined. The etiology of pediatric cataracts can be broadly classified in the following groups:
- Hereditary
- Autosomal dominant, autosomal recessive, and X-linked patterns of inheritance have been identified.
- Autosomal dominant transmission is responsible for 75% of hereditary cataracts; however, in populations with a high degree of consanguinity, autosomal recessive inheritance is seen more commonly.
- Metabolic
- Galactosemia can be caused by a galatose-1-phosphate uridyl transferase (GALT), a galactokinase, or an epimerase deficiency.
- Mutation in exon 6 of the GALT gene on chromosome 13 is found in 2/3 of children with transferase deficiency (Elsas et al, 1995).
- Buildup of galactose-1-phosphate→conversion to galactitol in the lens→influx of water into the lens by osmosis disrupts the packing of lens fibers
- Initial changes are reversible with elimination of galactose from the diet.
- If left untreated, lamellar cataract can transform into total cataract.
- Glucose 6-phosphate dehydrogenase deficiency, hypoglycemia, and hypocalcemia can lead to the development of lamellar cataracts.
- Traumatic
- Secondary cataracts
- The most common cause is uveitis associated with juvenile idiopathic arthritis.
- Other secondary causes include intraocular tumor and radiation therapy, chronic retinal detachment, steroid use, and laser for ROP.
- Infectious
- Cataracts can occur in infants after maternal infection during pregnancy.
- The most common cause is congenital rubella syndrome in countries where rubella vaccine is not available.
- Toxoplasmosis, toxocariasis, varicella virus, and cytomegalovirus can also cause cataracts.
- Cataracts associated with syndromes
- Infantile cataracts occur with a number of hereditary syndromes that also have associated systemic disease.
- They occur almost universally with X-linked oculocerebrorenal syndrome of Lowe and Hallermann-Streiff-François syndromes.
- They occur less commonly with trisomy 21 syndrome.
- Take note of any abnormal features including unusual facial features, extra digits, unusual skin, short stature, developmental delay, and microcephaly or hydrocephaly.
- Idiopathic
- Most nontraumatic unilateral cataracts are idiopathic.
- This is a diagnosis of exclusion.
Unilateral Cataracts
- The presence of a unilateral lenticular abnormality is less often associated with systemic disease and most often is the result of local dysgenesis.
- Unilateral cataracts are most often idiopathic.
- Possible etiologies include:
- Persistent fetal vasculature (PFV)
- Posterior lenticonus or lentiglobus
- Trauma should be considered in later postnatal onset
Bilateral Cataracts
- Bilateral congenital cataracts are associated with systemic disease more often than unilateral cataracts; an autosomal dominant inheritance is the etiology in more than 50% of these patients (Figure 13).
- More than 15 gene variants have been associated with pediatric cataract formation; these hereditary cataracts are most often autosomal dominant, though transmission can be X-linked or autosomal recessive.
- Systemic associations can include the following:
- Genetic abnormalities
- Metabolic associations
- Syndromic conditions
- Infections
- The most common causative infections accounting for bilateral congenital cataract include the following, which can be recalled with the acronym TORCHES: toxoplasmosis, rubella, cytomegalovirus (CMV), herpes simplex virus (HSV), and syphilis.
- Routine testing of all bilateral cataracts for TORCHES is recommended though such testing is generally uninformative in the absence of a history of maternal illness during pregnancy or systemic clinical indicators in the child.
- Cataracts associated with systemic disease can have characteristic morphologic features suggesting the etiology. However, congenital cataracts may present with an atypical appearance.
SIGNS/SYMPTOMS
- Asymmetric or whitened red reflex is the most commonly reported clinical finding (leukocoria, Figure 14).
- Figure 15 shows examples of less common morphologies.
- Children with visually significant unilateral cataract often develop strabismus when left untreated.
- Nystagmus can develop with development of amblyopia in bilateral cataracts when not treated within the first 3 months (critical period of vision development) of birth.
- The patient may fail to notice toys or faces or show a delay in meeting milestones.
EVALUATION
- History should include the following:
- A detailed pre- and postnatal history
- Review of systems
- Family history
- Family members should be examined when possible. Marked variability can be seen among affected family members, and cataracts can be so mild these family members may not be aware they have them.
Unilateral Cataracts
- For unilateral cataracts in an otherwise healthy child, an extensive laboratory workup is not required.
- Examination should include a thorough ophthalmic exam to understand anatomic and functional involvement.
- An ultrasound (B-scan ultrasonography) should be utilized if the posterior pole is not visible.
Bilateral Cataracts
- In addition to a thorough ophthalmic exam, the evaluation of bilateral cataracts should include:
- A pregnancy history
- A neonatal history
- A family history with examination of family members when possible
- If there is a clear autosomal dominant pattern and the child is healthy, further evaluation is not indicated.
- A thorough pediatric and developmental exam
- Recommended lab workup includes the following:
- If the patient presents in a population where rubella vaccination is not routine they should be screened for congenital rubella infection with IgM titers.
- TORCH titers and Venereal Disease Research Laboratory (VDRL) test
- Male infants who present with bilateral cataract associated with hypotonia or delayed milestones should be screened for Lowe syndrome, which is done through analysis of urine amino acids and serum electrolytes and confirmed with an inositol polyphosphate 5-phosphatase enzyme assay.
- Infants with bilateral cataracts and failure to thrive should receive a full metabolic workup in conjunction with a pediatrician and geneticist, if possible. This includes but is not limited to these tests: fasting blood glucose levels, serum calcium and phosphorus levels, urine-reducing substances after a milk feeding, RBC transferase, and galactokinase. Though most infants with galactosemia will be identified neonatally, there may be false negatives; therefore, a complete metabolic workup remains an important consideration.
- Testing is available to screen children with bilateral congenital cataracts for known mutations, though this is not widely available and may not inform treatment in the absence of other dysmorphic features.
MANAGEMENT
- Once pediatric cataract is diagnosed, the visual significance should be promptly assessed.
- Cataract extraction is the preferred treatment for most visually significant infantile cataracts.
- A comprehensive ophthalmic exam should be performed to identify possible ocular comorbidities.
- Consider the trade-off of loss of accommodation after cataract removal versus clarity of the visual axis in milder cataracts, especially bilateral ones.
Treatment
- Children with congenital cataract may lose vision due to a number of causes; therefore, all of the factors need to be considered carefully and addressed clinically or surgically. These reasons include direct deprivation as well as amblyopia and anisometropia due to high degrees of astigmatism.
- Treatment is generally surgical but nonsurgical options have to be considered too.
- Management is dictated by:
- Laterality
- Degree of amblyopia assessed through visual performance
- Morphology, size, position and density of the lenticular opacity
- Visual potential (associated ocular findings) and the central nervous system status
Nonsurgical Treatment
- Occlusion may be necessary in unilateral or asymmetric cases that present a risk for amblyopia. This may delay the need for surgery up to the time that an intraocular lens (IOL) can be implanted with less refractive uncertainty.
- Small and visually insignificant cataracts at birth or in infancy can progress throughout childhood; therefore, these patients should be carefully monitored. If significant decrease in vision develops with a change in cataract morphology, surgical intervention should be considered.
- Morphology can be informative regarding the need for surgery. In a recent study, it was shown that anterior polar cataracts (APCs) and cortical cataracts are less amblyogenic, and suitable refractive care and periodic follow up may suffice.
Surgical Treatment
Timing of Surgery
- For visually significant congenital opacities, the latency period should be noted.
- Unilateral cataracts: before 6 weeks.
- Bilateral cataracts: before 10 weeks.
- The age range for visual plasticity extends roughly from birth to age 7–9 years, but there is a 3-month critical period following birth.
- Some suggest a delay in surgery past 4 weeks of age to decrease the risk of glaucoma.
- Bilateral simultaneous surgery is controversial but is routine in some parts of the world.
- Earlier surgery leads to quicker rehabilitation, and a recent study from Sweden showed that maternity ward screening leads to more and earlier detection and thus earlier treatment compared to well-baby visits or no screening (Magnusson et al, 2003).
Biometry and Keratometry
- When planning to place an IOL, the surgeon needs axial length and keratometry values.
- For older cooperative children, in the office noncontact partial coherence interferometry provides consistent and accurate readings.
- For younger children, intraoperative measurements including manual keratometry and A-scan (contact or immersion) are necessary.
Technique, Lensectomy Surgery
- A bimanual small incision is made through clear cornea or pars plana.
- Children have more elastic anterior capsules than adults do, making anterior capsulotomy challenging, and equatorial extensions more likely. A curvilinear continuous capsulorrhexis can be made using standard techniques, or a rhexis can be made using the vitrector (vitrectorhexis).
- Trypan blue dye may be utilized to increase capsule stiffness and perform more controlled anterior capsulorrhexis (Haritoglou CJ et al. Cataract Refract Surg. 2013;39[11]:1749-52).
- Primary posterior capsulorrhexis is a recommended yet technically challenging step that facilitates in-the-bag IOL placement and is associated with long-term IOL stability.
- With the advent and availability of femtosecond laser devices, creation of the anterior and posterior capsulorrhexis with desired sizes will likely be achieved. However, at the present time, the high initial and maintenance costs of this technology limits its widespread use.
- The lens material is aspirated using bimanual irrigation and aspiration (I&A) or the bimanual anterior vitrector.
- Occasionally, intraocular forceps may be helpful to crush or extract intralenticular calcific excrescences and intraocular cautery may be used in eyes with persistent fetal vasculature to prevent intraocular hemorrhage.
- Virtually all children will develop posterior capsular opacification after cataract surgery. For children unlikely to be able to sit for YAG laser capsulotomy in the next few years, primary posterior capsulotomy, and anterior vitrectomy should be considered. This can be performed either before or after IOL implantation using the vitrector through clear cornea or pars plana incisions.
- When placing an IOL, the surgeon places the lens in the capsular bag or the ciliary sulcus (with or without IOL optic capture). The lens is commonly a monofocal acrylic 1-piece or 3-piece lens. If there is no capsular support (eg, trauma, Marfan syndrome, ectopia lentis, surgical complication), a sutured IOL, scleral-fixated IOL, anterior chamber IOL (ACIOL), or iris-claw lens may be considered.
- All wounds should be suture closed at the end of surgery.
Intraocular Lens Implantation
- Considered by many to be the standard of care after 1 year of age if no contraindications.
- The Infant Aphakia Treatment Study (IATS) demonstrated equivalent visual outcomes between infants treated with IOL placement versus aphakia with contact lens in the first 6 months of life but a higher rate of re-operation in the IOL group. Therefore, the recommendation was made to leave a child aphakic with a contact lens if operated on prior to 6 months of age (IATSG, 2014).
- Aphakia in infancy has been associated with increased risk of aphakic glaucoma. A study has demonstrated glaucoma in 17% of aphakic patients versus 4% of pseudophakic patients (Trivedi et al, 2006). The increased risk of glaucoma in aphakic infants has been replicated in other studies, though a recent IATSG study failed to find a significant association. Recent attention to central corneal thickness has shown that frequently the readings are overestimates due to the thicker cornea in the aphakic/pseudophakic eye. Nevertheless, increased risk of glaucoma in aphakic infants remains an important issue for future study.
- Due to rapid growth of the eye during the first 2 years of life, IOL power calculations will vary significantly from the time of implantation to adulthood.
- Studies have shown that rapid axial length change of approximately 0.62 mm/month occurs in the first 6 months of life. This reduces to 0.19 mm/month from 6 to 18 months and then continues at a rate of 0.01 mm/month until 18 years of age.
- Holladay 1 and SRK/T formulae reported to give equally good results in children IATS report: Vanderveen DK et al. Am J Ophthalmol. 2013;156(6):1252-1260.
- Several factors related to axial length measurement, formulae related errors, postoperative healing and ocular growth are related to poor long-term predictability of IOL power calculations.
- Calculation of IOL power is based on the child’s age and predicted ocular growth. Generally, hyperopia is aimed for, assuming the eye will grow and tend towards emmetropia as the child gets older.
- Alternatively, emmetropia may be aimed for at the time of surgery to provide a clear retinal image during the period of visual development and aid in amblyopia management with the expectation that myopia will result as the child grows.
- Figure 16 shows the suggested postoperative target refraction based on age in years.
Visual Rehabilitation
- For bilateral aphakia, aphakic glasses or contact lenses are both good options.
- For unilateral aphakia, a contact lens is the preferred method of visual rehabilitation to minimize aniseikonia induced by glasses from anisometropia.
- If pseudophakic, glasses or contact lenses are options.
- Generally, the clinician should overplus to leave child slightly myopic to ensure relatively better vision for near and intermediate distances until around the age of 3–5 years, when a bifocal segment may be prescribed.
- Amblyopia treatment should be started immediately, and its importance must be emphasized to the family at every visit.
- Patching regimens vary drastically amongst physicians.
- The amount of patching is related to the duration of deprivation amblyopia and age of the patient.
- Patching regimen in the IATS study for unilateral cataracts: 1 hour/day for every month of age up to 8 months, then patch 50% of all waking hours.
- Long-term occlusion therapy will be necessary for patients who have undergone unilateral cataract extraction.
- Assessment of compliance with patching at every visit is critical.
Evaluate re-opacification of the visual axis which may occur even after anterior vitrectomy.
Secondary Intraocular Lens
- Consider a secondary IOL in children not tolerating aphakic contact lens or glasses.
- The location of IOL depends on capsular support.
- Good support 360°
- Place IOL on the rim of residual lens capsule after freeing it from the iris with scissors or an iris spatula.
- Sulcus IOL
- Poor capsular support
- Anterior chamber IOL (ACIOL)or iris-claw (e.g. Artisan)
- IOL sutured into the ciliary sulcus
- Scleral-fixated IOL
Regional Information (AFRICA)
- A unique challenge of childhood blindness in Africa is delayed presentation to a hospital, delayed intervention, and poor follow-up.
- Mwende et al interviewed the parents of children presenting to KCMC Hospital or CCBRT Hospital with congenital or developmental cataracts and found that the average delay in presentation to a hospital is 34 months (Mwende et al, 2005).
- Factors contributing to this challenge include:
- Lack of transportation
- Cost
- Ignorance on the part of the caregivers
- Cultural beliefs and stigmatization
- Taboos against eye surgery and wearing eyeglasses
- Early detection of and treatment for cataracts in an African child is closely associated with a strong collaboration within the community.
- The “key informant” method has been very successful in many communities not only for identifying the cases but also for referral encouragement.
- It is imperative to involve the parents of children who underwent successful treatment for cataracts as motivators for other families to seek care.
- This can be accomplished through direct contact or recorded or written materials distributed in the clinics in local languages.
- As routine immunization during the first year of life is well-accepted by many mothers, health workers who administer immunizations should be trained in the red reflex test so that they may refer children suspected of having cataracts to the proper facility.
- Though genetic testing may not be available in many regions in Africa, it is important to provide counseling to parents of families in which a genetic condition is suspected.
IMAGE LIBRARY
Differential Diagnosis
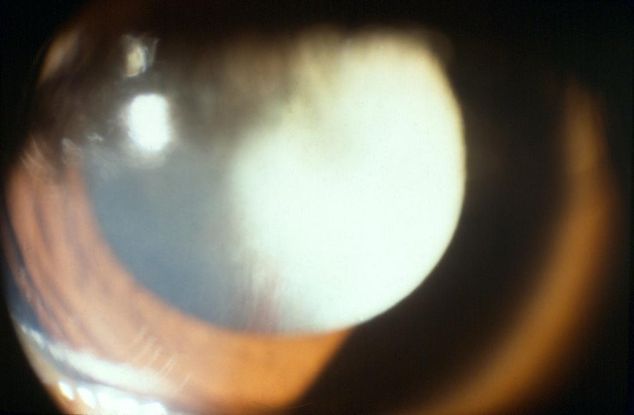
Figure 1. Persistent fetal vasculature (PFV) retrolental membrane. (© 2015 American Academy of Ophthalmology, www.aao.org.)
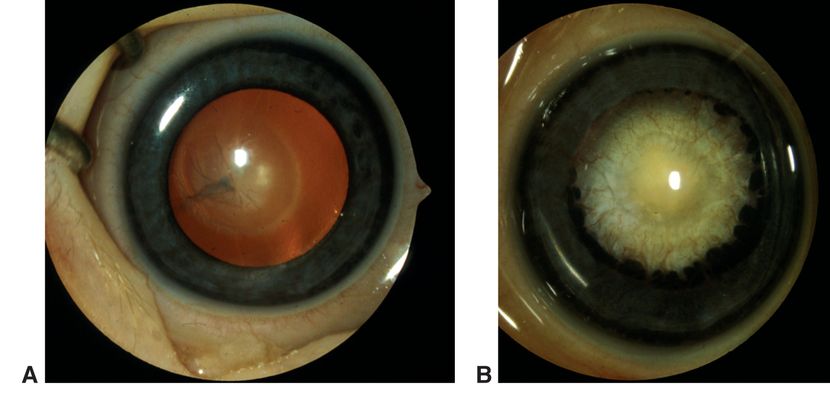
Figure 2. Persistent fetal vasculature (PFV). A. Mild variant with central retrolental membrane. B. Severe PFV variant with traction on ciliary processes. (© 2015 American Academy of Ophthalmology, www.aao.org. Courtesy of David A. Plager, MD.)
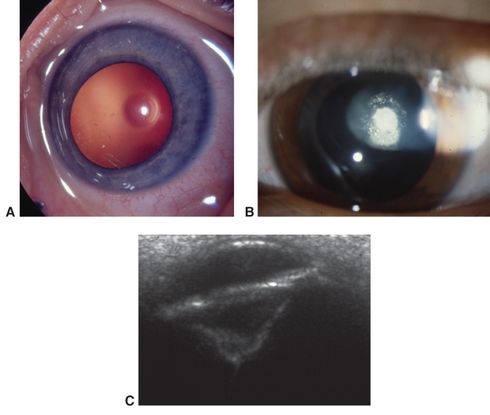
Figure 3. Posterior lenticonus/lentiglobus. A. Early central clear defect in posterior capsule. B. Early opacification of central defect. C. Ultrasound biomicroscopy of advanced posterior lenticonus. (© 2015 American Academy of Ophthalmology, www.aao.org. Part A courtesy of Edward L. Raab, MD; part B, David A. Plager, MD; and part C, Ken K. Nischal, FRCOphth.)
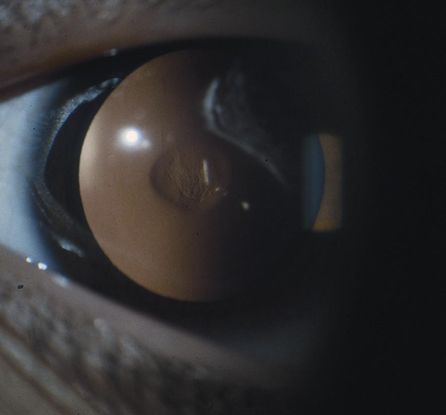
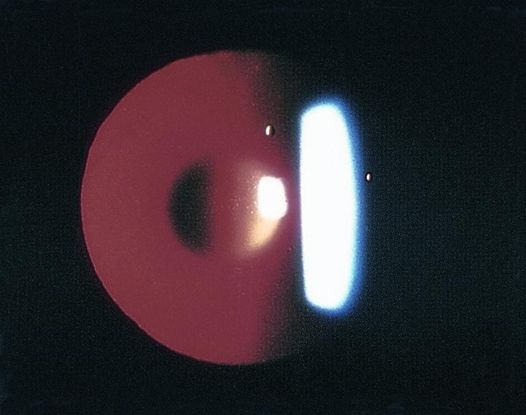
Figure 4. Examples of posterior lenticonus. (© 2015 American Academy of Ophthalmology, www.aao.org. Part A courtesy of David A. Plager, MD.)
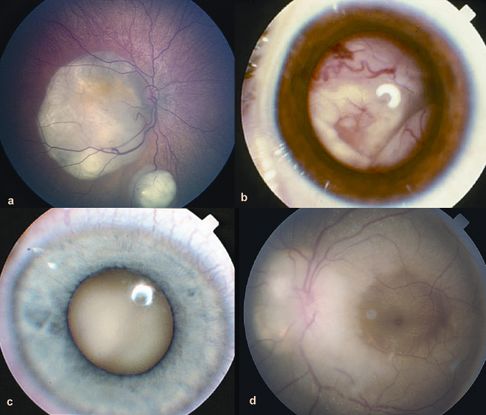
Figure 5. Growth patterns of retinoblastoma. A. Two foci of dome-shaped retinoblastoma. Feeder vessels that dip into the mass and drainer vessels that emerge from the lesion’s periphery. B. Exophytic retinoblastoma that has grown under the retina producing exudative retinal detachment. C. Endophytic retinoblastoma that has grown into the vitreous cavity. The endophytic mass is characterized by the absence of visibility of retinal vessels. D. Diffuse infiltrating retinoblastoma that presents as an ill-defined whitish infiltration of the retina. (© 2015 American Academy of Ophthalmology, www.aao.org.)

Figure 6. Retinoblastoma. (Reproduced from Retina Gallery. Courtesy of Chalamala Jangaiah.)
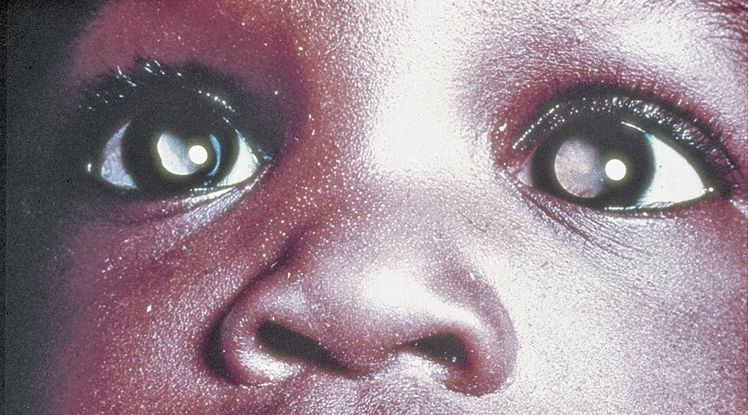
Figure 7. Retinopathy of prematurity, Stage 5 ROP. (© 2015 American Academy of Ophthalmology, www.aao.org.)
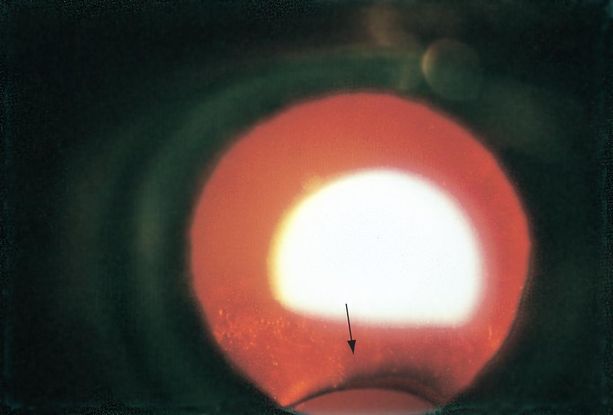
Figure 8. Coloboma of the lens (arrow) viewed by retroillumination. (© 2015 American Academy of Ophthalmology.)
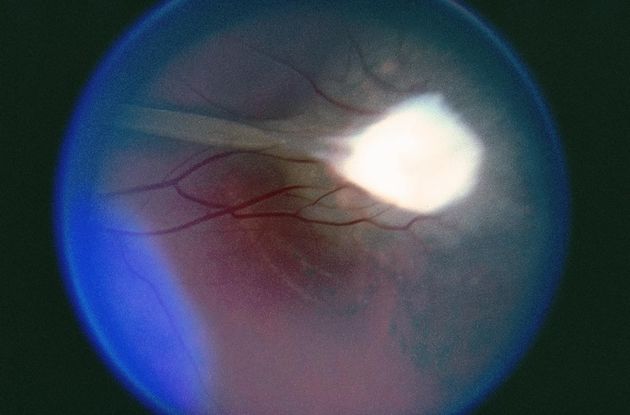
Figure 9. Toxocariasis. (© 2015 American Academy of Ophthalmology, www.aao.org.)
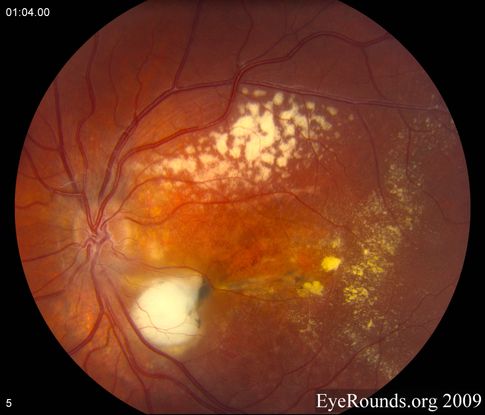
Figure 10. Coats disease. (Reproduced, with permission, from Brinton JP et al. Coats disease. EyeRounds Online Atlas of Ophthalmology.)
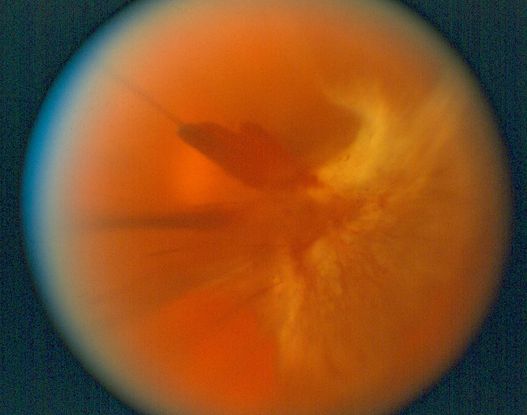
Figure 11. Vitreous hemorrhage. (© 2015 American Academy of Ophthalmology, www.aao.org.)
Pathophysiology/Definition
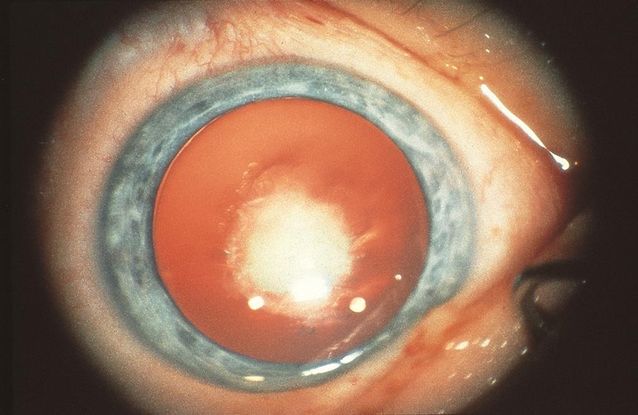
Figure 12. Congenital nuclear cataract. (Reproduced from Day SH. “Understanding and Preventing Amblyopia.” Eye Care Skills for the Primary Care Physician slide script series. San Francisco: American Academy of Ophthalmology, 1987.)

Figure 13. Bilateral congenital cataracts. (© 2015American Academy of Ophthalmology, www.aao.org.)
Signs, Symptoms
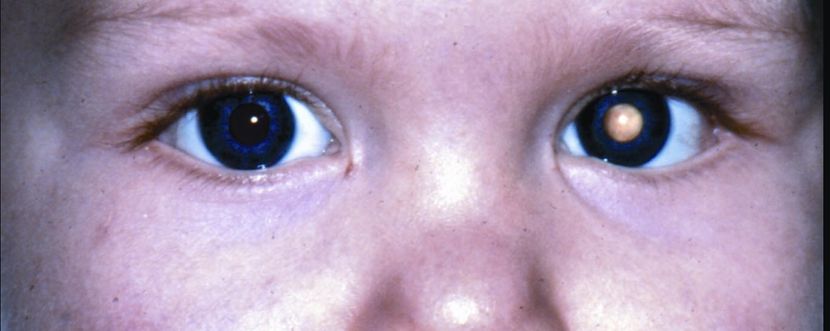
Figure 14. Leukocoria. (© 2015 American Academy of Ophthalmology, www.aao.org.)
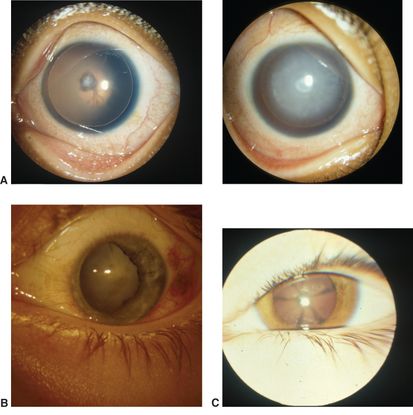
Figure 15. Less common morphologies of cataracts in childhood. A. Aniridia, mild (left) and advanced (right). B. Traumatic. C. Starfish cataract. (© 2015 American Academy of Ophthalmology, www.aao.org. Courtesy of David A. Plager, MD.)
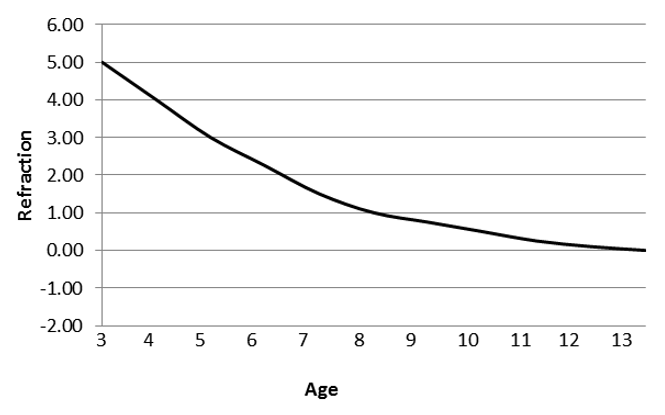
Figure 16. Suggested postoperative target refraction based on age in years (Reproduced, with permission, from Plager et al. Refractive change in pediatric pseudophakia: 6-year follow-up. J Cataract Refract Surg. 2002;28(5):810–5.)
REFERENCES
Agervi P, Kugelberg U, Kugelberg M, Zetterström C. Refractive and visual outcome of paediatric cataract surgery in the Ukraine. Acta Ophthalmol Scand. 2006;84(5):674–8.
Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996;37(8):1532–8.
Chan WH, Biswas S, Ashworth JL, Lloyd CI. Congenital and infantile cataract: aetiology and management. Eur J Pediatr. 2012;171(4):625-30.
Courtright P, Williams T, Gilbert C, et al. Measuring cataract surgical services in children: an example from Tanzania. Br J Ophthalmol. 2008;92(8):1031–4.
Donaldson KE, Gorscak JL, Budenz DL. Anterior chamber and sutured posterior chamber intraocular lenses in eyes with poor capsular support. J Cat Refrac Surg. 2005;903–9.
Edmonds LD, Layde PM, James LM, Flynt JW, Erickson JD, Oakley GP Jr. Congenital malformations surveillance: two American systems. Int J Epidemiol. 1981;10(3):247–52.
Elder MJ, DeCock R. Childhood blindness in the West Bank and Gaza Strip: prevalence, aetiology and hereditary factors. Eye. 1993;7:580–83.
Elsas LJ, Langley S, Steele E, et al. Galactosemia: a strategy to identify new biochemical phenotypes and molecular genotypes. Am J HumGen. 1995;56(3):630–9.
Foster A, Gilbert C. Cataract in children. Acta Paediatrica. 2003;92(12):1376–8.
Foster A, Gilbert C, Rahi J. Epidemiology of cataract in childhood: a global perspective. J Cataract Refract Surg. 1997;23:601–4.
Infant Aphakia Treatment Study Group. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. J AMA Ophthalmol. 2014;132(6):676–82.
James LM, McClearon AB, Waters GD. Congenital malformation surveillance data for birth defects prevention: Metropolitan Atlanta congenital defects program (MACDP) 1968–1991 and birth defects monitoring program (BDMP) 1970–1991. Teratology. 1993;48:545–709.
Johar SR, Savalia NK, Vasavada AR, Gupta PD. Epidemiology based etiological study of pediatric cataract in western India. Indian J Med Sci. 2004;58(3):115–21.
Kalua K, Patel D, Muhit M, Courtright P. Causes of blindness among children identified through village key informants in Malawi. Can J Ophthalmol. 2008;43:425–7.
Lambert SR, Lynn MJ, Reeves R, Plager DA, Buckley EG, Wilson ME. Is there a latent period for the surgical treatment of children with dense bilateral congenital cataracts? J AAPOS. 2006;10:30–6.
Latif, Kanwal, et al. Outcomes of congenital cataract surgery in a tertiary care hospital. Pak J Ophthalmol. 2014;30(1):28.
Lim Z, Rubab S, Chan YH, Levin AV. Management and outcomes of cataract in children: The Toronto experience. J AAPOS. 2012;16:249–54.
Magnusson G, Jaobsson P, Kugelberg U, et al. Evaluation of screening procedures for congenital cataracts. Acta Paediatrica. 2003;92(12):1468–73.
Medsinge A, Nischal KK. Pediatric cataract: challenges and future directions. Clin Ophthalmol. 2015;9:77–90.
Mostafa MS, Temtamy S, El-Gammal MY, Sayed SI, Abdel-Salam M, El-Baroudy R. Genetic studies of congenital cataract. Metab Pediatr Ophthalmol. 1981;5(3-4):233–42.
Mwende J, Bronsard A, Mosha M, Bowman R, Geneau R, Courtright P. Delay in presentation to hospital for surgery for congenital and developmental cataract in Tanzania. Br J Ophthalmol. 2005;89(11):1478–82.
Plager DA, Kipfer H, Sprunger DT, Sondhi N, Neely DE. Refractive change in pediatric pseudophakia: 6-year follow-up. J Cataract Refract Surg. 2002 May;28(5):810–5.
Rahi JS, Dezateux C. Congenital and infantile cataract in the United Kingdom: underlying or associated factors. Invest Ophthalmol Vis Sci. 2000;41(8):2108–14.
Ram J, Gupta N, Sukhija JS, et al. Outcome of cataract surgery with primary intraocular lens implantation in children. Br J Ophthalmol. 2011;95(8):1086–90.
Rana AM, Ali R, Waseem A. Congenital cataracts; its laterality and association with consanguinity. Pak J Ophthalmol. 2014;30(4):187.
Thylefors B. A global initiative for the elimination of avoidable blindness. Community Eye Health. 1998;11(25):1–3.
Trivedi RH, Wilson ME Jr, Golub RL. Incidence and risk factors for glaucoma after pediatric cataract surgery with and without intraocular lens implantation. J AAPOS.2006;10:117–23.
Trivedi RH, Wilson ME. Selection of an initial contact lens power for infantile cataract surgery without primary intraocular lens implantation. Ophthalmology. 2013;120:1973–76.
Trumler AA. Evaluation of pediatric cataracts and systemic disorders. Curr Opin Ophthalmol. 2011;22:365–79.
World Health Organization (WHO). Preventing blindness in children: report of WHO/IAPB scientific meeting. Programme for the Prevention of Blindness and Deafness, and International Agency for Prevention of Blindness. Geneva: WHO, 2000 (WHO/PBL/00.77).
Yorston D, Wood M, Foster A. Results of cataract surgery in young children in east Africa. Br J Ophthalmol.2001;85(3):267–71.