EPIDEMIOLOGY
Global Information
- Polypoidal choroidal vasculopathy (PCV) primarily affects pigmented individuals, especially Asians and African-Americans; however, it is now also recognized in the white population.
- Typically presents in 6th to 7th decade of life, though it may present earlier than other forms of macular degeneration
- Whites are affected to a lesser extent and with different clinical features
- Table 1 highlights the observed differences in PCV characteristics across ethnicities.
Table 1: Polypoidal Choroidal Vasculopathy Demographics and Characteristics |
|
Ethnicity
|
Mean Age
|
% Male
|
% Bilateral
|
% Central Macula Involving
|
% Peripapillary Involving
|
|
White
|
60-70
|
<50
|
20-80
|
25-68
|
35-75
|
|
Asian
|
60-70
|
>60
|
<10
|
>85
|
<15
|
Regional Information (Asia)
- Japan
- 23%-55% of those with age-related macular degeneration (AMD) have signs of PCV
- 92% of patients with PCV have central macula-involving lesions
- 71% male
- 14% bilateral
- Singapore
- 33%-61.1% of those with AMD have PCV
- 58.1%-62.4% male
- Up to 87.8% unilateral
- South Korea
- 24.6% of those with AMD have PCV
- 78.5% male
- 75.9% unilateral
- China and Taiwan
- 24.5%-49% of those with AMD have PCV
- 71.4% male
- 91.8% unilateral
- The Beijing Eye Study reported a PCV prevalence of 0.3%
DIFFERENTIAL DIAGNOSIS
RISK FACTORS/BIOMARKER
- Shared risk factors with AMD-CNV
- Age, male gender, smoking, hypertension, coronary artery disease, and hyperlipidemia
- Female gender was found to be a protective factor
- Large study in Korea: Age, body mass index (BMI), and higher education to be more strongly associated with PCV than AMD-CNV
- Ocular risk factors
- A history of CSC
- Systemic biomarkers
- C-reactive protein (CRP)
- Homocysteine
- Matrix metalloproteinase (MMP)
- Aqueous/vitreous biomarkers
- a. Interleukin-1b (IL-1b)
- VEGF
- Pigment epithelium derived factor (PEDF)
PATHOPHYSIOLOGY/DEFINITION
PCV is a neovascular and hemorrhagic disorder of the choroid.
Histology
- Degeneration of the walls of arterioles, capillaries, and large, dilated venules
- Large vascular channels and “polypoid” formations (Figure 9)
- Thickened capillary basement membranes
- Hyalinization of choroidal vessels
- Exudation of fibrin and plasma
Genetic Factors
- Gene HTRA1 is associated with PCV as well as neovascular AMD
- Complement factor H (CFH) variants rs3753394 and rs 800292 are associated with PCV
- CRP levels are higher in PCV patients and in AMD patients than in control patients
- I62V variant of CFH is associated with PCV in Japanese patients
- ARMS2 gene for mitochondrial photoreceptor protein is associated with PCV in Japanese patients
- CETP genetic variants to be associated with a high risk of PCV and is concordant with reports of elevated HDL as a risk factor for AMD, suggesting possible involvement of the high-density lipoprotein pathway
- Recent meta-analysis (2015)
- Complement cascade (CFH Y402H SNP rs1061170, CFH I62V SNP rs800292, C2 SNP rs547154, CFB SNP rs4151657, RDBP SNP rs3880457, and SKIV2L SNPs rs2075702 and rs429608)
- Inflammatory pathway (TNFRSF10A-LOC389641SNP rs13278062 and BEST- C4orf14-POLR2B-IGFBP7 SNP rs1713985)
- Extracellular matrix/basement membrane regulation pathway (ARMS2 A69S SNP rs10490924 and HTRA1 promoter SNP rs11200638) and lipid metabolism pathway (CETP SNP rs3764261)
- Previously associated with PCV (ARMS2, HTRA1, C2, CFB, ELN, LIPC, LPL, ABCA1, VEGF-A, TLR3, LOXL1, SERPING1, and PEDF) were not significantly associated with PCV in this meta-analysis
- Genotype associated with retreatment response
- ARMS2 LOC387715 rs10490924 variant: larger lesion size, higher likelihood of vitreous hemorrhage, and worse visual outcome after PDT or combination therapy
- ARMS2 A69S risk genotype :higher risk for second eye involvement
- CFH I62V polymorphism: associated with choroidal thickness
- ARMS2 A69S (rs10490924), HTRA1-rs11200638, PEDF gene polymorphism (SERPINF1 rs12603825): poor outcome after PDT
Vascular endothelial growth factor (VEGF) plays an uncertain role in the pathophysiology of PCV.
- VEGF has been localized to the retinal pigment epithelium (RPE) in patients with PCV
- Aqueous levels of VEGF were higher in PCV eyes compared to controls, but were significantly lower than aqueous VEGF levels in CNV-AMD
- VEGF has not been seen in the vascular endothelium on immunostaining of surgical specimens
- Anti-VEGF treatment has been well-reported to be effective in treating fluid associated with PCV; however, it has shown inconsistent results in resolving the polypoid vascular lesions
- While VEGF related angiogenesis processes were important in PCV pathogenesis, different angiogenesis pathways and cascades may be implicated as well
Natural Course
- Depends on factors including location (peripapillary vs macular), size of the lesion, and associated bleeding and exudation
- Polypoidal structures may also involute spontaneously
- Recurrent serosanguinous detachments of the RPE or neurosensory retina
- Microtears of RPE
- RPE atrophy
- Favorable course was seen in half of all patients with PCV while the other half had recurrences and eventual visual loss.
- 11% cumulative incidence of second-eye involvement in PCV in five years
Definition
- The Japanese Study Group of PCV
- Definite PCV: protruded orange-red elevated lesions on fundus examination and/or characteristic polypoidal lesions on ICGA
- Probable PCV: only an abnormal vascular network or occurrence of recurrent hemorrhagic and/or serous detachment of RPE were observed
- EVEREST study
- Subretinal focal ICGA hyperfluorescence with presence of at least one of the following
- Branching vascular networks (BVNs)
- Pulsatile polyp
- Nodular appearance on stereoscopic viewing
- Hypofluorescent halo
- Orange subretinal nodule on fundus photo
- Presence of massive submacular hemorrhage
SIGNS/SYMPTOMS
Clinical Characteristics
- Serosanguinous detachments of the RPE and neurosensory retina
- PCV lesions and choroidal neovascularization (CNV) (→The hallmark of PCV is the polypoidal lesion, which may be visible as an orange-red nodule on fundus examination)
- Typically peripapillary or central macula location
- Relatively preserved retinal architecture
- Better-than-expected visual acuity
- Pigment epithelial detachment associated with polypoid lesions
- Chronic lipid deposition
- Occasional spontaneous resolution with residual pigment mottling
- 2 types of PCV
- Exudative, characterized by serous PED and retinal detachment
- Hemorrhage, characterized by hemorrhagic PED and subretinal hemorrhage at the macula
Clinical characteristics may differ in white patients;
- Lesions are primarily peripapillary
- Drusen may also be seen in white patients
Imaging
Fluorescein angiogram (FA) (Figure 10)
- Branching vascular networks are often located in Bruch’s membrane (occult CNV)
- Polypoidal lesions can at times be difficult to see on FA. Lesions may manifest as either classic or occult leakage patterns
- Atrophy of RPE
Indocyanine green angiography (ICG) (Figure 11)
- Gold standard for diagnosis of PCV
- Choroidal vessel hyperpermeability
- Pulsatile polypoidal vessels
- Choroidal vascular networks
Optical coherence tomography (OCT) (Figure 12)
- Sub-RPE polypoidal lesions
- Pigment epithelial detachments
- Notch in the margin of large PED indicates the site of polypoidal lesions
- “Double layer sign”: two hyper-reflective lines represent separation of the RPE from Bruch’s membrane
- Higher rates of choroidal thickening
Fundus autofluorescence (FAF)
- Central hypo-autofluorescence with a circumferential hyper- autofluorescent ring corresponding to polypoidal lesions
- Granular hyperautofluorescence, corresponding to the branching choroidal vascular network
- Useful, non-invasive adjunct to ICGA and OCT for diagnosis and monitoring of treatment
OCT angiography
- The combination of en face and cross-sectional OCT angiographic images provides anatomical information about polypoidal structures
- More detailed view of type 1 CNV or branching vascular network complicated with PCV than ICGA
- Detection rate of polypoidal structures is lower than ICGA
- Only 50% of polyps were detected by OCT angiography from recent study performed in Korea
MANAGEMENT
- Photocoagulation
- Effective for treating extrafoveal polyps, but success rate is variable
- Persistent exudation from branching vascular network reported in 28.5%-37.7%
- Yuzawa reported that only 10% of eyes worsened if both the polyp and network were lasered, compared to 54% of eyes worsened after laser to polyps only. However, lasering the whole network is often impossible due to the large area involved
- Combined with anti-VEGF, photocoagulation has been shown to confer additional benefit in reducing exudation and hemorrhage from the network, while focal laser can be targeted mainly to the polyps.
- Treatment of the entire lesion is effective in resolving exudative lesions and preventing further vision loss
- Treatment of only the polypoid lesion does not prevent recurrence or progression
- Laser photocoagulation is reserved for extrafoveal polyps. Treating the entire lesion including the branching vascular network may not always be possible.
- Anti-VEGF
- The pathophysiology of polypoidal lesions may not be a VEGF-driven process
- Intravitreal bevacizumab has been shown to be effective treatment for fluid associated with PCV, but has not been shown to improve polypoidal lesions (25%-40% of polypoidal lesion regression rate)
- BVNs do not appeared to be affected by anti-VEGF monotherapy
- Early results of intravitreal aflibercept suggest that aflibercept may be more effective at achieving polypoidal lesion closure (55%-70%) than other ant-VEGF agents.
- Photodynamic therapy (PDT)
- PDT has been shown to be effective in preserving or improving visual acuity (80% of patients)
- PDT may be more effective for PCV than for wet AMD
- PDT has been shown to be effective in closure of polypoidal lesions (>70%), but, ineffective in causing regression of the BVN
- Retreatment rates are much lower than anti-VEGF therapy
- Complications: acute vision loss, progressive RPE atrophy, RPE tears, subretinal hemorrhage and vitreous hemorrhage
- Combination therapy (PDT+anti-VEGF)
- Anti-VEGF may also counteract the potential upregulation of VEGF after the application of PDT
- EVEREST study, the combination arm achieved the best angiographic and visual outcome, with the highest proportion of complete polypoidal lesion regression (78%) and highest mean gain in vision (+10.9 letters)
- Fujisan study reported the similar visual and anatomical improvements at 1 year between initial and deferred PDT combined with ranibizumab
- Anti-VEGF vs PDT:
- Many studies reporting up to 12-month follow-up have described stabilization of vision and improvement of exudation
- Inoue et al reported better vision at month 18 and 24 in eyes treated with intravitreal ranibizumab compared to those treated with PDT
- LAPTOP study based in Japan (Phase IV, prospective RCT) reported better visual outcomes in eyes treated with ranibizumab than eyes treated with PDT at month 12 and month 24
- Concern remains related to incomplete polyp regression, which has been thought to lead to recurrence
- Higher recurrence rate has been reported in anti-VEGF-treated eyes (64.9%) compared to PDT treated eyes (47.4%) at 2 years in a study based in Korea
- EVEREST study: A multicenter RCT reported higher polyp closure rate in eyes treated with PDT (77.8% combined with ranibizumab and 71.4% PDT alone) compared to eyes treated with ranibizumab alone (28.6%). However, visual acuity gain in the ranibizumab group was numerically higher than PDT monotherapy group (9.2 letters vs 7.5 letters, not statistically significant)
- Some centers (particularly in Japan) recommend reserving PDT to eyes with poor vision to minimize the risk of visual deterioration that may be seen in PDT
- Other treatment options for PCV
- Radiation therapy: stereotactic radiation therapy (SRT) has been shown 83% polypoidal lesion regression rate and improvement of 7.6 ETDRS letters at month 12 after a single dose of 16-Gy SRT and PRN ranibizumab in one study
- Management of submacular hemorrhage
- 20%-60% of cases, PCV has been found to be the cause of submacular hemorrhage
- Pneumatic displacement and recombinant tissue plasminogen activator (rtPA) injection with vitrectomy
- Anti-VEGF monotherapy alone may be effective for thin submacular hemorrhage
- Peripapillary PCV
- Anti-VEGF monotherapy represent the safest treatment option
- The optimal therapy for PCV is still unclear. Currently there are two clinical trials comparing anti-VEGF alone with combination therapy (anti-VEGF and PDT).
- EVEREST 2: ranibizumab monotherapy vs. ranibizumab+PDT
- PLANET: aflibercept monotherapy vs. aflibercept with adjunctive photodynamic therapy
Treatment Algorithm
- Workup: clinical history, dilated fundus exam (FA/ICG)
- If visual acuity is 20/30 or better: Observation, though treatment initially with anti-VEGF may be considered in patients with good visual acuity but significant exudate
- If visual acuity is 20/40 or worse: Consider treatment for symptomatic patients
- PDT is often effective initial treatment for PCV where available
- If the polypoidal lesion is associated with significant exudate or fluid, consider PDT + anti-VEGF therapy
- Recurrent associated fluid that is visually significant and responsive to anti-VEGF intravitreal injection may require repeat anti-VEGF injections to maintain stable vision
- If lesions are not responsive to repeat injections of an initial anti-VEGF agent, a switch to a different agent may be effective (eg, switching from ranibizumab to aflibercept)
- If PDT is not an available treatment option, laser photocoagulation of polypoidal lesions outside of the foveal avascular zone may be an effective treatment strategy; however, laser photocoagulation has been associated with macular hemorrhage and should be performed only if the entire lesion can be treated
CASE STUDY: Japan/Asia
History of Present Illness
- A 69-year-old Japanese male patient with no past medical history or ocular history presented to a clinic complaining of decreased visual acuity of the right eye for 2 months
Examination
- Visual acuity: 20/100 right eye; 20/30 left eye
- Intraocular pressure (IOP): 15 mm/Hg both eyes
Slit-lamp examination
- Lens: mild nuclear sclerotic cataract of both eyes
Fundus examination
- Macula: Drusen. Parafoveal polypoidal vascular lesions beneath the RPE in the right eye
Testing
- FA and ICG showed polypoidal choroidal neovascularization in the right eye
- Macular OCT showed serous pigment epithelial detachments and subretinal pigment epithelium polypoidal lesion of the right eye
Treatment
- PDT
- Anti-VEGF therapy: The patient was treated with ranibizumab monthly injections for 3 months, followed by prn injections for a total of 4 injections in 6 months
Follow-up
- After 6 months, visual acuity was 20/40 in the right eye. The polypoidal neovascular lesions showed signs of regression on FA and ICG. The serous exudative detachment was significantly improved.
IMAGE LIBRARY
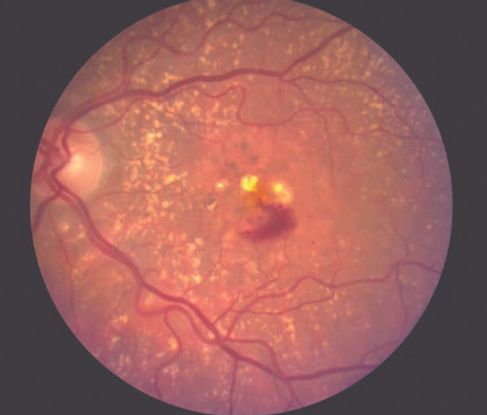
Figure 1. Age-related macular degeneration (AMD) ©American Academy of Ophthalmology, 2014.
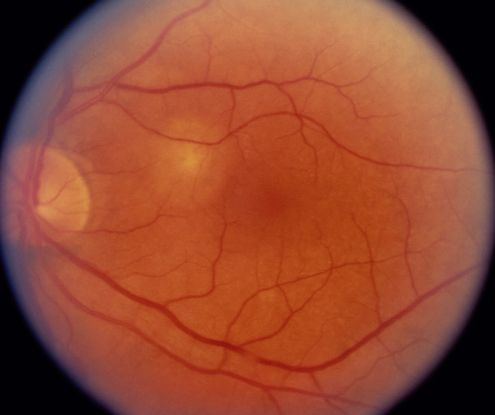
Figure 2. Choroidal neovascularization (CNV) ©American Academy of Ophthalmology, 2014.
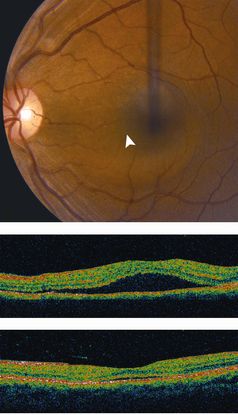
Figure 3. Central serous chorioretinopathy (CSC) ©American Academy of Ophthalmology, 2014.
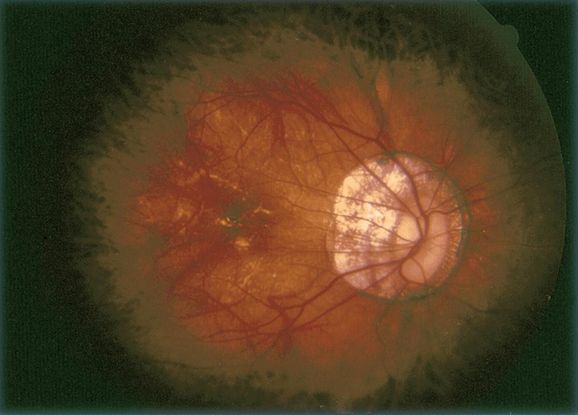
Figure 4. Pathologic myopia ©American Academy of Ophthalmology, 2014.
Figure 5. Melanocytoma ©American Academy of Ophthalmology, 2014.
Figure 6. Choroidal tumors (hemangioma, melanoma, etc) ©American Academy of Ophthalmology, 2014.
Figure 7. Tilted disc syndrome ©American Academy of Ophthalmology, 2014.
Figure 8. Choroidal osteoma ©American Academy of Ophthalmology, 2014.
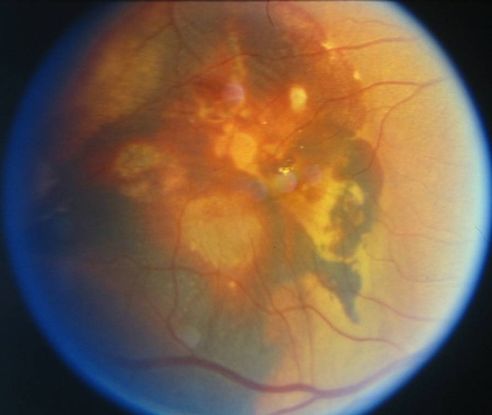
Figure 9. Large vascular channels and “polypoid” formations ©American Academy of Ophthalmology, 2014.
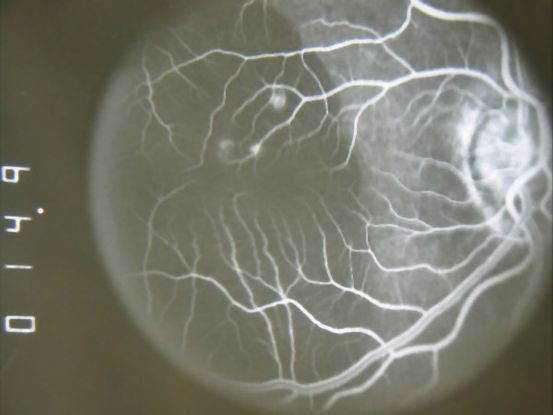
Figure 10. Fluorescein angiogram (FA) ©American Academy of Ophthalmology, 2014.
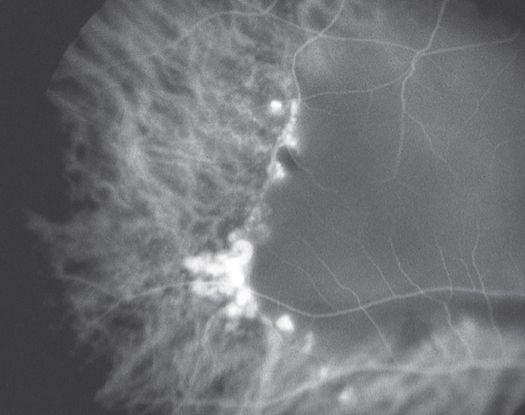
Figure 11. Indocyanine green angiography (ICG) ©American Academy of Ophthalmology, 2014.
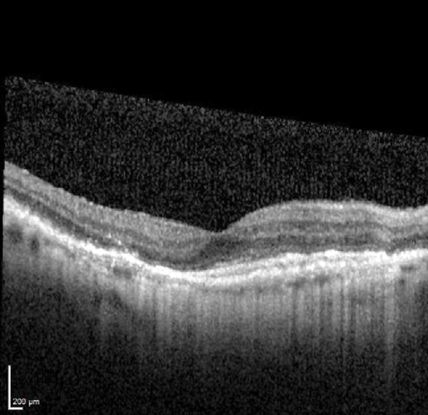
Figure 12. Optical coherence tomography (OCT) ©American Academy of Ophthalmology, 2014.
CASE IMAGES (Asia Pacific)
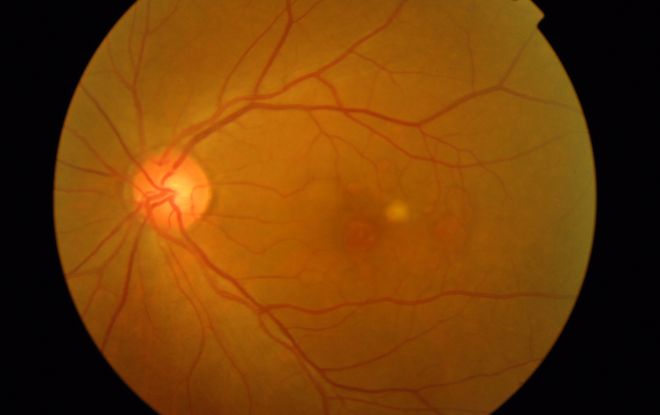
Figure 4a
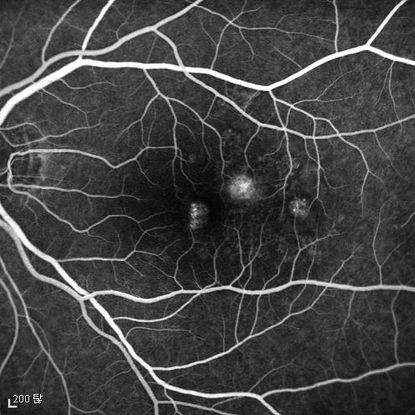
Figure 4b
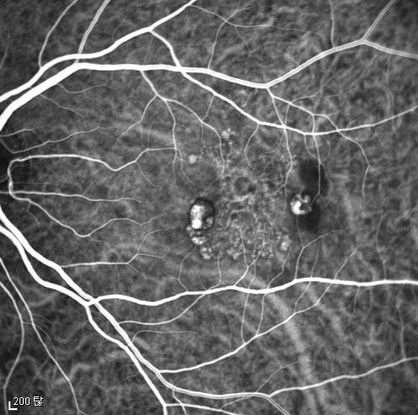
Figure 4c
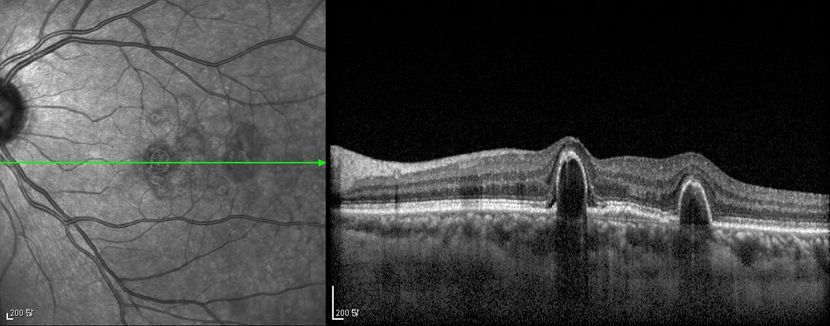
Figure 4d
Figures 4a-4d. Case Images Asia. A 58-year-old woman with reduced visual acuity for 1 month. BCVA: 20/40. Orange round lesions at the fovea in color photograph (A) represents polyps that are revealed in fluorescein angiography (B) and in indocyanine green angiography (C). Branching vascular networks are obvious in ICG. Section through the polyps in OCT (D) reveals local pigment epithelial detachments (Case and images courtesy of Young Hee Yoon MD, Asan Medical Center, Ulsan University, Seoul, Korea.)
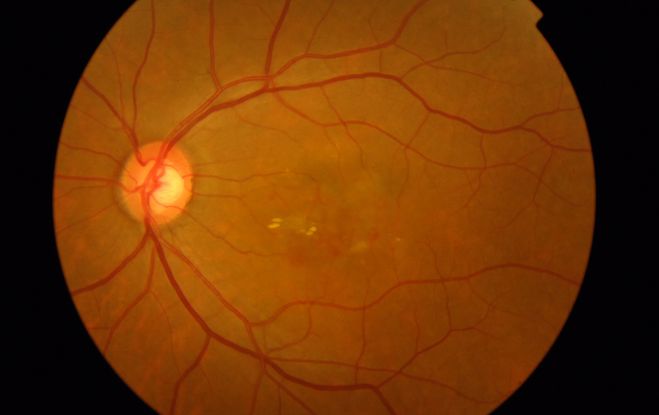
Figure 5a
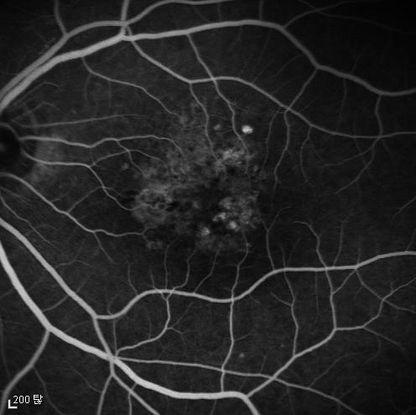
Figure 5b
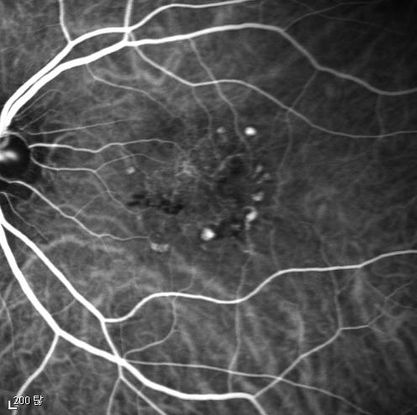
Figure 5c
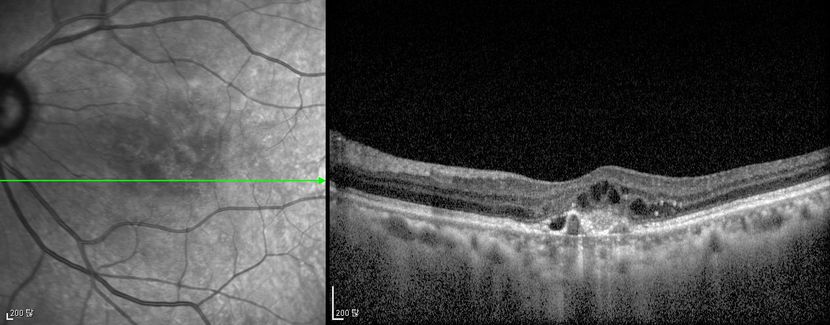
Figure 5d
Figures 5a-5d. Case Images Asia. A 60- year-old woman with reduced visual acuity for 2 weeks. BCVA: 20/80. Retinal hemorrhage with exudate at the fovea in color photograph (A) represents polyps that are revealed in fluorescein angiography (B) and in indocyanine green angiography (C). Branching vascular networks are obvious in ICG. Section through the polyps in OCT (D) reveals local pigment epithelial detachments and fluid collection at subretinal and intraretinal space (Case and images courtesy of Young Hee Yoon MD, Asan Medical Center, Ulsan University, Seoul, Korea.)
REFERENCES
Ahuja RM, Stanga PE, Vingerling JR, Reck AC, Bird AC. Polypoidal choroidal vasculopathy in exudative and haemorrhagic pigment epithelium detachments. Br J Ophthalmol. 2000; 84(5): 479-484.
Bozzoni Pantaleoni F, Magliari Galante V, Da Dalt S, Pecorella I. Localizing polypoidal choroidal vasculopathy for laser treatment. Acta Ophthalmol Scand. 2007; 85(4): 456-458.
Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009; 148 (1): 70-78.
Gemmy Cheung CM, Yeo I, Li X, et al. Argon laser with and without anti- vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy. Am J Ophthalmol . 2013; 155: 295- 304 e291.
Gomi F, Sawa M, Sakaguchi H, et al. Efficacy of intravitreal bevacizumab for choroidal vasculopathy. Br J Ophthalmol. 2008; 92(1): 70-73.
ImamuraY, Engelbert M, Iida T, Freund KB, Yannuzzi LA. Polypoidal Choroidal Vasculopathy: A Review. Surv Ophthalmol. 2010: 55(6):501-515.
Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term efficacy of intravitreal aflibercept in treatment naïve patients with polypoidal choroidal vasculopathy. Retina. 2014; 34 (11):2178-2184.
Jeon S, Lee WK, Kim KS. Adjusted retreatment of polypoidal choroidal vasculopathy after combination therapy: results at 3 years. Retina. 2013; 33: 1193-1200.
Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A. LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol. 2007; 144(4): 608- 612.
Kwok AK, Lai TY, Chan CW, Neoh EL, Lam DS. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol. 2002; 86(6): 892-897.
Lafaut BA, Leys AM, Snyers R, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000; 238(9): 751-759.
Lee KY, Vithana EN, Mathur R, et al. Association analysis of CFH, C2, BF, and HTRA1 gene polymorphisms in Chinese patients with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49(6): 2613-2619.
Lee MW, Yeo I, Wong D, Ang CL. Argon laser photocoagulation for the treatment of polypoidal choroidal vasculopathy. Eye (Lond.). 2009; 23(1):145-148.
Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007; 144(1): 15- 22.
Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinico-pathological findings of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49(11): 4729-4737.
Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol. 2002; 86(10): 1093-1098.
Reche-Frutos J, Calvo-Gonzales C, Donate-Lopez J, Garcia-Feijoo J, Leila M, Garcia-Sanchez J. Short- term anatomic effect of ranibizumab for polypoidal choroidal vasculopathy. Eur J Ophthalmol. 2008; 18(4): 645- 648.
Saito M, Kano M, Itagaki K, Oguchi Y, Sekiryu T. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. 2014; 34(11):2192-2201,
Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121: 1392- 1396.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995; 15: 100-110.
Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002; 86(3): 321-327.
Tsujikawa A, Sasahara M, Otani A, et al. Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2007; 143(1): 102-111.
Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003; 47(4): 379-384.
CONTRIBUTORS
Executive Editor:
R. V. Paul Chan, MD, FACS, Weill Cornell Medical College, New York, New York
Section Editor:
Asia-Pacific:
Timothy Y. Lai, MBBS, MD, MMedSc, FRCS, FRCOphth, FHKAM, Department of Ophthalmology & Visual Sciences, The Chinese University of Hong Kong
Associate Editors:
Jeff Pettey, MD, University of Utah Department of Ophthalmology and Visual Sciences, John Moran Eye Center's Residency Program Director
Grace Sun, MD, Weill Cornell Eye - Lower Manhattan, Weill Cornell Medical College Residency Program Director
Assistant Editors:
Samir Patel, BS, Weill Cornell Medical College, New York, New York
Peter Coombs, MD, Weill Cornell Medical College; New York, New York
Regional Contributors:
Asia Pacific:
Dr. Gemmy Cheung, Consultant Ophthalmologist, Singapore National Eye Centre
Dr. Andrew Fok, Hong Kong Eye Hospital, Hong Kong
Dr. Lim Tock Han, NHG Eye Institute, Tan Tock Seng Hospital, Singapore
American Academy of Ophthalmology
P.O. Box 7424
San Francisco, CA 94120-7424
415.561.8500
Copyright © 2014 American Academy of Ophthalmology®. All Rights Reserved.