EPIDEMIOLOGY
Global Information
- Glaucoma is the most common cause of preventable, irreversible blindness worldwide.
- Primary open-angle glaucoma (POAG) is responsible for 12.3% of blindness worldwide.
- In people older than 40 years old, the prevalence of primary open-angle glaucoma (POAG) is 1.86%.
- People in their 70s have a 3 to 8 times higher prevalence of POAG. As the population ages, the total number of glaucoma patients will continue to rise.
Regional Information
- POAG prevalence is estimated to be highest among Chinese people, intermediate in Japanese, and lower in Europeans and Indians.
- In a rural East African population-based study, 3,268 underwent ophthalmic examination. POAG was diagnosed in 3.1%.
- A hospital-based survey of the glaucomas in Ghana reported that the most common form of glaucoma was primary open-angle glaucoma (44.2%).
- See Table 1 for additional prevalence data and Table 2 for incidence data.
|
Table 1. The Prevalence of Definite Open-Angle Glaucoma as Reported in Other Studies
|
|
Study
|
Racial/Ethnic Group
|
Age-Specific Prevalence
Age Groups (years)
|
|
40–49
|
50–59
|
60–69
|
70–79
|
80+
|
Total
|
|
Baltimore Eye Study1
|
African American
|
1.27
|
4.15
|
6.19
|
8.88
|
12.87
|
4.97
|
|
Barbados Eye Study2
|
Afro-Caribbean
|
1.4
|
4.1
|
6.7
|
14.8
|
23.2
|
6.8
|
|
LALES
|
Latino
|
1.32
|
2.92
|
7.36
|
14.72
|
21.76
|
4.74
|
|
Proyecto VER3
|
Latino
|
0.50
|
0.59
|
1.73
|
5.66
|
12.63
|
1.97
|
|
Baltimore Eye Study1
|
Non-Hispanic White
|
0.18
|
0.32
|
1.53
|
3.33
|
1.94
|
1.44
|
|
Blue Mountains Eye Study4
|
Non-Hispanic White
|
0.4*
|
1.3
|
4.7
|
11.4
|
3.0
|
|
Visual Impairment Project5
|
Non-Hispanic White
|
0.5
|
1.5
|
4.5
|
8.6
|
9.9
|
3.4
|
|
Beaver Dam Eye Study6
|
Non-Hispanic White
|
|
|
|
|
|
2.1
|
|
Roscommon7
|
Non-Hispanic White
|
|
0.72
|
1.76
|
3.2
|
3.05
|
1.88
|
- Tielsch JM, Sommer A, Katz J, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374.
- Leske MC, Connell AM, Schachat AP, Huyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol .1994;112:821–829.
- Quigley HA, West SK, Rodriguez J, Munoz B,Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–1826.
- Mitchel P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–1669.
- Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998;105;733–739.
- Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma: the Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–1504.
- Coffey M, Reidy A, Wormald R, Xian WX, Wright L, Courtney P. Prevalence of glaucoma in the west of Ireland. Br J Ophthalmol. 1993; 77:17–21.
LALES = Los Angles Latino Eye Study.
*The study combined ages 40–59 into one group.
NOTE: The studies reporting prevalence used different definitions of disease; therefore, caution should be exercised when comparing these studies.
Reprinted, with permission, from Varma R, Ying-Lai M, Francs B, et al, Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1445. Also appearing in American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern® Guidelines. Primary Open-Angle Glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2010. Available at: www.aao.org/ppp.
|
|
Table 2. Incidence of Primary Open Angle Glaucoma in Population-Based Studies
|
|
Country
|
Race
|
Age
|
Incidence (5-Year Risk %)
|
|
Sweden1
|
White
|
≥55
|
1.2
|
|
Sweden2
|
White
|
65–74
|
3.1
|
|
Barbados3
|
Black
|
≥40
|
2.8
|
|
Australia4
|
White
|
≥40
|
1.1
|
|
Netherlands5
|
White
|
≥55
|
1.8
|
|
1 Bengtsson BO. Incidence of manifest glaucoma. Br J Ophthalmol. 1989;73:483–487.
2 Ekström C. Elevated intraocular pressure and pseudoexfoliation of the lens capsule
as risk factors for chronic open-angle glaucoma. A population-based five-year follow-up
study. Acta Ophthalmol (Copenh). 1993;71:189–195.
3 Leske MC, Connell AM, Wu SY, et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. The Barbados Eye Studies Group. Arch Ophthalmol. 2001;119:89–95.
4 Mukesh BN, McCarty CA, Rait JL, Taylor HR. Five-year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology. 2002:109;1047–1051.
5 de Voogd S, Ikram MK, Wolfs RC, Jansonius NM, Hofman A, de Jong PT. Incidence of open-angle glaucoma in a general elderly population: the Rotterdam Study. Ophthalmology. 2005;112:1487–1493.
|
DIFFERENTIAL DIAGNOSIS
- Secondary open-angle glaucoma (another disease like eye injury or inflammation leads to increased eye pressure)
- Ocular hypertension
- Physiologic optic nerve cupping
- Static appearing nerve after optic nerve insult (prior glaucomatous nerve damage from steroid induced glaucoma, uveitic glaucoma, trauma or pigmentary glaucoma with the inducing cause now absent)
- Optic atrophy (Figure 1)
- Congenital optic nerve defects
- Optic nerve drusen (Figure 2)
- Intermittent intraocular pressure elevation (secondary to angle-closure or glaucomatocyclitic crisis)
- Coloboma (Figure 3)
- Optic nerve pit
- Compressive optic nerve lesions
- Anterior ischemic optic neuropathy
- Toxic or nutritional optic neuropathy
PATHOPHYSIOLOGY/DEFINITION
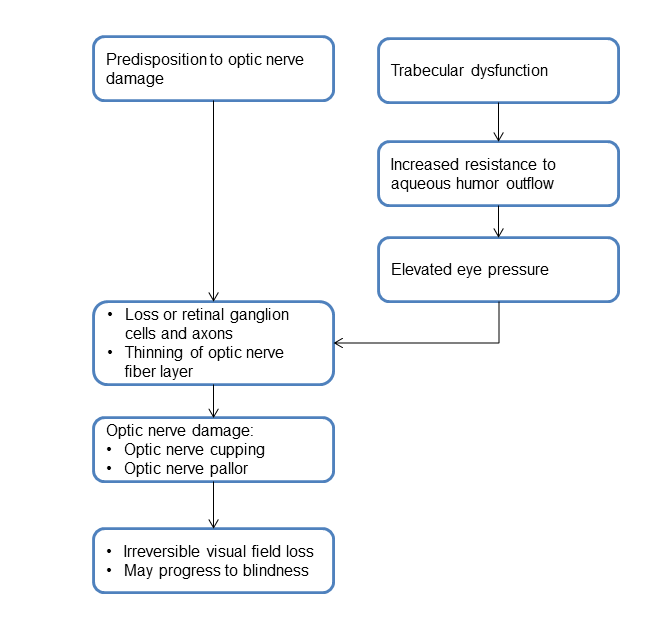

Chart 1. Pathophysiology.
SIGNS/SYMPTOMS
- The patient may present without pain or ocular symptoms.
- Optic nerve abnormalities include the following:
- Increased cup to disc (C:D) ratio (Figure 4)
- Vertical elongation with vertical C:D >0.4
- Rim notching
- Asymmetry of C:D between the eyes greater than 0.2 (Figure 5)
- Disk or disk edge hemorrhages (Figure 6)
- Intraocular pressure may be elevated greater than 22 mmHg or may be normal.
- Note that this cutoff will miss 30% to 50% of people with glaucoma and the complete clinical evaluation is necessary to rule in or out the diagnosis of POAG.
- Repeated testing at different times of the day is needed for some patients (IOP will vary from time of day—usually higher in morning and lower in early evening).
- Gonioscopy demonstrates open angle (Figure 7).
- Visual field testing may demonstrate visual field defects that do not respect the vertical meridian but do respect the horizontal meridian.
- Visual field loss that begins mid-peripherally (Figure 8)
- Optic disc imaging (e.g. Optical coherence tomography, Scanning laser ophthalmoscopy or Scanning laser polarimetry) demonstrate thinning of the retinal nerve fiber layer and/or optic nerve head rim.
The screening for glaucoma should include the following:
- Family history of glaucoma
- Central corneal thickness
- Intraocular pressure
- Gonioscopy
- Dilated fundus exam with C:D ratio noted with any indication of disk hemorrhage
- Fundus photos (important for comparison)
- Humphrey visual field 24-2, SITA Standard or Fast strategy
- Optic disc imaging if possible
MANAGEMENT
Medical Management
For intraocular pressure > 21mmHg or optic nerve changes with or without visual field defects):
- Prostaglandin analogs (latanoprost, travoprost, bimatoprost, tafluprost)
- Possibly not for uveitic glaucoma or pregnant women
- Can cause iris and lid skin pigmentation, eyelash growth and enophthalmos.
- Topical beta-blockers (non-selective: timolol, levobunolol, relatively selective: betaxolol)
- Use with caution in patients with reactive pulmonary disease, asthma, arrhythmias, heart block or myasthenia gravis (wider safety margin with relatively selective agents, but less effective).
- Alpha-agonists (apraclonidine, brimonidine)
- Contraindicated if using a monoamine oxidase inhibitor
- Contraindicated in infants and children
- Topical carbonic anhydrase inhibitors (dorzolamide, brinzolamide)
- Oral carbonic anhydrase inhibitors (acetazolamide)
- Cannot be used in patients with sulfa allergy or renal failure.
- Use with caution if the patient is on diuretics or digitalis.
- Miotics (pilocarpine)
- Accommodative spasm and dimness of vision have reduced frequency of use, despite effectivenss.
Laser Therapy: Lasertrabeculoplasty (LTP)
- Options for laser therapy include Argon laser trabeculoplasty (ALT), diode laser trabeculoplasty, and selective laser trabeculoplasty (SLT).
- ALT is performed with a goniolens used to apply a 50-mm laser for 0.1 seconds at the junction of the anterior nonpigmented and posterior pigmented edge of the trabecular meshwork. Power is titrated, starting at 300 mW up to 1000 mW until blanching of the trabecular meshwork occurs. 40-50 applications are applied over 180o.
- The diode laser uses a 75-mm laser at 600–1000 mW for 0.1 seconds.
- SLT targets intracellular melanin using a 532-nm Q switched Nd:YAG laser at 0.4 to 1.0 mJ for 0.3 ns to create a 400-mm spot size.
- Contraindications include inflammatory glaucoma, iridocorneal endothelial syndrome (ICE), neovascular glaucoma, synechial angle closure or developmental glaucoma.
- Risks of LTP include transient rise in intraocular pressure in up to 20% of patients, a low-grade iritis, hyphema, or the formation of posterior anterior synechiae.
- Effects begin 4 to 6 weeks after treatment with 80% of patients achieving success with lower IOP for at least one year, 50% with lower IOP for 3–5 years and 30% with success last ing at least 10 years.
- LTP may be repeated, but repeat treatments may have decreased success rate.
Surgical Intervention
Surgical intervention if medical management fails:
- Trabeculectomy: 50%–95% success
- Molteno, Baerveldt, and Ahmed: 50%–85% success with medications
- Cyclocryotherapy: 33% success with high complications
- Endoscopic cyclophotocoagulation: 50% success
- Cycloablation/YAG used for resistant cases
Patient-Specific Considerations
- The management of glaucoma patients needs to be individualized and generally depends on the presenting IOP, the age of the patient, the severity of damage and the socioeconomic status of the patient.
- Patients ≤40 years (ie, juvenile open-angle glaucoma patients) are often scheduled for surgery within the first 2–4 weeks of presentation. These patients often present with advanced disease and very high IOP.
- Generally, trabeculectomy is done for patients with high intraocular pressure at presentation (IOP≥30mmHg) and advanced disease.
- Patients with early to moderate disease and presenting IOP <22 mmHg are often counseled regarding initial treatment and may be managed medically or with laser therapy. Careful discussion regarding the risks and benefits to either approach can help lead a patient to a decision which is best for his or her clinical profile.
- Patients are offered trabeculectomy when IOP remains uncontrolled, when there is progression of the visual fields, or when there are issues of nonadherence or affordability of medications is an issue. Trabeculectomies are augmented with antimetabolites (5-FU and/or mitomycin C).
- Glaucoma drainage devices (eg, Molteno, Baervelt, Ahmed devices) are used as second- or third-line surgical management after a failed trabeculectomy and trabeculectomy revision, although some specialists employ them as their first-line surgical procedure. Cyclocryotherapy and cyclophotocoagulation are often reserved for painful blind eyes and intractable glaucoma.
CASE STUDY
History of Present Illness
A 75-year-old male was sent to the Glaucoma Clinic as a glaucoma suspect because of his optic nerve appearance. Glaucoma had not been suspected before. Years before, he had retinal focal photocoagulation in both eyes for retinal tears, without complications. His past medical history included a prostatic hypertrophy, for which he was taking medical treatment.
Examination:
- His ophthalmological examination revealed best corrected visual acuity of 20/25 in both eyes, using -2.50-1.50 x 25º on his right eye and -2.50-0.50x145º on his left eye. He had nuclear sclerotic cataract grade I on both eyes, and no other relevant findings at the slit lamp examination. His intraocular pressure was 20 on both eyes. Gonioscopy showed open angles on both eyes.
- Figure 9 shows his optic nerves.
- The peripheral retina showed focal photocoagulation scars surrounding retinal tears at the temporal superior quadrant on both eyes.
- His HRT confirmed the findings shown in the photographs: severe thinning of the neural rim on the right eye with abnormal stereometric parameters. On left eye the stereometric parameters were normal. (Figure 10)
- His visual fields showed on the right side altitudinal superior and inferior deep arcuate scotomas while on the left, there was a general depression along with superior and inferior arcuate scotomas. (Figure 11)
- Pachymetry was right 515 µm and left 525 µm.
Treatment
The patient was started with therapy using 2 topical aqueous suppressants. Intraocular pressure dropped to 12 mmHg on both eyes. Eight months later progression of visual field loss persisted, so, a second topical agent was added to the patient’s regimen (Figure 12).
IMAGE LIBRARY
Differential Diagnosis
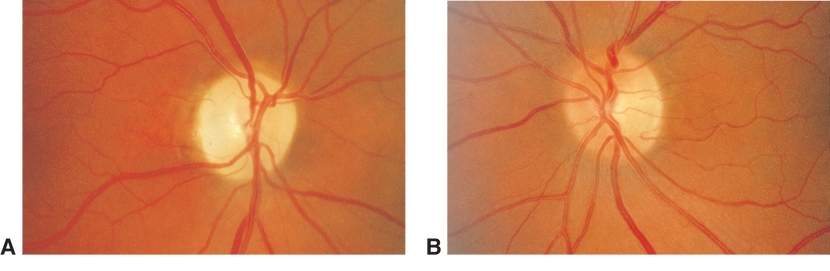
Figure 1. Fundus photographs demonstrating diffuse optic atrophy (A) compared with normal optic disc appearance (B).(Reproduced, with permission, from Foorozan R. Neuro-Ophthalmology. Basic and Clinical Science Course, Section 5, American Academy of Ophthalmology, 2013–2014. Courtesy of Steven A. Newman, MD.)
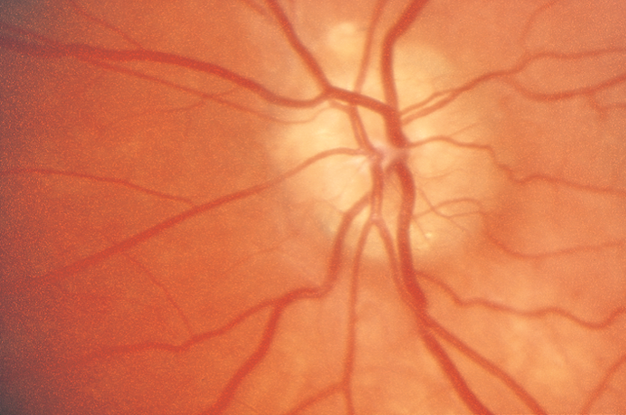
Figure 2. Optic nerve drusen. (Reproduced, with permission, from Foorozan R. Neuro-Ophthalmology. Basic and Clinical Science Course, Section 5, American Academy of Ophthalmology, 2013–2014.)
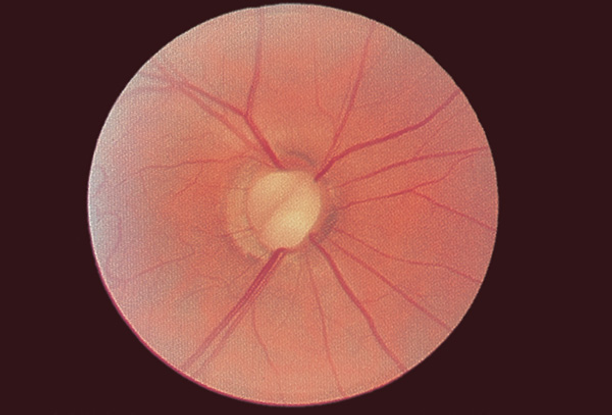
Figure 3. Optic nerve coloboma. (Reproduced, with permission, from Raab EL. Pediatric Ophthalmology and Strabismus, Section 6, Basic and Clinical Science Course American Academy of Ophthalmology, 2013–2014.)
Signs/Symptoms
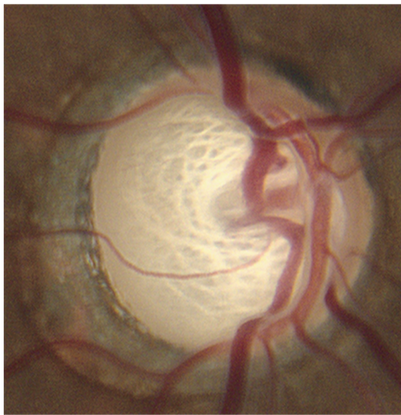
Figure 4. Optic nerve abnormalities include increased cup to disc (C:D) ratio. (© 2013 American Academy of Ophthalmology, Courtesy of Ronald L. Gross, MD.)
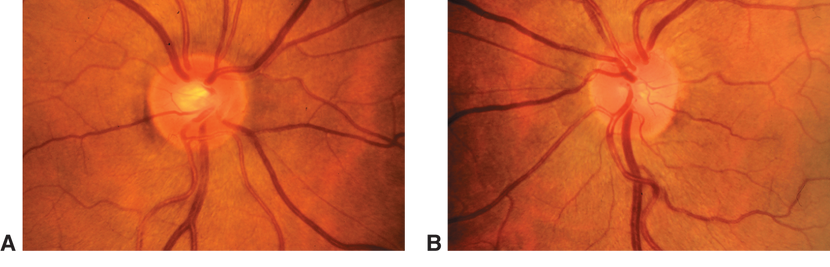
Figure 5. Optic nerve abnormalities include asymmetry of C:D between both eyes greater than 0.2. (© 2013 American Academy of Ophthalmology. Courtesy of G. A. Cioffi, MD.)
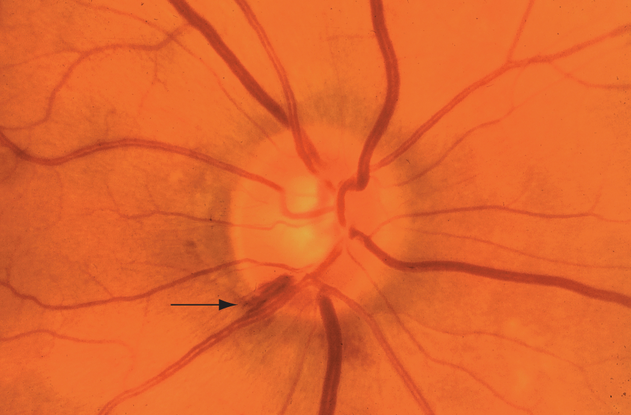
Figure 6. Hemorrhage (arrow) of the right optic nerve at the 7-o’clock position in a patient with early open-angle glaucoma. (© 2013 American Academy of Ophthalmology. Courtesy of G. A. Cioffi, MD.)
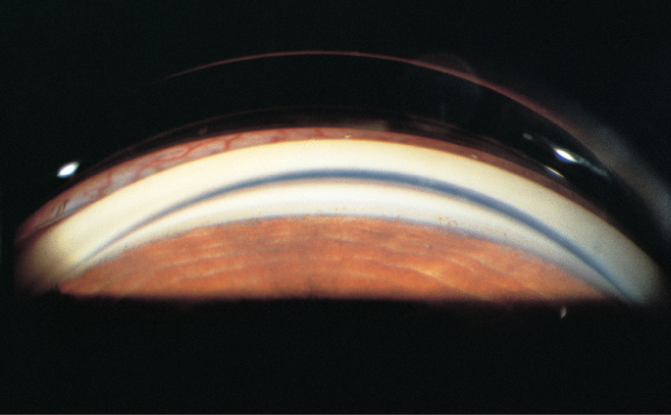
Figure 7. Gonioscopy demonstrates open angle. (© 2013 American Academy of Ophthalmology. Courtesy of Elizabeth A. Hodapp, MD.)
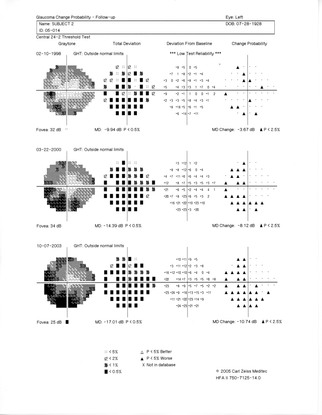
Figure 8. Visual field testing may demonstrate visual field defects that do not respect the vertical meridian. (© 2013 American Academy of Ophthalmology. Courtesy of Ronald L. Gross, MD.)
Case Study
A 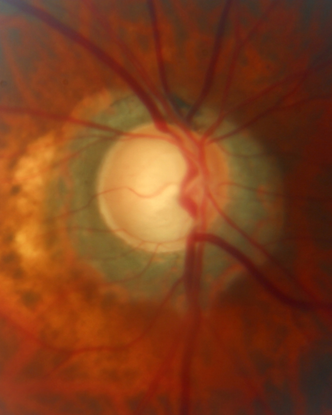 B
B 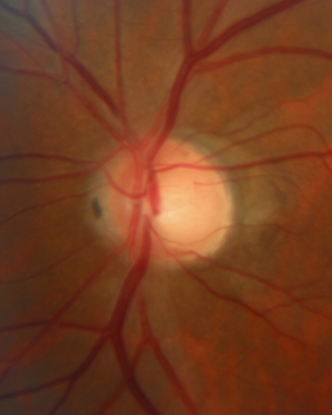
Figure 9. Optic nerve cupping of right (A) and left (B) eyes with peripapillary atrophy. (Courtesy of Sandra Belacazar-Rey, MD.)
A 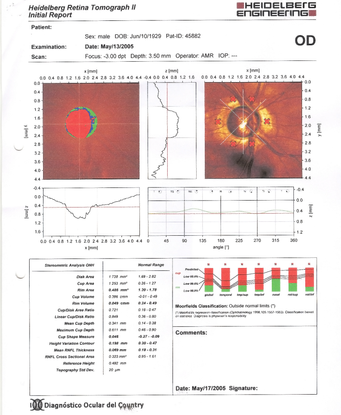 B
B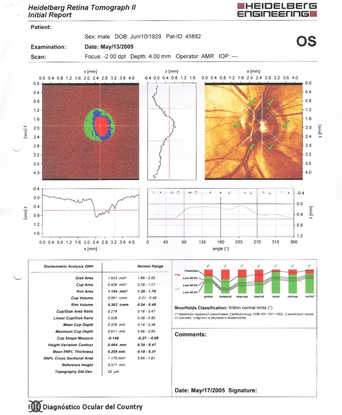
Figure 10. Severe thinning of the neural rim on the right eye with abnormal stereometric parameters (A). On the left eye the stereometric parameters were normal (B). (Courtesy of Sandra Belacazar-Rey, MD.)
A 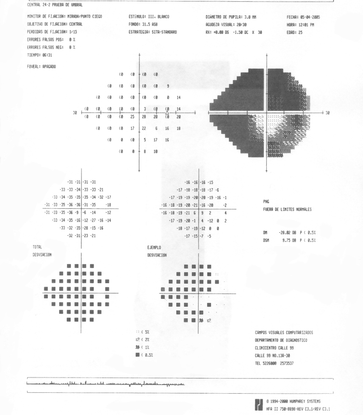 B
B 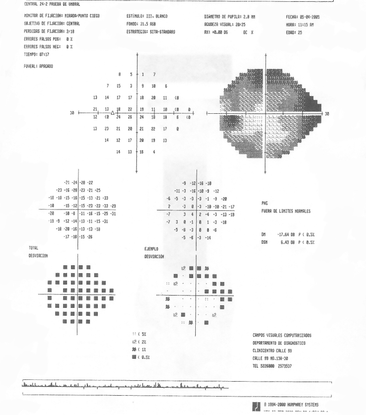
Figure 11. The patient's visual field showed altitudinal superior scotoma and inferior deep arcuate scotoma on the right eye (A). On the left eye, there was a general depression and superior and inferior arcuate scotoma (B). (Courtesy of Sandra Belacazar-Rey, MD.)
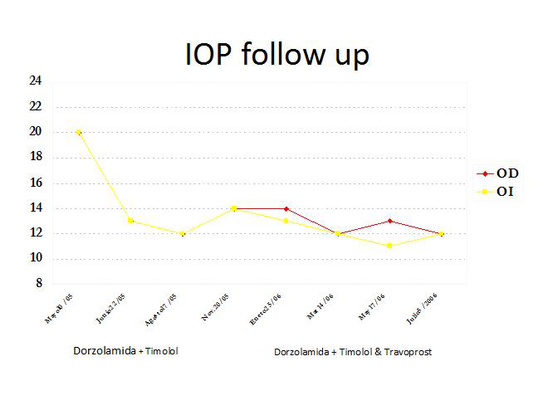
Figure 12. The patient's intraocular pressure decreased in both eyes after treatment with aqueous suppressants was initiated. (Courtesy of Sandra Belacazar-Rey, MD.)
REFERENCES
American Academy of Ophthalmology Glaucoma Panel. Preferred Practice Pattern®Guidelines. Primary Open-Angle Glaucoma. San Francisco: American Academy of Ophthalmology, 2010. Available at: www.aao.org/ppp.
Anton A, Andrada, M, Mujica V, Calle MA, Portela J, Mayo A. Prevalence of primary open-angle glaucoma in a Spanish population: the Segovia study. J Glaucoma. 2004;13:371–376.
Bengtsson BO. Incidence of manifest glaucoma. Br J Ophthalmol. 1989;73:483–487.
Buhrmann RR, Quigley HA, Barron Y, West SK, Oliva MS, Mmbaga BB. Prevalence of glaucoma in a rural East African population. Invest Ophthalmol Vis Sci. 2000;41:40–48.
Coffey M, Reidy A, Wormald R, Xian WX, Wright L, Courtney P. Prevalence of glaucoma in the west of Ireland. Br J Ophthalmol. 1993; 77:17–21.
de Voogd S, Ikram MK, Wolfs RC, Jansonius NM, Hofman A, de Jong PT. Incidence of open-angle glaucoma in a general elderly population: the Rotterdam Study. Ophthalmology. 2005;112:1487–1493.
Ekström C. Elevated intraocular pressure and pseudoexfoliation of the lens capsule
as risk factors for chronic open-angle glaucoma. A population-based five-year follow-up
study. Acta Ophthalmol (Copenh). 1993;71:189–195.
Friedberg M, Rapuano C. Wills Eye Manual: Office and Emergency Room Diagnosis and treatment of Eye Disease, 5th ed. New York: Lippincott Williams & Wilkins, 2008.
Glaucoma. Basic and Clinical Science Course, Section 10, 2011–2012. San Francisco: American Academy of Ophthalmology, 2011:139–158.
Herndon LW, Challa P, Ababio-Danso B, et al. Survey of glaucoma in an eye clinic in Ghana, West Africa. J Glaucoma. 2002;11:421–425.
Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma: the Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–1504.
Kocur I, Resnikoff S. Visual impairment and blindness in Europe and their prevention. Br J Ophthalmol. 2002;86:716–722.
Leske MC, Connell AM, Schachat AP, Huyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol .1994;112:821–829.
Leske MC, Connell AM, Wu SY, et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. The Barbados Eye Studies Group. Arch Ophthalmol. 2001;119:89–95.
Mitchel P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–1669.
Mukesh BN, McCarty CA, Rait JL, Taylor HR. Five-year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology. 2002:109;1047–1051.
Olawoye O, Sarimiye T. Is angle closure glaucoma a problem in Nigeria? Niger J Clin Pract. [In press. October 4, 2013.]
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267.
Quigley HA, West SK, Rodriguez J, Munoz B,Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–1826.
Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851.
Tielsch JM, Sommer A, Katz J, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374.
Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998;105;733–739.
CONTRIBUTORS
Executive Editor:
R. V. Paul Chan, MD, FACS, Weill Cornell Medical College, New York, New York
Section Editors:
Asia-Pacific:
Timothy Lai, MD
Manish Nagpal MBBS, FRCS, MS
Europe:
Christiane Isolde Falkner-Radler, MD
Latin America:
Sandra Belalcázar Rey, MD
North Africa/Middle East:
Ebtisam Kadhim Jaffar AlAlawi, MD,
Sub-Saharan Africa:
Bolutife Olusanya, MBBS(Ib), MSc, FWACS, FMCOphth
Assistant Editors:
Swetangi D. Bhaleeya, MD, Weill Cornell Medical College; New York, New York
Kristin Chapman, MD, Weill Cornell Medical College, New York, New York
Peter Coombs, MD, Weill Cornell Medical College; New York, New York
Michael Klufas, MD, Weill Cornell Medical College, New York, New York
Samir Patel, medical student, Weill Cornell Medical College; New York, New York
Region Contributors:
Middle East:
Khatoon Ali Husain, MD, Senior Resident, Ophthalmology, Salmaniya Medical Complex, Bahrain
Europe:
Jan Darius Unterlauft, MD, Klinik & Poliklinik für Augenheilkunde, Universitätsklinikum Leipzig, Leipzig, Germany
Latin America: Sandra Belalcázar Rey, MD
Sub-Saharan Africa:
Olusola Olawoyem, MD, College of Medicine, University of Ibadan and University College Hospital, Ibadan, Nigeria
Copyright © 2013 American Academy of Ophthalmology®. All Rights Reserved.