Download PDF
Tanya Jones,* a 24-year-old Black woman, had decided that enough was enough. She’d had headaches previously, but this one was much worse than anything she had ever experienced. It just was not going away.
After two weeks, she went to the emergency department. She was told that it was simply a migraine, and she was given pain medications. Over the next week, she developed blurry vision with flashes and floaters in both eyes.
After another week of these visual symptoms, she woke up one morning with significant ocular pain in both eyes. She went to see her local optometrist, and he referred her to us.
We Get a Look
Ms. Jones had no past ocular, medical, or surgical history. She had recently given birth to her first child, who was born with septo-optic dysplasia.
Physical exam. We saw no redness of the eyes and no proptosis. But we did note that one of her cheeks had a small patch of hypopigmentation, which had been hidden under her COVID-19 face mask. The lesion was flat and it was roughly 1 × 1 cm in size.
Vision exam. On examination, Ms. Jones’ best-corrected visual acuity (BCVA) was 20/200 in her right eye and 20/100 in the left. Her intraocular pressures were 17 mm Hg and 16 mm Hg in the right and left eyes, respectively. There was no afferent pupillary defect, and her ocular motility was intact. Confrontation visual fields showed inferior hemifield loss in the right eye and no frank deficit in the left eye.
On slit-lamp exam, the anterior chamber cell count was rare in both eyes.
Dilated fundus exam. On ophthalmoscopy, we noted extensive subretinal fluid in both eyes along with optic disc edema, as well as a serous retinal detachment in the inferior periphery of the right eye.
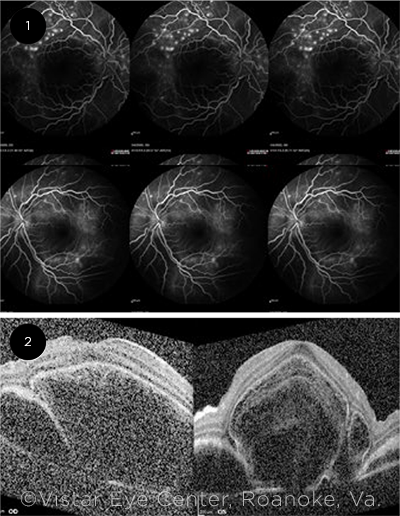
Imaging. Fluorescein angiography revealed areas of punctate hyperfluorescence superior to the macula in both eyes (Fig. 1). In later phases, the same hyperfluorescent areas enlarged over time.
OCT studies of the macula demonstrated large areas of subretinal fluid bilaterally with trace intraretinal fluid (Fig. 2).
 |
|
IMAGING. (1) Fluorescein angiography in the midcirculatory phase shows punctate hyperfluorescence along the arcade vessels in both eyes, more pronounced in the right eye (top row). (2) OCT imaging demonstrates large subretinal cystic spaces and sub-RPE fluid in both eyes.
|
Differential Diagnosis
Ms. Jones presented with an acute onset of decreased visual acuity associated with a headache and ocular pain. On exam, she had rare cell in the anterior chamber, extensive subretinal fluid, and a patch of hypopigmentation on her face.
Given these findings we thought of both autoimmune and infectious causes of uveitis such as syphilis, tuberculosis, sarcoidosis, lupus, sympathetic ophthalmia, and Vogt-Koyanagi-Harada (VKH) disease. Because Ms. Jones lived in an area that was endemic for Lyme disease, we decided to include that condition in our differential.
In addition, we considered other diseases that cause extensive subretinal fluid, including central serous chorioretinopathy.
Narrowing the Diagnosis
A complete uveitis panel was ordered. This included:
- a complete blood count (CBC);
- basic metabolic panel (BMP);
- urinalysis (UA);
- human leukocyte antigen B27 (HLA-B27) typing;
- tests for angiotensin-converting enzyme (ACE), antinuclear antibody (ANA), beta-2 microglobulin, Lyme antibody, and syphilis antibody;
- QuantiFERON Gold; and
- a chest X-ray.
All results came back either negative or normal.
The clinical picture of bilateral uveitis with exudative retinal detachments and optic disc edema, along with no history of trauma and negative testing for several common causes of uveitis, was most consistent with VKH disease. Characteristic findings of VKH on OCT include internal limiting membrane (ILM) fluctuation, increased central retinal thickness, and a subretinal septum.
VKH is a clinical diagnosis, based on criteria that were published in 2001 (see “Revised Diagnostic Criteria for Vogt-Koyanagi-Harada Syndrome”).
Revised Diagnostic Criteria for Vogt-Koyanagi-Harada Syndrome
|
Complete Vogt-Koyanagi-Harada syndrome
I. No history of penetrating ocular trauma or surgery
II. No clinical or laboratory evidence of other ocular or systemic disease
III. Bilateral ocular disease (either A or B below must be met):
A. Early manifestations
1. Diffuse choroiditis as manifested by either: a. Focal areas of subretinal fluid, or
b. Bullous serous subretinal detachments
2. With equivocal fundus findings, then both:
a. Fluorescein angiography showing focal delayed choroidal perfusion, pinpoint leakage, large placoid areas of hyperfluorescence, pooling of dye within subretinal fluid, and optic nerve staining
b. Ultrasonography showing diffuse choroidal thickening without evidence of posterior scleritis
B. Late manifestations
1. History suggestive of findings from IIIA, and either both 2 and 3 below, or multiple signs from 3
2. Ocular depigmentation
a. Sunset-glow fundus, or
b. Sugiura sign
3. Other ocular signs
a. Nummular chorioretinal depigmentation scars, or
b. Retinal pigment epithelium clumping and/or migration, or
c. Recurrent or chronic anterior uveitis
IV. Neurologic/auditory findings (may have resolved by time of examination):
A. Meningismus
B. Tinnitus
C. Cerebrospinal fluid pleocytosis
V. Integumentary findings (not preceding central nervous system or ocular disease):
A. Alopecia
B. Poliosis
C. Vitiligo
Incomplete Vogt-Koyanagi-Harada syndrome
Criteria I to III and either IV or V from above
Probable Vogt-Koyanagi-Harada syndrome
Criteria I to III from above must be present Isolated ocular disease
|
|
Published in the 2021-2022 Basic and Clinical Science Course as Table 9-2. Adapted from Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131(5):647–652.
|
About the Disease
Epidemiology. While VKH disease is most prevalent among individuals with dark skin pigmentation, it can present in any racial or ethnic group. It has a female predominance, and it most commonly presents in individuals in the second to fifth decades of life. It represents less than 5% of uveitis diagnoses in the United States.
It is generally accepted that the condition’s pathophysiology is due to an autoimmune reaction to melanocyte antigens.1 Melanocytes are found in the uvea as well as the meninges, skin, and inner ear.
Individuals with certain genetic backgrounds, including the genetic marker HLA-DR4, are at an increased risk of developing VKH disease.1
Clinical presentation. The clinical presentation of VKH disease can be separated into three phases: prodromal, acute uveitic, and chronic/convalescent phases.
Prodromal phase. This phase typically lasts three to five days. It is characterized by a flu-like illness with symptoms of meningismus, periorbital pain, or tinnitus.
Acute uveitic phase. A bilateral posterior granulomatous uveitis is the most typical finding in the acute phase. It presents as extensive exudative retinal detachments in the posterior pole, vitritis, and/or optic disc edema. Some patients with VKH will also develop anterior uveitis, giving them panuveitis. VKH disease involves both eyes in 94% of cases.
Chronic/convalescent phase. Depigmentation is the hallmark of the chronic phase. Choroidal depigmentation gives the characteristic “sunset glow” fundus. Perilimbal depigmentation carries the eponym of Sugiura sign. Depigmentation of the skin and hair leads to vitiligo and poliosis, respectively. During this chronic phase, it also is possible for patients to relapse into the acute uveitic phase.
Role of imaging in making the diagnosis. While VKH is a clinical diagnosis, characteristic imaging findings can aid in the diagnosis for cases in which the clinical picture isn’t clear
FA. On fluorescein angiography (FA) numerous foci of pinpoint leakage can be seen at the retinal pigment epithelium (RPE) level in the early phase. The dye remains in separate lobules and does not coalesce. In later phases the dye will start to coalesce and leak into subretinal fluid, outlining neurosensory detachments.2
OCT. OCT can show extensive subretinal fluid leading to an average retinal thickness of 750 μm. Other common findings in patients with VKH disease include ILM fluctuations, RPE folds, subretinal septa, and bacillary detachments.3-5 A bacillary detachment involves a separation of the photoreceptor inner segment myoid and ellipsoid layers.4
Prognosis. The prognosis of VKH disease is related to the number and duration of uveitic episodes. Complications of chronic VKH include cataracts, glaucoma, subretinal atrophy, choroidal atrophy, posterior synechiae, and optic atrophy.
Treatment. The mainstay of treatment is early intervention with high-dose steroids and/or immunosuppressants. High-dose steroids are used for two to four weeks, followed by a slow taper over the course of a year or longer.
Immunosuppressive agents can be considered in patients with chronic or recurrent VKH who are intolerant or refractory to steroids.1,6
 |
|
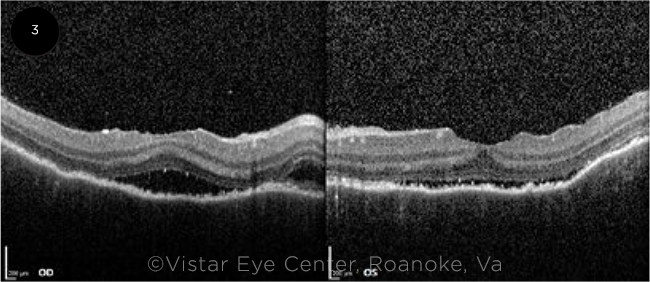
THREE WEEKS LATER. After three weeks of treatment with oral steroids, a marked reduction in the subretinal fluid was seen on OCT in both eyes.
|
Our Patient
Ms. Jones was started on high-dose oral prednisone taper therapy, starting with 60 mg daily.
She then returned to the clinic three weeks later. Her symptoms had improved with BCVA of 20/40 in both eyes. On exam, the subretinal fluid was markedly reduced bilaterally. This was seen clearly on OCT (Fig. 3). We tapered the oral steroids, but she was then lost to follow-up.
We will consider systemic steroid sparing immunosuppressive therapy versus local (intra/periocular) steroid treatments in the future.
___________________________
* Patient name is fictitious.
___________________________
1 Du L et al. Prog Retin Eye Res. 2016;52:84-111.
2 Arellanes-García L et al. Int Ophthalmol. 2007;27(2-3):155-161.
3 Lin D et al. BMC Ophthalmol. 2014;14:87.
4 Cicinelli MV et al. Ophthalmol Retina. 2020;4(4):454-456.
5 Agarwal A et al. Retina. 2021;41(4):774-783.
6 Lai T et al. Eye (Lond). 2009;23(3):543-548.
___________________________
Dr. Weber is a preliminary resident (PGY-1) at the University of Chicago Medical Center. Dr. John is an assistant professor of surgery at the Virginia Tech Carilion School of Medicine in Roanoke, Va., and is a retina surgeon at Vistar Eye Center, which has offices located throughout southwest Virginia. Financial disclosures: None.