By Jong G. Park, MD, Ramsudha Narala, MD, and Prithvi Mruthyunjaya, MD, MHS
Edited By: Ahmad A. Aref, MD, MBA
Download PDF
Megan March,* a 38-year-old woman with no significant past medical or ocular history, saw a local optometrist for a routine eye examination. He told her that the exam had revealed a freckle in the back of her left eye, and she was referred to our clinic for further evaluation.
We Get a Look
Ms. March reported having eye strain when using the computer for a long time, but she had not experienced blurred vision, flashes or floaters, eye pain, or photophobia. Further review of systems was negative, including headache, weakness or tingling in the extremities, chest pain, shortness of breath, and joint pain.
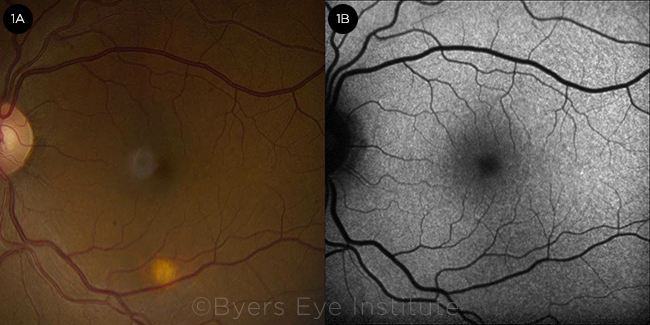
Ms. March’s visual acuity was 20/25 in both eyes, and the anterior segment examination was unremarkable. The posterior segment examination revealed a solitary round, flat, amelanotic choroidal lesion approximately 0.5 disc diameter in size, located in the inferior macula of her left eye (Fig. 1A). There were no signs of vitritis, overlying lipofuscin, or surrounding subretinal fluid.
 |
|
FUNDUS FINDINGS. (1A) Color fundus photograph of the left eye demonstrating a solitary amelanotic flat lesion in the inferior macula. (1B) Fundus autofluorescence of the left eye showing stippled hypoautofluorescence overlying the lesion.
|
Differential Diagnosis and Workup
At this point our differential diagnosis was broad and included amelanotic choroidal nevus or a very early melanoma, choroidal osteoma, choroidal granuloma, choroidal metastasis, solitary idiopathic choroiditis, sclerochoroidal calcification, and posterior scleritis.
We used multimodal imaging to narrow our differential diagnosis. Fundus autofluorescence revealed stippled hypoautofluorescence overlying the lesion (Fig. 1B). B-scan ultrasound demonstrated a lesion measuring 1.5 mm (base) × 0.9 mm (height) that did not have the characteristic “collar stud” or dome-shaped configuration of a choroidal melanoma (Fig. 2A). The A-scan ultrasound showed high internal reflectivity, which is also atypical for a melanoma, although it is sometimes seen in a nevus or metastatic lesion. The ultrasound was also useful in ruling out calcific lesions such as choroidal osteoma or sclerochoroidal calcification, which are classically hyperechoic with posterior shadowing. There was no T-sign around the optic nerve to suggest posterior scleritis.
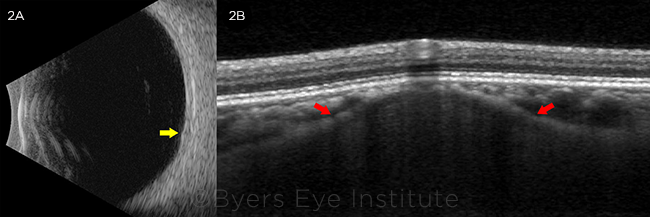 |
FURTHER STUDIES. (2A) B-scan ultrasonography (transverse 6 o’clock) of the left eye revealing an approximately 1.5-mm × 0.9-mm minimally elevated lesion without acoustic shadowing (yellow arrow). (2B) EDI-OCT of the left eye demonstrating a moderately hyporeflective dome-shaped lesion originating from the sclera, with anterior bowing of the inner sclera (red arrows). The choriocapillaris overlying the lesion was thinned, with preservation of the retinal layers.
|
Making the Diagnosis
We ultimately arrived at our diagnosis with enhanced depth imaging (EDI) OCT (Spectralis, Heidelberg), which revealed that the lesion was actually a scleral abnormality.
We identified a dome-shaped lesion arising from the sclera, with anterior bowing of the inner sclera producing focal compression of the overlying choriocapillaris, but not obliteration or compaction as seen in choroidal nevi or melanoma (Fig. 2B). The scleral lesion was less reflective than the normal adjacent sclera. There was no subretinal fluid or drusen. With EDI-OCT to confirm that the lesion originated from the sclera rather than choroid, we diagnosed our patient with solitary idiopathic choroiditis (SIC).
Since SIC is a diagnosis of exclusion in the absence of systemic disease, routine laboratory testing for tuberculosis (TB), syphilis, and sarcoidosis was performed.
Ms. March’s QuantiFERON-TB Gold test was positive, suggesting possible exposure to tuberculosis. On further workup, her chest x-ray was normal; and because she had no systemic or ocular signs of TB, we did not feel that she required treatment to address this ocular lesion.
Discussion
SIC is a diagnosis of exclusion in the absence of systemic disease, and the etiology is unknown. It was first described by Hong et al. as a yellow-white solitary choroidal mass termed unifocal helioid choroiditis.1 Shields et al. redefined the condition as SIC and characterized these lesions as being found more commonly in white individuals and females. They also noted that SIC lesions tended to be posterior to the equator.2 They reported that a minority of patients had active inflammation, and, in these cases, systemic corticosteroids were effective in controlling the inflammation.2
Refining the definition. A follow-up study by Fung et al. using EDI-OCT further characterized SIC lesions as being located deeper than previously thought, and the authors found that SIC involves the sclera in addition to the choroid. They postulated that such lesions may represent a focal scleritis or scleral fibrosis following previous choroiditis.3
In the most recent and extensive study to date, Fung et al. concluded that these lesions in fact originate from the sclera rather than the choroid, and they proposed a new name: focal scleral nodule (FSN).4
EDI-OCT, which demonstrated that our patient’s lesion originated from the sclera rather than the choroid, was crucial in helping us differentiate SIC/FSN from a solitary choroidal granuloma. In all 10 patients initially evaluated by Fung et al. by means of EDI-OCT, the lesions were less reflective than the adjacent normal sclera, and there was overlying compression of the choroid. In some cases, the outer retina and retinal pigment epithelium were disrupted. On infrared reflectance imaging, the lesions showed a nearly uniform hyperreflective area in 9 of 10 patients. The authors thought that this phenomenon was secondary to scleral collagen not blocked by overlying choroidal pigment.3
The follow-up study by Fung et al., which was published last year,4 reported that these lesions were confined to the sclera, and not the choroid, in all 63 patients. The authors proposed that the yellow-white color of the lesions was due to atrophy of the overlying tissues and unmasking of the sclera.
Confounding conditions. Solitary lesions mimicking SIC/FSN have been associated with TB, syphilis, sarcoidosis, and—less commonly—cat-scratch disease, toxoplasmosis, toxocariasis, histoplasmosis, blastomycosis, coccidioidomycosis, aspergillosis, herpes simplex, herpes zoster, Lyme disease, and other inflammatory conditions. Therefore, a thorough ocular examination should be performed to look for active inflammation, and we recommend that a basic uveitis workup be pursued in all cases.
Shields et al. found that while 67% of patients with SIC had no evidence of inflammation, 33% had subretinal fluid, vitritis, or exudation.2 Our patient had a positive QuantiFERON-TB test, and we knew that TB could also manifest as a single choroidal granuloma.5 However, choroidal tuberculomas have not been shown to involve the sclera, which is now considered a defining feature of SIC/FSN.
Conclusion
Our case highlights how multimodal imaging can be invaluable in narrowing down a broad differential diagnosis of an amelanotic fundus lesion. In our patient, EDI-OCT was particularly helpful in making the diagnosis of SIC/FSN given recent advances in the understanding of this condition.
For all patients suspected of having SIC/FSN, we recommend pursuing a uveitis workup to include TB, syphilis, and sarcoidosis. Diagnosing a patient with one of these potentially life-threatening diseases is an important role for us as ophthalmologists.
___________________________
*Patient name is fictitious.
___________________________
1 Hong PH et al. Arch Ophthalmol. 1997;115(8):1007-1013.
2 Shields JA et al. Arch Ophthalmol. 2002;120(3):311-319.
3 Fung AT et al. Ophthalmology. 2013;120(4):852-858.
4 Fung AT et al. Ophthalmology. 2020;127(11):1567-1577.
5 Cangemi FE et al. Ophthalmology. 1980;87(3):252-258.
___________________________
Dr. Park is an ophthalmology resident at the Byers Eye Institute, Stanford University School of Medicine, Palo Alto, Calif. Dr. Narala is a vitreoretinal surgeon at the Retina-Vitreous Associates Medical Group, Los Angeles. Dr. Mruthyunjaya is associate professor of ophthalmology at Stanford University School of Medicine and director of the ocular oncology service at the Byers Eye Institute. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Mruthyunjaya Aura: C; Castle Biosciences: C.
Dr. Narala None.
Dr. Park None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|