Download PDF
Eight-year-old Emily* had had numerous appointments with her optometrist for accommodative esotropia with 20/20 vision. This time, her mother brought her in for right eye pain described as a foreign body sensation. Emily incidentally mentioned to the optometrist that, for the last few weeks, she could no longer see her alarm clock with her left eye.
The optometrist found that her right eye had 20/20 visual acuity (VA) and mild corneal staining consistent with a small corneal epithelial defect. However, the VA in her left eye had declined to 20/40, and confrontation testing demonstrated a nasal visual field loss in that eye. Dilation revealed a large bulbous white lesion protruding from the temporal retina of the left eye. The optometrist had seen Emily four months earlier and was puzzled—and very worried—about this new, large growth. Emily was immediately transferred to the closest pediatric hospital.
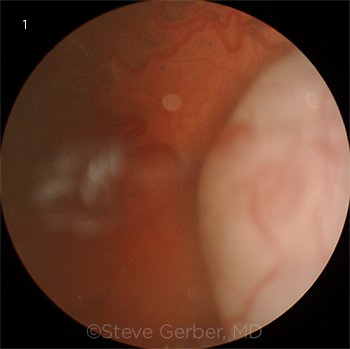 |
|
MYSTERIOUS MASS. Large temporal tumor in the patient’s left eye.
|
We Get a Look
We examined her that same day. For an 8-year-old, Emily allowed us to perform a very thorough examination, though she would not let us do tonometry. Initial VA was 20/20 in her right eye and 20/200 in her left. Pinhole and manifest refraction did not improve her vision, and she had a left afferent pupillary defect. Eye movements were full. The anterior segment exam was normal in both eyes. The lens was clear, as was the vitreous in both eyes.
Examination of the fundus was normal in the right eye. In the left eye, the retina in the posterior pole appeared to be elevated with subtle subretinal fluid throughout the macula, with the elevation extending temporally and inferiorly to the location of the tumor. There were also areas of subretinal pigment clumps. A midtemporal area of retina was more elevated and appeared detached; anterior to this was a huge, whitish temporal mass extending into the vitreous, with engorged and tortuous feeder blood vessels (Fig. 1). A number of hyperreflective lesions were visible in the retina (Fig. 2). Optical coherence tomography confirmed the macular detachment.
She was immediately admitted to the hospital. Magnetic resonance imaging (MRI) and computed tomography (CT) both showed a temporal mass on the left eye with fluid under the retina extending posteriorly from the lesion (Fig. 3). No calcification was noted on these scans.
Next Steps
Retinoblastoma is always a consideration in a new tumor in a child. However, Emily’s case was atypical for retinoblastoma, as she was older than the usual age of presentation, and no calcification was present. Over 80% of retinoblastomas have calcification, and 80% are diagnosed prior to age 4.
Because of the serious retinal issue and the diagnostic uncertainty, she was sent to the nearest academic medical center to see a retina specialist for further evaluation. Fluorescein angiography (FA) was performed; no autofluorescence was seen, and there was early staining of the lesion and leakage in the late phases. This was not typical of Coats disease, one of the more common masquerade conditions for retinoblastoma. B-scan ultrasonography showed a tumor with surrounding retinal detachment and no calcification. The tumor measured 16 mm at its base and 5.6 mm in thickness.
The ambiguities introduced by the lack of calcification and Emily’s age prompted referral that same day to a pediatric retinal physician, who confirmed that the tumor was retinoblastoma. Emily was then referred to an ocular oncologist for treatment.
Differential Diagnosis
Several other retinal disorders have clinical features similar to those of retinoblastoma, leading to possible misdiagnosis. In children older than 5 years of age, the differential diagnosis includes Coats disease, toxocariasis, and familial exudative vitreoretinopathy (FEVR).
Coats disease. Coats disease remains one of the most difficult conditions to differentiate from retinoblastoma—and also one of the most misdiagnosed. Although Coats disease is more typically seen in patients older than those with retinoblastoma, the age ranges can overlap.
In this case, the primary differentiating factor between Coats disease and retinoblastoma was the presence of a solid mass in the retina on CT. In contrast with retinoblastoma, Coats disease causes a yellow, lipid-laden mass rather than a white lesion, and there is associated telangiectatic neovascularization.1
FA can be helpful, as the leakage in retinoblastoma occurs on multiple levels. In Coats disease, there are focal telangiectasias of small- to medium-sized vessels, with “light bulb” aneurysms and profuse subretinal leakage. Although the presence of calcium often helps to identify retinoblastoma, this finding is not universal, as demonstrated in Emily’s case.
Toxocariasis. Ocular toxocariasis results from infection with the parasite Toxocara canis and can also present with a mass in the eye similar to retinoblastoma.2 Even though ocular toxocariasis presents with a unilateral mass without calcifications, it is also characterized by subretinal granuloma formation or inflammation, especially in the vitreous, which did not occur in our patient.2
FEVR. The retinal findings in FEVR can be similar to those noted in Coats disease, with massive subretinal exudation. In addition, there may be avascular areas of peripheral retina and radial retinal folds. FEVR is a form of hereditary retinal dysplasia, inherited in an autosomal dominant pattern. Thus, there is often a known family history of the disease.
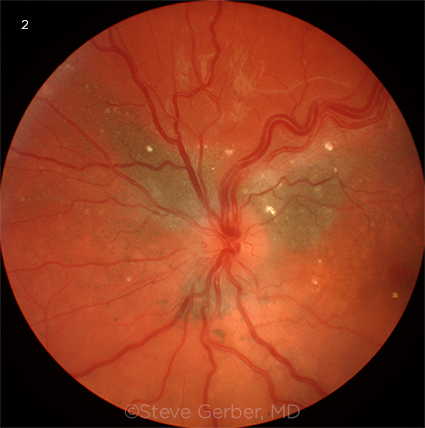 |
|
FUNDUS FINDINGS. Posterior pole showing focal hyperreflective retinal deposits, subretinal pigment, and tortuous, dilated retinal veins.
|
Discussion
Retinoblastoma is the most common form of childhood eye cancer, generally found in children younger than 5 years old. Emily’s case is unusual because approximately 80% of retinoblastoma cases are diagnosed before the age of 4 years, with a median age at diagnosis of 2 years.3 There are some reports of adults being diagnosed with retinoblastoma, but this is extremely rare.
Pathogenesis. Retinoblastoma has been characterized on a genetic level. The gene responsible, Retinoblastoma 1 (RB1), is a tumor suppressor gene.3 A mutation of the gene leads to uncontrolled cell growth and creates the tumor seen in patients with retinoblastoma. While inherited mutations of RB1 have been described, it is also possible for this mutation to happen sporadically. A “two-hit” hypothesis was described stating that after two different mutations occurred in that gene, a tumor would begin to grow.3 In children with a family history of retinoblastoma, it is often the case that one hit was already inherited, making them prone to developing a tumor. However, it is still possible to obtain those two mutations sporadically.
The disease is unilateral in approximately two-thirds of patients and bilateral in one-third. Patients diagnosed with retinoblastoma are classified by whether the mutation is germline (present in all cells of the body) or somatic (present in the tumor only).
The germline mutation is what leads to an inheritable form of the disease, whereas the somatic mutation would only exist in that person.3 Laterality can predict whether the retinoblastoma is caused by a germline or somatic mutation; bilateral tumors most often indicate a germline mutation. Although it was not confirmed whether Emily’s tumor was caused by a germline or somatic mutation, based on presentation it can be assumed that it was mostly likely somatic.
Staging. Currently, the most commonly used method for staging retinoblastoma is the International Classification for Intraocular Retinoblastoma, which grades the tumor in order of increasing severity from A to E. This system is based on tumor size and location and whether vitreous or subretinal seeding is present. Emily’s tumor was considered to be Group D because there was diffuse subretinal seeding more than 3 mm from the tumor and extensive subretinal fluid.
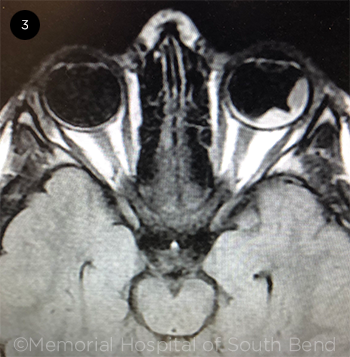 |
|
CLUES FROM MRI. MRI shows the darkened temporal tumor mass, along with the lighter subretinal fluid.
|
Treatment
The treatment plan for a retinoblastoma differs depending on whether the tumor is diagnosed as intraocular or extraocular. Intraocular tumors that are not metastatic can be treated locally, while metastatic intra- or extraocular tumors extending beyond the wall of the eye require either radiation or chemotherapy. Patients with smaller tumors can be treated with cryotherapy or laser therapy. Enucleation can also be part of management depending on vision and treatment potential. Emily’s tumor was diagnosed as intraocular, based on the MRI.
Systemic chemotherapy. The drugs usually administered for retinoblastoma are vincristine, carboplatin, and etoposide, which can be given separately or in combination. Although chemotherapy is an effective means of reducing tumor growth, it carries the risk of multiple systemic adverse effects.
Radiation. Radiation therapy is a broad category that includes many different types of treatment options. Radioactive plaque treatment is used for focal tumors not in the macula. The benefits of this approach are that it helps preserve the patient’s vision and diminishes radiation exposure to other parts of the body. Adverse effects of radiation therapy include damage to other parts of the eye that may lead to glaucoma, retinal detachment, bleeding, and second tumors later in life.
Intra-arterial chemotherapy. Previously, in advanced cases such as Emily’s, the eye was enucleated because there was little hope of salvaging vision. However, intra-arterial chemotherapy (IAC) has revolutionized treatment by saving eyes that would otherwise have been enucleated while also sparing—or at least decreasing—the amount of chemotherapy and radiation needed.4 Melphalan is the agent most commonly injected directly into the ophthalmic artery, resulting in tightly focused delivery of chemotherapy to the tumor.
Multiple studies have shown that IAC is effective as a primary treatment. In particular, it is used in advanced tumors. In a 2012 study, the effectiveness of IAC treatment was evaluated in 76 eyes of 67 patients with retinoblastoma. In treatment-naive eyes, the two-year probability of ocular salvage was 83% for eyes with subretinal seeding only, 64% for eyes with vitreous seeding only, and 80% for eyes with both.5
Among eyes that had previously been treated but had progressed, the two-year probability of ocular salvage was 50% for eyes with subretinal seeding only, 76% for eyes with vitreous seeding only, and 54% for eyes with both. The study concluded that, unlike radiation or systemic chemotherapy, IAC can usually obviate the need for enucleation.5
Adverse effects of IAC include eyelid edema, vitreous hemorrhage, hyperemia of the forehead, and temporary loss of the eyelashes. Complications include retinal artery occlusion, enophthalmos, choroidal occlusion, neovascular glaucoma, and direct toxicity to the retina.
Our Patient’s Course
Emily had three treatments with IAC, which initially resulted in complete resolution of the tumor elevation and the subretinal fluid. Unfortunately, submacular fibrosis limited her final VA to 20/200. On a subsequent follow-up, she had new evidence of retinal seeding and received two additional IAC treatments at a higher dose. This treatment was selected to avoid radiation, large doses of chemotherapy, and enucleation. Recurrence of retinal seeding after these two additional IAC treatments led to the decision to enucleate the eye.
___________________________
*Patient name is fictitious.
___________________________
1 Ghorbanian S et al. Ophthalmologica. 2012;227(4):175-182.
2 Fan CK et al. Clin Microbiol Rev. 2015;28(3):663-686.
3 Corrêa ZM, Berry JL. Review of retinoblastoma. April 28, 2016. aao.org/disease-review/review-of-retinoblastoma. Accessed Sept. 27, 2019.
4 Zanaty M et al. Scientific World Journal. 2014;2014:869604.
5 Abramson DH et al. Br J Ophthalmol. 2012;96(4):499-502.
___________________________
Mr. Kalafatis is a third-year medical student at the Indiana University School of Medicine in Indianapolis, Ms. Myers is a senior undergraduate at St. Mary’s College in South Bend, Ind., and Dr. Gerber is in practice at Advanced Ophthalmology and is clinical professor of ophthalmology at the Indiana University School of Medicine in South Bend. Financial disclosures: None.