What's Happening
New AAOE Website
 |
| UPDATED SITE. The redesigned website www.aao.org/aaoe is a valuable tool for finding and utilizing practice management information, programs, products, and services designed to address the needs of ophthalmic administrators, office managers, managing physicians, billers, coders, and others. |
The American Academy of Ophthalmic Executives (AAOE), the practice management division of the Academy, announces the launch of its new web-site: www.aao.org/practice-management. The revamped site delivers a variety of valuable information, education, and resources for physicians and administrators. It is easy to navigate, offering quick access to critical insights in business management areas such as coding and billing, PQRS, e-prescribing, EHR adoption and meaningful use attestation, financial management, and human resources.
The site offers a number of helpful practice management tools.
- Educational materials specifically for physicians. You’ll find useful articles such as “The Managing Partner,” outlining the leadership skills that drive a practice’s development and success. There is also advice on building your practice, contracting with accountable care organizations, negotiating contracts, and much more. And a section is dedicated to Young Ophthalmologists. (Go to www.aao.org/practice-management and select “Physicians.”)
- Newly redesigned EHR Central. EHR Central contains guidance on planning your transition to EHR, vendor selection, and implementation. You’ll also find a list of system features essential to ophthalmology, as well as the Academy’s Meaningful Use Attestation Guide. AAOE’s EHR Central is the only ophthalmology-specific EHR web-site currently in existence. (Go to www.aao.org/practice-management and select “EHR Central.”)
- The new AAOE Practice Forms Library. This section features more than 100 forms needed to run an ophthalmic practice, specifically in the areas of practice administration, finance, HIPAA, human resources, examination, patient education, testing services, and surgery. (Go to www.aao.org/practice-management and select “Managing a Practice” and then “Practice Operations and Efficiency.”)
- A primer on effective online reputation management. If an ophthalmologist’s practice has few online reviews, it is at increased risk of having a low rating; a higher volume of reviews correlates to a higher average rating. How do you get those reviews, and where should they be placed? AAOE’s article “Protect Your Online Reputation and Grow Your Patient Base” will answer these questions to ensure your online reviews work for you, not against you. (Go to www.aao.org/practice-management and select “Managing a Practice,” then “Building Your Practice,” and click “Marketing.”)
Note that EHR Central is available to Academy members with their Academy login. However, most other sections of the AAOE website are available exclusively to AAOE members. Academy members are invited to join AAOE; details are online.
To check out AAOE’s practice management resources, visit www.aao.org/practice-management.
EyeWiki Contest—Read the Four Winning Articles
EyeWiki is the Academy’s collaborative online encyclopedia where physicians, patients, and the public can view content written by ophthalmologists covering the spectrum of eye disease, diagnosis, and treatment. All ophthalmologists, residents, and fellows are invited to expand this valuable online resource by submitting articles.
In 2012, the Academy invited U.S. residents and fellows-in-training to contribute topics to EyeWiki throughout the year for an article contest. After the judging in December and January, four authors won free trips to the Mid-Year Forum in Washington, D.C.
- Hubert H. Pham, MD, Washington Hospital Center, Georgetown University Hospital: Onchocerciasis (African River Blindness)
- Yasser M. Elshatory, MD, University of Oklahoma, Dean McGee Eye Institute: Age-Related Macular Degeneration
- Crystal Hung, MD, Jules Stein Eye Institute: Glaucomatocyclitic Crisis (Posner-Schlossman Syndrome)
- Rao V. Chundury, MD, St. Louis University: Nonspecific Orbital Inflammation (Idiopathic Orbital Inflammation, Orbital Inflammatory Syndrome, Orbital Pseudotumor)
Read the winning articles at www.aao.org/eyewiki.
 ONE SPOTLIGHT: Maintenance of Certification 101. Visit the MOC Essentials on the Ophthalmic News and Education Network for resources and helpful information about the American Board of Ophthalmology (ABO) Maintenance of Certification (MOC) exam requirements. The MOC Essentials area includes everything you need to prepare for the exam, from Academy-developed study tools to information about the Practicing Ophthalmologists Curriculum (POC), online registration for the 2013 MOC Exam Review Course, answers to frequently asked questions, links to the ABO website, and a glossary of MOC acronyms. ONE SPOTLIGHT: Maintenance of Certification 101. Visit the MOC Essentials on the Ophthalmic News and Education Network for resources and helpful information about the American Board of Ophthalmology (ABO) Maintenance of Certification (MOC) exam requirements. The MOC Essentials area includes everything you need to prepare for the exam, from Academy-developed study tools to information about the Practicing Ophthalmologists Curriculum (POC), online registration for the 2013 MOC Exam Review Course, answers to frequently asked questions, links to the ABO website, and a glossary of MOC acronyms.
For more information, visit www.aao.org/MOCOverview. |
Take Notice
Ask the Ethicist: FDA Warning Letters
Q: I read about the December 2012 FDA Warning Letters sent to five ophthalmology practices stating that their advertising violated sections of the Federal Food, Drug, and Cosmetic Act (the Act) because it provided incorrect or incomplete information about the specific lasers named. The letters noted that failure to correct the violation could result in “seizure, injunction, and civil money penalties.” I use very similar information in my advertising and worry that I may receive one of these FDA Warning Letters. What should I do? I don’t understand what the issue is with naming specific lasers in my advertising.
A: In the Warning Letters, the FDA noted that the providers’ advertisements did not supply adequate information about risks and potential adverse events associated with refractive surgery. The lasers were considered misbranded because the advertising failed to reveal information that a reasonable person would find significant in influencing their decision-making process (known as material facts). This includes any consequences that may result from use of the device, per the Act.1,2
For example, if an advertisement names a specific LASIK device or manufacturer, the advertisement must include “a brief statement of the intended uses of the device and relevant warnings, precautions, side effects, and contraindications” as found on the laser’s FDA-approved labeling. This statement should include the following, especially regarding contraindications for LASIK:
- The risks of dry eye syndrome
- The possible need for glasses or contact lenses after surgery
- Visual symptoms including halos, glares, starbursts, and double vision
- Loss of vision
The Ethics Committee advises you to review additional material (see the Further Reading box, below), meet with your advertising staff, and rewrite your ad copy—either deleting all references to specific lasers or laser companies, or adding the required risk and contraindication information. Then, have a layperson read your draft ad and provide their interpretation of it. Based on what they say, you may have to go back to the drawing board to ensure that there are no potentially false, deceptive, or misleading words or claims in your ad. If uncertainties remain, consider consulting with an experienced health care attorney. You certainly want to avoid the FDA penalties for misbranding or false labeling of a device—up to $15,000 per violation and up to $1 million for all violations resolved in a single proceeding.3
You should know that the FDA specifically regulates the advertising and labeling of restricted medical devices (such as lasers used in refractive procedures), and the FTC regulates advertising in general. To prove a violation of the Act, the FDA requires the labeling or advertising to be false or misleading in any way, while the FTC must show that a misleading statement is materially misleading.
To submit a question, contact the Ethics Committee staff at ethics@aao.org.
___________________________
1 Federal Food, Drug, and Cosmetic Act, 21 U.S.C. §502(q)
2 Federal Food, Drug, and Cosmetic Act, 21 U.S.C. §201(n)
3 Federal Food, Drug, and Cosmetic Act, 21 U.S.C. §333(f)
Customized Solutions to Practice Management Issues
When you have a practice management problem, visit the American Academy of Ophthalmic Executives (AAOE) Consultant Directory. It provides a listing of experts with experience on many issues related to ophthalmology. You can search on a range of topics, including the following:
- accountable care organizations
- practice management software
- web design and management
- electronic health records
- locum tenens
- optical dispensing
- chart auditing
- social media management
For more information, visit www.aao.org/consultant.
D.C. Report
CMS Identifies Ophthalmic Codes
The Centers for Medicare & Medicaid Services (CMS) has identified several potentially misvalued ophthalmic codes in the 2013 Medicare Physician Fee Schedule. They are from three families of ophthalmic codes, which were identified as part of a CMS effort to target codes that have annual charges greater than $10 million and have never been reviewed.
At CMS’s request, the Academy, together with related ophthalmic subspecialty societies, is surveying members as part of the review process to determine and/or justify Medicare pay for the newly targeted codes. They will then defend their find findings before the American Medical Association/Specialty Society Relative Value Scale Update Committee (RUC) in 2013. The RUC reviews of the Academy’s recommendations must be initiated in 2013 for the 2014 fee-schedule process.
The reviews. In April, the Academy and the America Glaucoma Society presented compelling evidence that supported increased work values for CPT codes 66180 and 66185, the aqueous shunt codes. Additionally, eight entropion and ectropion repair codes were reviewed at the end of April and presented with the American Society of Ophthalmic Plastic and Reconstructive Surgery. Six vitrectomy codes will be presented before the RUC in October. These reviews of codes can have a real impact. Last year, for instance, CMS identified codes with significant increases in utilization that had not been reviewed in the last five years, which resulted in the examination of cataract procedures. That review found that the procedures now take less time to perform, and this finding resulted in reduced valuations for them.
The review back story. The RUC reviews society-recommended work and practice expense values, and then forwards its final determinations to CMS. In the past, the agency accepted about 95 percent of the RUC’s findings. However, complaints lodged by primary care physician organizations and the Medicare Payment Advisory Commission about the RUC process—including bias and lack of transparency—have decreased CMS’s acceptance rate to less than 85 percent. CMS made significant cuts in a number of RUC recommendations for procedures in the 2013 fee schedule, including several recommendations that the Academy had argued for. Physician time and/or work values were reduced for CPT code 65222 Removal of corneal foreign body and CPT code 67810 Incisional eyelid biopsy. While saying that it believed there was duplication in pre- and post-service physician time, CMS did not provide any rationale or methodology for the substituted lower times and values. The Academy believes other arbitrary and unfounded decreases could occur again this year.
The results of the RUC reviews will not be known until CMS publishes its final 2014 Medicare Physician Fee Schedule later this year. In the meantime, the Academy will defend ophthalmology’s recommended values and oppose any inappropriate reductions.
Meeting Matters
Get Ready for the Annual Meeting
Next month, opportunities to prepare for the Annual Meeting begin in full force.
- Online Program Search. Starting in early June, use the Online Program Search to view 2013 Annual Meeting instruction courses, symposia, and other events by day, topic, type of event/course, special interest, or presenter. You can also log in to begin building your personal calendar. Visit www.aao.org/2013, click on “Meeting Program” in the left navigation bar, then click “Online Program Search.”
- Registration and housing. On June 26, registration and housing opens for Academy and AAOE members; it opens for nonmembers on July 10. Find complete registration information online starting next month. Visit www.aao.org/registration.
- Tour Program. The 2013 Tour Program will be available online in June. Join us in exploring the rich history, traditions, music, and food of New Orleans. Visit www.aao.org/2013.
- EyeNet’s “Destination New Orleans.” This special EyeNet series begins in the June issue, with program directors sharing their tips for this year’s Annual Meeting. Visit www.eyenet.org next month.
Exchange Ideas at the Annual Meeting
The Annual Meeting provides a valuable opportunity to collaborate with your peers. Take advantage of the informal, small group learning experiences at the Annual Meeting such as Breakfasts With the Experts and gatherings at the Learning Lounge and the Academy Café. Or if you prefer to set up a small meeting with a few colleagues and need a comfortable place to meet, there are several options:
- Senior ophthalmologists can visit the SO Lounge.
- Members in training and ophthalmologists in their first five years of practice have access to the YO Lounge.
- International attendees may use the International Center.
- All are welcome to drop by one of the two Rest Stops or grab lunch at Bistro AAO on the exhibit hall floor.
Subspecialty Day 2013: Start Planning
Information about the seven Subspecialty Day meetings in New Orleans is now online. View meeting titles and descriptions, faculty, and program directors. Be sure to watch for program details and updates throughout the summer and fall.
This year’s Subspecialty Day takes place on Friday, Nov. 15, and Saturday, Nov. 16, and features:
- Two Friday/Saturday meetings—Refractive Surgery and Retina.
- Five Saturday-only meetings—Cornea, Glaucoma, Neuro-Ophthalmology, Oculofacial Plastic Surgery, and Pediatric Ophthalmology.
Explore all seven meetings. If you register for a Saturday-only meeting, you may attend any of the Subspecialty Day meetings that take place on that day. If you register for a two-day meeting, you can also attend any of the Friday sessions.
Visit the Annual Meeting exhibit hall. On Saturday, your Subspecialty Day badge entitles you to visit the Annual Meeting Exhibition.
Visit www.aao.org/2013, and click “Subspecialty Day” in the left navigation bar.
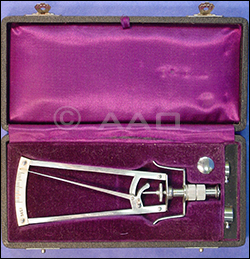 MUSEUM: THIS MONTH IN OPHTHALMIC HISTORY. On May 12, 1905, ophthalmologist and professor Hjalmar A. Schiøtz, MD (1850-1927), invented his indentation tonometer: the Schiøtz tonometer. It was not the first of its kind, but it quickly became the most clinically useful. For 50 years after its introduction, the Schiøtz tonometer was the instrument most commonly used to measure intraocular pressure and study glaucoma. Today, this test is not as accurate as applanation tonometry and therefore is not used frequently by ophthalmologists, although physicians in other specialties may still use it. MUSEUM: THIS MONTH IN OPHTHALMIC HISTORY. On May 12, 1905, ophthalmologist and professor Hjalmar A. Schiøtz, MD (1850-1927), invented his indentation tonometer: the Schiøtz tonometer. It was not the first of its kind, but it quickly became the most clinically useful. For 50 years after its introduction, the Schiøtz tonometer was the instrument most commonly used to measure intraocular pressure and study glaucoma. Today, this test is not as accurate as applanation tonometry and therefore is not used frequently by ophthalmologists, although physicians in other specialties may still use it.
To learn more, visit www.museumofvision.org/about, then select “News Articles.” |
Members at Large
Council Brings Decorative Contacts Issue to Forefront
 |
| COUNCIL IN ACTION. The Academy's Council during the advisory recommendation hearing. |
The Academy Council is the policy advisory body to the Academy Board of Trustees. It consists of 102 ophthalmologists representing 52 state societies and 29 subspecialty and specialized interest societies. To help educate the public about the dangers of circle decorative lenses, which extend past the iris, the Council submitted a Council Advisory Recommendation (CAR) to the Board in April of 2011. Two years later, public awareness about the hazards of these types of contact lenses has vastly increased.
Council requests priority action. “By bringing the issue of circle lenses to the attention of the Academy Board, the Council ultimately provided the vehicle for many positive outcomes,” noted Thomas “Tim” L. Steinemann, MD, councilor for CLAO. “This is truly an issue that all ophthalmologists should care about, as it has a significant impact on our younger patients.”
The CAR pointed out that circle decorative lenses are not FDA approved. They are sold illegally online—without a medical contact lens fitting or contact lens prescription, without placement and removal instructions, without lens care instructions, and without ongoing involvement of an eye care professional. After Dr. Steinemann introduced the CAR on behalf of CLAO to the Council, it was forwarded for priority action to the Board.
A campaign is launched. This resulted in a successful and collaborative national media campaign led by the Academy in partnership with CLAO and others. In 2012, the Academy’s public-facing website, EyeSmart, launched a campaign warning consumers that lenses worn with Halloween costumes could seriously impact vision if purchased without a prescription. Decorative lens safety messages were developed for print, online, radio, and television channels. The Academy worked with targeted state ophthalmology societies on a broad range of messaging, resulting in more than 69 million total media impressions.
Further action is taken. The Canadian Ophthalmological Society (COS) is also represented as an organization on the Academy Council. It benefited from participating in the Council’s debate of the matter, after which the COS initiated an effort to enact Bill C-313 to amend the Canadian Food and Drugs Act so it would require noncorrective (cosmetic/decorative) contact lenses to be treated as class II medical devices. This law passed in December 2012 and brought the rules for decorative lenses in Canada —where they had previously been unregulated—closer to legislation enacted in the United States. Since 2005, the FDA has considered such lenses to be medical devices requiring a prescription from a licensed eye care professional.
“I’m gratified that the FDA is now seriously looking at websites offering these illegal lenses,” said Dr. Steinemann. He hopes that “we continue the conversation on decorative lens regulation on a worldwide basis, as these lenses are largely unregulated in many Pacific Rim countries, for example.”
“We applaud Dr. Steinemann for bringing this issue forward on behalf of CLAO. This is just one example of how the Council can help bring issues to the attention of the Academy Board for action,” said Russell Van Gelder, MD, PhD, Academy Council chairman. Dr. Steinemann was recently recognized with a Memorandum of Understanding by the Ohio Optical Dispensers Board for “encouraging Congress to amend federal law in 2005 to mandate that all contact lenses, including cosmetic or colored contacts, are classified as restricted medical devices requiring a prescription from a medical professional for purchase.”
To view a list of councilors—along with the state, subspecialty, or specialized interest society that each represents, visit www.aao.org/about, choose “Governance” in the left navigation bar, then click “Council.”
People
Joan W. Miller, MD, has been elected to Academia Ophthalmologica Internationalis (AOI), a university-centered international organization that limits active membership to 70 people. Dr. Miller is professor and chairwoman of ophthalmology at Harvard Medical School and chief of ophthalmology at Massachusetts Eye and Ear Infirmary and Massachusetts General Hospital. She is the second woman from the United States to join the AOI.
On March 18, Castle Connolly Medical presented Jerry A. Shields, MD, with a National Physician of the Year Award for Clinical Excellence. This award recognizes those whose work has improved the lives of people worldwide and who dedicate themselves to their patients and the practice of medicine. Dr. Shields is director of the oncology service at Wills Eye Institute and professor of ophthalmology at Thomas Jefferson University in Philadelphia.
On Feb. 12, V.K. Raju, MD, was awarded the Dr. Nathan Davis International Award in Medicine, which honors physicians for outstanding international service. The 2013 awards were presented at the Grand Hyatt Washington Hotel in Washington, D.C., in conjunction with the National Advocacy Conference. Dr. Raju is the founder and medical director of the Eye Foundation of America, an organization that provides eye care in 21 developing countries to combat avoidable childhood blindness. He is also a clinical professor of ophthalmology at West Virginia University.
Passages
The Academy’s 2006 Laureate, Lorenz E. Zimmerman, MD, died on March 16. He was 93.
Dr. Zimmerman was known as the founder of modern ophthalmic pathology. His long and prolific career began upon completion of his general pathology residency at the Walter Reed Army Medical Center in 1950. Dr. Zimmerman then became the pathologist in charge of a field hospital pathology laboratory in Korea, and was awarded the Bronze Star and the Legion of Merit. In 1954, he became the chairman of ophthalmic pathology at the Armed Forces Institute of Pathology and remained in that position for 52 years.
Dr. Zimmerman was the coauthor of Ophthalmic Pathology, considered the preeminent text in the field, and he received numerous awards and honors for his contributions to ophthalmology. He also made pivotal contributions to the practice of ophthalmology, including the recognition of various entities causing leukocoria and the management of ocular melanoma. “Dr. Zimmerman’s passing should make every ophthalmologist pause and reflect on the impact one person can have on a profession,” said Academy Executive Vice President and CEO David W. Parke II, MD. “One of only 14 Academy Laureates, Dr. Zimmerman set a very high bar for scholarship and for professional dedication to teaching and mentoring. The field of ophthalmic pathology in large measure can trace its lineage to his contributions. We have all lost a great colleague. Our condolences go out to the Zimmerman family.”